Skin Improvement Effects of Phytosterol Ester Derived from Rice Bran
Article Information
Mizuka Kobayashi, Shiori Nakagawa, Toshio Nakamura*, Takuo Tsuno
TSUNO RICE FINE CHEMICALS Co., ltd, Ito-gun, Katsuragi-cho, Chonomachi 2283, Wakayama, Japan
*Corresponding Author: Toshio Nakamura, TSUNO RICE FINE CHEMICALS Co., ltd, Ito-gun, Katsuragi-cho, Chonomachi 2283, Wakayama, Japan
Received: 23 December 2023; Accepted: 29 December 2023; Published: 30 January 2023
Citation: Mizuka Kobayashi, Shiori Nakagawa, Toshio Nakamura, Takuo Tsuno. Skin Improvement Effects of Phytosterol Ester Derived from Rice Bran. Journal of Food Science and Nutrition Research 6 (2023): 17-23.
View / Download Pdf Share at FacebookAbstract
Rice bran contains an abundance of phytosterols. We have prepared phytosterol ester extract (PEE), which consists of a phytosterol ester and fatty acids made from the by-product of extracting oil from rice bran. PEE has been registered as a cosmetic ingredient in rice bran-derived products, and its physical functions, such as its high water retention and emollient effects, are evident, although its biological functions remain poorly understood. In this study, we clarified the biological effectiveness of PEE. Treatment of 0.01% PEE to normal human fibroblasts cell (Hs68) resulted in increased amounts of hyaluronic acid and type III collagen. On the other hand, in the face application test with human volunteers, a significantly reduced amount of trans-epidermal water loss (TEWL) was found in the 8th and 12th weeks. By image analysis, improvements in facial redness and pore troubles were also observed using a visual analog scale (VAS) questionnaire. It is suggested that PEE improves barrier function and maintains the repair of the dermis structure.
Keywords
Rice bran, Oryza sativa, Phytosterol ester, Hyaluronan, Type III collagen, Barrier function
Rice bran articles; Oryza sativa articles; Phytosterol ester articles; Hyaluronan articles; Type III collagen articles; Barrier function articles
Rice bran articles Rice bran Research articles Rice bran review articles Rice bran PubMed articles Rice bran PubMed Central articles Rice bran 2023 articles Rice bran 2024 articles Rice bran Scopus articles Rice bran impact factor journals Rice bran Scopus journals Rice bran PubMed journals Rice bran medical journals Rice bran free journals Rice bran best journals Rice bran top journals Rice bran free medical journals Rice bran famous journals Rice bran Google Scholar indexed journals Oryza sativa articles Oryza sativa Research articles Oryza sativa review articles Oryza sativa PubMed articles Oryza sativa PubMed Central articles Oryza sativa 2023 articles Oryza sativa 2024 articles Oryza sativa Scopus articles Oryza sativa impact factor journals Oryza sativa Scopus journals Oryza sativa PubMed journals Oryza sativa medical journals Oryza sativa free journals Oryza sativa best journals Oryza sativa top journals Oryza sativa free medical journals Oryza sativa famous journals Oryza sativa Google Scholar indexed journals Phytosterol ester articles Phytosterol ester Research articles Phytosterol ester review articles Phytosterol ester PubMed articles Phytosterol ester PubMed Central articles Phytosterol ester 2023 articles Phytosterol ester 2024 articles Phytosterol ester Scopus articles Phytosterol ester impact factor journals Phytosterol ester Scopus journals Phytosterol ester PubMed journals Phytosterol ester medical journals Phytosterol ester free journals Phytosterol ester best journals Phytosterol ester top journals Phytosterol ester free medical journals Phytosterol ester famous journals Phytosterol ester Google Scholar indexed journals Hyaluronan articles Hyaluronan Research articles Hyaluronan review articles Hyaluronan PubMed articles Hyaluronan PubMed Central articles Hyaluronan 2023 articles Hyaluronan 2024 articles Hyaluronan Scopus articles Hyaluronan impact factor journals Hyaluronan Scopus journals Hyaluronan PubMed journals Hyaluronan medical journals Hyaluronan free journals Hyaluronan best journals Hyaluronan top journals Hyaluronan free medical journals Hyaluronan famous journals Hyaluronan Google Scholar indexed journals Type III collagen articles Type III collagen Research articles Type III collagen review articles Type III collagen PubMed articles Type III collagen PubMed Central articles Type III collagen 2023 articles Type III collagen 2024 articles Type III collagen Scopus articles Type III collagen impact factor journals Type III collagen Scopus journals Type III collagen PubMed journals Type III collagen medical journals Type III collagen free journals Type III collagen best journals Type III collagen top journals Type III collagen free medical journals Type III collagen famous journals Type III collagen Google Scholar indexed journals Barrier function articles Barrier function Research articles Barrier function review articles Barrier function PubMed articles Barrier function PubMed Central articles Barrier function 2023 articles Barrier function 2024 articles Barrier function Scopus articles Barrier function impact factor journals Barrier function Scopus journals Barrier function PubMed journals Barrier function medical journals Barrier function free journals Barrier function best journals Barrier function top journals Barrier function free medical journals Barrier function famous journals Barrier function Google Scholar indexed journals food additives articles food additives Research articles food additives review articles food additives PubMed articles food additives PubMed Central articles food additives 2023 articles food additives 2024 articles food additives Scopus articles food additives impact factor journals food additives Scopus journals food additives PubMed journals food additives medical journals food additives free journals food additives best journals food additives top journals food additives free medical journals food additives famous journals food additives Google Scholar indexed journals cell bank articles cell bank Research articles cell bank review articles cell bank PubMed articles cell bank PubMed Central articles cell bank 2023 articles cell bank 2024 articles cell bank Scopus articles cell bank impact factor journals cell bank Scopus journals cell bank PubMed journals cell bank medical journals cell bank free journals cell bank best journals cell bank top journals cell bank free medical journals cell bank famous journals cell bank Google Scholar indexed journals
Article Details
Introduction
Rice is a grain cultivated in many countries worldwide, particularly in East and Southeast Asia. Rice bran, a non-edible part of rice, accounts for approximately 10% of its weight and is known to contain various functional ingredients [1]. Phytosterols and their esters are among the functional components of rice bran. Phytosterols are used as food additives, such as natural emulsifiers and processing aids, and are widely found in pharmaceuticals and cosmetics. Their biological functions have been studied in medicine and food, and one of their effects is the suppression of blood cholesterol [2-4]. Phytosterols are also known to exert anti-inflammatory effects in cosmetics [5]. However, the biological functions of the ester forms of phytosterols have not been elucidated.
Phytosterols differ depending on the plant species. Rice bran contains large amounts of the plant sterols β-sitosterol and campesterol, as well as the triterpene alcohols cycloartenol and 24-methylenecycloartenol [6,7]. We developed a method for producing PEE from by-products of the rice bran oil refining process. PEE is an oil rich in esters of phytosterols, triterpene alcohols, and fatty acids that has been confirmed to have high water-retention and emollient effects [8]. However, there are few examples of biological verification of the cosmetic effects of phytosterols using cultured skin cells. Therefore, in this study, we examined whether phytosterol esters derived from rice bran have effects on the skin.
Materials and Methods
Samples
The PEE used in this study was provided by Tsuno Food Industry Co., Ltd (Wakayama, Japan) and was dissolved in DMSO before being added to the medium.
Cell culture
Hs68, a normal human fibroblast, was purchased from the JCRB cell bank. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Fuji Film Wako Pure Chemical Corporation, Osaka, Japan) containing 10% fetal bovine serum (FBS; Biosera, Nuaille, France) and 1% penicillin streptomycin (Fuji Film Wako Pure Chemical Corporation, Osaka, Japan). After 24 h pre-cultivation, the cells were suspended and moved on to a 24-well plate (WATSON, Tokyo, Japan) with 5.0 × 104 cells/well, and pre-cultured overnight at 37 °C under 5% CO2 atmosphere.
mRNA expression level
The PEE was dissolved in DMEM containing 1% FBS to a final concentration of 0.1 or 0.01% (w/v). Pre-cultured cells were treated with the PEE medium and cultured under 5% CO2 at 37 °C for 24 h. Total RNA was extracted from the cells using ISOGEN II (Nippon Gene, Tokyo, Japan). Total RNA dissolved in Milli-Q water was fluorescently labeled using the Qbit RNA BR Assay kit (Thermo Fisher Scientific, Tokyo, Japan), and RNA concentration was calculated using Qbit (Thermo Fisher Scientific, Waltham, MA, USA). Each RNA solution was adjusted to 50 μg/μL. Reverse transcription was performed in a thermal cycler (Thermo Fisher Scientific, Waltham, MA, USA) using the Prime Script RT Reagent kit (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. The cDNA obtained was used as a template. Real-time PCR was performed using the StepOne system (Thermo Fisher Scientific, Waltham, MA, USA) with specific primers. Real-time PCR detected the glyceraldehydes 3-phosphatedehydrogenase (GAPDH), hyaluronan synthase 2 (HAS2), and type III collagen (COL3) genes using the Power Up SYBR Green Master Mix (Takara Bio, Shiga, Japan). The expression levels of the target genes were calculated by comparing with that of the housekeeping gene GAPDH using the ΔΔCT method. Primer sequences of HAS2, COL3, and GAPDH were as follows: HAS2, F5'-GTGGATTATGTACAGGTTTGTGA-3' and R5'-TCCAACCATGGGATCTTCTT-3'; COL3, F5'-TGGTGCCCCTGGTCCTTGCT-3' and R5'-TACGGGGCAAAACCGCCAGC-3'; and GAPDH, F5'-CTCCTGTTCGACAGTCAGCC-3' and R5'-TCGCCCCACTTGATTTTGGA-3' (Seo et al., 2019, Tong et al., 2019).
ELISA
PEE dissolved in DMSO was added to DMEM containing 10% FBS and the PEE concentration was adjusted to 0.01% (w/v). The PEE medium was poured onto pre-cultured cells, and the cells were cultured under 5% CO2 at 37 °C for 48 h. The amounts of hyaluronic acid and type III collagen in the treated culture supernatants were quantified using enzyme-linked immunosorbent assay (ELISA). The examinations were performed using ELISA kits for hyaluronic acid (HA) and collagen type III (COL3) (Cloud-Clone, Houston, TX, USA), respectively. Specifically, PEE-reacted media were added to the wells of a specific antibody-coated 96-well plate to capture hyaluronic acid and type III collagen. Then, the trapped targets were reacted with a specific adsorbent bound to western wasabi peroxidase. The detection was performed by the colorimetric method using tetra-hydroxy benzidine.
Clinical trial subjects
This study was conducted according to the guidelines of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects proposed by the Japan Ministry of Health, Labor, and Welfare. All subjects provided written informed consent before the commencement of the study, which was approved by the Ethics Committees at Tsuno Food Industry Co., Ltd. (approval number: Rin 21-002). In addition, we provided sufficient explanation to test participants during the briefing session. The participants in this study confirmed the registration/selection criteria and did not violate the exclusion criteria. In addition, we selected subjects who confirmed that they were healthy, did not have skin diseases by questionnaire, and did not correspond to the statistical outliers in the measurement items.
Test cream product
The composition of the creams used in this study is shown in table 1. The PEE cream contained 0.5% PEE, and the placebo cream contained the same amount of water instead of PEE.
|
Component |
|
|
Placebo cream |
Water, triethylhexanoin, butylene glycol, stearyl alcohol, behenyl alcohol, PEG- 20 sorbitan cocoate, glyceryl stearate, methylparaben, citric acid |
|
PEE cream |
Placebo cream 0.5% PEE |
Table 1: Test cream product
Clinical trial design
This clinical trial was performed as a placebo-controlled, single-blind test. Application to half of the face was continued for 12 wk. Each cream was applied twice daily in the morning and evening. The subjects were assigned so that the group applying the PEE-containing cream (PEE cream) on the right side and the group applying the placebo cream on the right side were evenly distributed.
Measurements
Measurements were performed every 4 wk after the start of the application (4th, 8th, and 12th weeks). Before the measurement, the subjects washed their faces and remained for 20 min or more in a room with constant temperature and humidity (22 ± 2 °C, RH% = 40 ± 5). The cheeks of the subjects were measured using a Cutometer DUAL MPA580 (Courage + Khazaka, Cologne, Germany) for skin viscoelasticity, and a Tewameter TM Hex (Courage + Khazaka, Cologne, Germany) for transdermal water evaporation (TEWL), which is one of the indicators of skin barrier function. In addition, wrinkles and redness were analyzed using VISIA evolution (Canfield Scientific, Parsippany, NJ, USA) for facial image analysis. Subjects responded to a VAS questionnaire at the time of measurement [9].
Statistical Methods
In this study, the values are shown as mean ± standard error, and only the age of the subjects is shown as mean ± standard deviation. The results of the cell experiments and the VAS questionnaire were analyzed using Dunnett's test, and if a risk rate of 5% or less occurred, it was considered a significant difference. The clinical test results were compared between before and after the application of test creams, and were analyzed by Paired t-test. Cases with a risk rate of 5% or less were determined to have a significant difference. MEPHAS, a statistical analysis program for pharmaceutical data provided by the Center for Genetic Information Experiments at Osaka University, was used.
Results and Discussion
Promotion of HAS2 gene expression and hyaluronic acid synthesis
The effect of PEE on hyaluronic acid (HA) promotion was investigated. HA is widely present in the skin and maintains its hydrophilicity. In this study, we confirmed the expression of the HAS2 gene, which is an enzyme that synthesizes HA from fibroblasts. The change in the expression of the HAS2 gene by PEE was verified by RT-qPCR. HAS2 expression increased in a concentration-dependent manner with the addition of 0.01% or 0.1% PEE (Figure 1a). We also examined the quantitative changes of hyaluronic acid produced in the culture medium using ELISA. Hyaluronic acid production in the medium was greatly increased by the addition of 0.01% PEE, and the concentration was approximately 10 times higher than that in the steady state (Figure 1b). The amount of hyaluronic acid synthesized increased substantially with respect to the degree of enhancement of the HAS2 gene. HAS2, a hyaluronic acid synthase, has been mainly expressed in fibroblasts, but the presence of HAS1 and HAS3 has also been confirmed [10]. It has been speculated that PEE may also increase the gene expression of hyaluronan synthases other than HAS2.
Promotion of COL3 gene expression and type III collagen synthesis
The effect of PEE on the promotion of type III collagen expression was investigated. PEE increased the expression of the COL3 gene in a concentration-dependent manner (Figure 1c). We also verified the quantitative change in type III collagen produced in the medium using ELISA. With the addition of 0.01% PEE, the amount of type III collagen produced in the medium greatly increased (Figure 1d). The dermis is mainly composed of an extracellular matrix, and collagen is one of its main components. There are approximately 30 types of human collagen, and type I collagen is widely distributed in adult skin. Type III collagen, which is thought to be involved in wound healing, is also widely distributed in the skin, especially in newborns, but its presence gradually decreases with age. The reduction of collagen leads to the loss of the structure-maintaining function of the skin [11]. The fatty acid composition of PEE derived from rice bran oil is 40% oleic acid [12]. Oleic acid and linoleic acid are important factors that promote wound healing, and have been confirmed to enhance the expression of type III collagen [13]. It was suggested that PEE treatment increased the amount of type III collagen and contributed to the improvement of sagging and wrinkles.
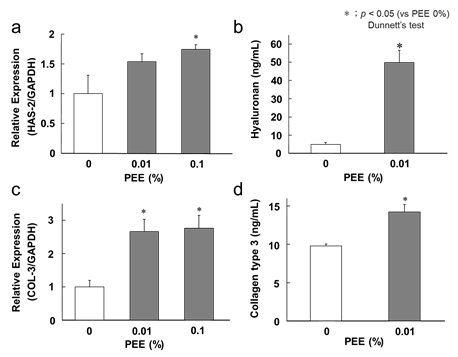
Figure 1: Related factors to hyaluronic acid and collagen
The white bar shows the placebo-treated sides and the PEE sides are shown in gray. a) The effect of enhancing HAS2 gene expression was verified by RT-qPCR. The vertical axis shows the HAS2 gene expression ratio to the housekeeping gene GAPDH, and the expression of no sample added was denoted as 1 (negative control). The gene expression ratio when PEE was added is shown. Error bars indicate the standard error. b) The quantitative change in hyaluronic acid produced in the medium by ELISA was verified. The vertical axis shows the concentration of hyaluronic acid (ng/mL) and the error bar shows the standard error. c) The effect of enhanced COL3 gene expression was verified by RT-qPCR. d) The quantitative change in type III collagen produced in the medium by ELISA was verified.
Result of subject grouping
The two groups were assigned to equalize the measured mean values, such as the skin viscoelasticity R2, trans-epidermal water loss (TEWL), and wrinkle score (Figure 2). There was no significant difference between the initial values on the PEE-applied and placebo-applied sides for all measurement items. The study design for topical application of PEE is shown in figure 3. Twenty-five subjects were assessed for eligibility; only one subject did not meet the inclusion criteria. Twenty-four subjects were divided into two groups: one group applied PEE cream on the right side of the face and the other group applied PEE cream on the left side. Two subjects dropped out during the test period, and the final analysis was conducted on 22 subjects with an average age of 38 ± 13.2 years, of which 10 women in their 40s and 50s had an average age of 51.5 ± 2.46 years.
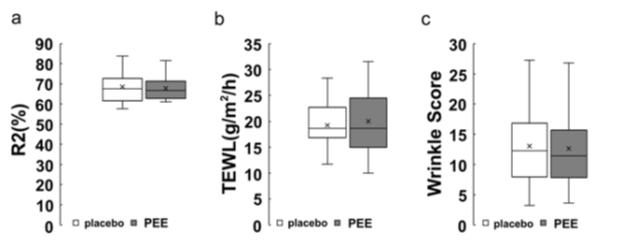
Figure 2: Box plot
A box plot for each measurement item after allocation is shown. The white bar shows the values of placebo treatment sides, and the gray bar shows the values of PEE treatment sides. a) vertical axis denotes R2 values in skin elasticity (%), b) cost transpiration amount of water through skin (g/m2/h), and c) wrinkle score (arbitrary unit) relative to number of wrinkles.
Figure 3: Design of clinical trial
Twenty-two subjects continued half-face application of PEE-containing cream or placebo cream application for 12 wk. The subjects were assigned such that the group applying PEE cream on the right side and the group applying the placebo cream on the right side were evenly distributed.
Effect on skin elasticity
The effect of PEE on the human face was verified using a placebo-controlled half-face application test. The R2 value was used as the index of elasticity. The R2 value is determined by the recovery rate (%) of the skin height after stretch/involution, and values closer it is to 100%, indicate greater elasticity. The viscoelasticity of the skin decreased from the initial measurement value, with a smaller decline in the PEE group than in the control group, and there was no significant difference between the measurement values and the groups (Figure 4a). As type III collagen is promoted in fibroblasts, it was expected that the elasticity of the cheeks would be improved, but no significant contribution to viscoelasticity was observed. Type I collagen accounts for the majority of adult dermal collagen, and the contribution of the increase in type III collagen to skin elasticity may be low.
Effect on barrier function
The amount of trans-epidermal water loss (TEWL), which is an index of skin barrier function, was measured. The TEWL amounts in both groups changed from the 0th week and increased slightly at the 4th week, but were suppressed at weeks 8 and 12. The TEWL of the PEE topical side decreased by -2.68 ± 0.93 at week 8 and by -2.31 ± 1.04 at week 12, and a significant suppression was observed between before and after treatment (Figure 4b). We considered that topical application of PEE to subjects increased the production of hyaluronic acid in the skin. Hyaluronic acid, a glycosaminoglycan, is abundant in the dermis and is involved in water retention. The water retention of human skin is enhanced by increasing the production of hyaluronic acid. Another factor is the increase in type III collagen due to topical application of PEE. Type III collagen is involved in wound healing, and when collagen production is enhanced in the dermis, the repair of the epidermis is accelerated. In mice, an increase in collagen suppresses TEWL and increases the water content of the stratum corneum [14]. Taken together, the enhancement of type III collagen maintains the dermis structure, contributes to the normalization of turnover of the stratum corneum, and improves the barrier function of the skin.
Effect on wrinkle score
The facial wrinkle score was calculated using the image analyzer VISIA evolution. Although the wrinkle score decreased on both face sides of the subjects, there was no significant difference in measurements between the initial and later stages, and between the PEE topical side and placebo side (Figure 4c). Although PEE enhanced type III collagen along with skin elasticity, it was suggested that the actual contribution to human skin wrinkles was small.
Effect on facial redness
The redness of the face was analyzed using VISIA evolution. The redness score in the 4th week of the PEE group decreased -1.15 ± 0.31 points, and significant suppression was observed (Figure 4d). It has been reported that rice bran components such as the plant sterol γ-oryzanol, which is an ester of triterpene alcohol, and ferulic acid, have various anti-inflammatory effects [15]. Anti-inflammatory effects, such as suppression of IL-6 and COX-2, have been reported in plant sterols such as hydrolyzate sitosterol and stigmasterol, as well as with triterpene alcohol [16,17]. Oleic acid, the main fatty acid in rice bran, has also been confirmed to be involved in skin wound healing and have an anti-inflammatory effect [18]. However, the anti-inflammatory action and pharmacokinetics of oleic acid and sterols in the ester form remain unclear. It is also possible that free phytosterols and free oleic acid in the PEE suppress inflammation. Dry skin, due to a decrease in barrier function, also induces chronic inflammation, such as with atopic dermatitis and dry diseases. The application of PEE improved the barrier function of the skin and made it possible to retain water, which improved the dry condition and suppressed the chronicity of the inflammatory reaction.
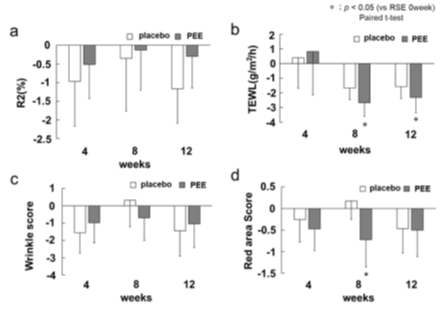
Figure 4: Results of skin measurements
a) amount of change of skin viscoelasticity from the 0th wk is shown. The white bar shows placebo treatment sides, and the gray bar shows the PEE treatment sides. The vertical axis is the total viscoelasticity R2 (%), and the horizontal axis is the number of weeks from the start of the application. Error bars indicate standard error. a) amount of change in water evaporation from the 0th The vertical axis shows the amount of cost water evaporation (g/m2/h). c) amount of change in the wrinkle score from the 0th wk. The vertical axis shows the wrinkle score (arbitrary unit), and the horizontal axis shows the number of weeks from the start of the application. d) amount of change in the redness score from the 0th wk. The vertical axis shows the redness score (arbitrary unit), and the horizontal axis shows the number of weeks from the start of the application.
Improvement of pore issues
The subjects were asked about pore troubles using the VAS questionnaire. The score was significantly increased for the side of the face treated with PEE by 1.39 ± 0.45 at 4 wk, 1.35 ± 0.39 at 8 wk, and 1.7 ± 0.36 at 12 wk compared to the values at 0 wk. At 12 wk, an increase of 1.43 ± 0.38 was observed on the placebo side, but the rate of increase was higher in those with the PEE applications (Figure 5). It is thought that the problems with pores are caused by an increase in sebum amount, an increase in hole volume due to clogging of keratin plugs, and a decrease in elasticity around the pores [19]. In our study, it appears that the improvement in pore issues was not caused by repairing the skin structure and improving elasticity around pores by collagen production [20], but by promoting turnover by improving TEWL. In addition, the suppression of chronic inflammation confirmed in the improvement of redness was also considered to reduce the problem of pores.
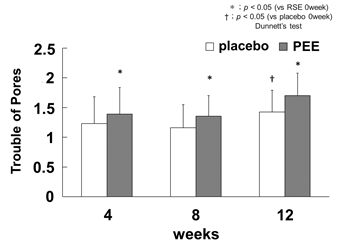
Figure 5: Improvement of pore troubles
The pore troubles by the VAS questionnaire is shown as the amount of change from the 0th wk. The white bar indicates the placebo application side and the gray bar indicates the PEE application. The vertical axis shows the pore trouble score (arbitrary unit) and the horizontal axis shows the number of weeks from the start of the application. The error bars are the standard error, and the significance test was compared with the 0th wk of each application group (* p <0.05, vs PEE 0week, † p <0.05 vs placebo 0week).
Wrinkle improvement
The subjects were asked about wrinkles using the VAS questionnaire. On the PEE application side, the score increased from week 4, but there was no significant difference in the multiple comparison test with week 0 (Figure 6a). Increased expression of type III collagen in culture fibroblasts had been suggested to have the effect of suppressing wrinkle formation in human skin, but the effect of PEE was not clear. However, in the VAS questionnaire, the average score for wrinkles was 4.53 ± 0.39, but for those subjects in their 40s and 50s , the score decreased to 2.32 ± 0.24. It has been reported that wrinkles on the face are only slightly observed in women in their 20s and 30s, and increase in women in their 40s and 50s [21]. For the wrinkle score in VISIA, the average value for subjects in their 20s and 30s was 10.33 ± 1.76, and the average value for subjects in their 40s and 50s was 15.86 ± 1.66, which is a difference of more than 5 points. Therefore, we re-analyzed the wrinkle score of the skin image analysis of subjects in their 40s and 50s (total number 10) (Figure 6b), and it was confirmed that the wrinkle score was significantly reduced to -2.82 ± 1.53 at 4 weeks. This indicates that PEE effectively improved wrinkles in higher-aged women. It is considered that the application of PEE promoted the production of type III collagen in skin parts with problems, repaired the support structure, and improved wrinkles. The effect was weaker in the subjects with fewer skin problems and those who were younger.
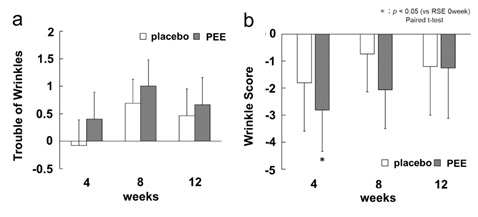
Figure 6: Effect on wrinkles
The white bar indicates placebo application sides, and the gray indicates the PEE application. The vertical axis shows the wrinkle trouble score (arbitrary unit), and the horizontal axis shows the number of weeks from the start of application. a) score of wrinkles by VAS questionnaire as the amount of change from the 0th wk. Error bars indicate standard error. b) wrinkle score by skin image analysis of 10 people in their 40s to 50s as the amount of change from the 0th wk. Error bars indicate standard error.
Conclusion
The plant sterol ester derived from rice bran was found to promote the synthesis of hyaluronic acid and reduce the amount of trans-epidermal water loss, suggesting that it improves the skin barrier function. Topical application of PEE is thought to increase the amount of synthesized hyaluronic acid and the water retention body, resulting in the retention of water that has transpired and the enhancement of the barrier function of the skin. It has also been speculated that PEE promotes type III collagen production, repairs skin structure via wound healing mechanisms, and improves wrinkles by normalizing epidermal turnover and enhancing barrier function.
In addition, a decrease in the redness score was observed after applying PEE, suggesting an anti-inflammatory effect. The phytosterols and triterpenes contained in PEE likely suppressed the inflammatory reaction and further improved the moisturizing property by enhancing barrier function, thereby preventing erythema and pore problems caused by inflammation.
In this study, the sterol ester derived from rice bran is expected to improve wrinkles in the skin and dermis structure, whose barrier function has deteriorated due to inflammation, and can be effective as a cosmetic raw material.
Acknowledgement
We would like to thank Editage (www.editage.com) for English language editing.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Ghosh M. Review on recent trends in rice bran oil processing. J. Am. Oil. Chem. Soc. 84 (2007): 315-324.
- Pollak OJ. Reduction of blood cholesterol in man. Circulation 7 (1953): 702-706.
- Saenjum C, Chaiyasut C, Chansakaow S, et al. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J. Med. Plant. Res 6 (2012): 1070-1077.
- Jones PJ, Raeini-Sarjaz M, Ntanios FY, et al. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J. Lipid. Res 41 (2000): 697-705.
- Vissers MN, Zock PL, Meijer GW et al. Effect of plant sterols from rice bran oil and triterpene alcohols from sheanut oil on serum lipoprotein concentrations in humans. Am. J. Lin. Nutr 72 (2000): 1510-1515.
- Loizou S, Paraschos S, Mitakou S, et al. Chios mastic gum extract and isolated phytosterol tirucallol exhibit anti-inflammatory activity in human aortic endothelial cells. Exp. Bio. Med 234 (2009): 553-561.
- Akihisa T, Yasukawa K, Oinuma H, et al. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry 43 (1996): 1255-1260.
- Shimotoyodome A and Okahara F. Anti-obese and anti-hyperglycemic effects of dietary triterpene alcohols and sterols from rice bran oil. Oleoscience 17 (2017): 269-276.
- Janeš D, Glava? NK. Modern Cosmetics, Ingredients of Natural Origin, A Scientific View, volume 1. Velenje: Širimo dobro besedo (2018).
- Grant S, Aitchison T, Henderson E, et al. A comparison of the reproducibility and the sensitivity to change of visual analogue scales, Borg scales, and Likert scales in normal subjects during submaximal exercise. Chest 116 (1999): 1208-1217.
- Siiskonen H, Oikari S, Pasonen-Seppänen S et al. Hyaluronan Synthase 1: a mysterious enzyme with unexpected functions. Front.Immunol 6 (2015): 43.
- Murakami Y, Adachi H, Sakaida T, et al. The reduction mechanism of the type III/I collagen ratio with aging: age-related change in meprin, a type III collagen propeptide cleavage enzyme. J.Soc. Cosmet. Chem. Jpn 47 (2013): 278-284.
- Takagi T and Itabashi Y. Glass capillary gas chromatography of fatty acids from refined vegetable oils. Journal of Japan Oil Chemists' Society 33 (1984): 23-28.
- Cardoso C, Favoreto S, Oliveira L, et al. Oleic acid modulation of the immune response in wound healing: a new approach for skin repair. Immunobiology 216 (2011): 409-415.
- Lee ES, Yujin Ahn Y, Bae IH, et al. Synthetic retinoid seletinoid G improves skin barrier function through wound healing and collagen realignment in human skin equivalents. Int. J. Mol. Sci 21 (2020): 3198.
- Saenjum C, Chaiyasut C, Chansakaow S, et al. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J. Med. Plant. Res 6 (2012): 1070-1077.
- Loizou S, Paraschos S, Mitakou S, et al. Chios mastic gum extract and isolated phytosterol tirucallol exhibit anti-inflammatory activity in human aortic endothelial cells. Exp. Bio. Med 234 (2009): 553-561.
- Pereda M DCV, Dieamant G, Nogueira C, et al. Sterol-standardized phytopharmaceutical from ground cherry: Corticoid-like properties on human keratinocytes and fibroblasts and its effects in a randomized double-blind placebo-controlled clinical trial. J Cosmet Dermatol 18 (2018): 1-13.
- Cardoso C, Favoreto S, Oliveira L, et al. Oleic acid modulation of the immune response in wound healing: a new approach for skin repair. Immunobiology 216 (2011): 409-415.
- Laneri S, Dini I, Tito A, et al. Plant cell culture extract of Cirsium eriophorum with skin pore refiner activity by modulating sebum production and inflammatory response. Phytother Res 35 (2021): 530-540.
- Schütz R, Rawlings AV, Wandeler E, et al. Bio-derived hydroxystearic acid ameliorates skin age spots and conspicuous pores. Int J Cosmet Sci 41 (2019): 240-256.
- Hayashi S, Matsuki T, Matsue K, et al. Changes of facial wrinkles by aging, sunlight exposure and application of cosmetics. J.Soc. Cosmet. Chem. Jpn 27 (1993): 355-373.?
