Should An Early Hospital Discharge Strategy Be Implemented Following A Successful Primary PCI For Acute ST Elevation Myocardial Infarction?
Article Information
Ofir Koren1,3*, Mohamad Mahamid1, Ehud Rozner1, Rony Hakim2, Yoav Turgeman1,3
1Heart Institute, Emek Medical Center, Afula, Israel
2Anesthesia departments, Emek Medical Center, Afula, Israel
3Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel
*Corresponding author: Ofir Koren, Heart Institute, Emek Medical Center, Afula, Israel & Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel
Received: 18 November 2019; Accepted: 25 November 2019; Published: 29 November 2019
Citation: Ofir Koren1, Mohamad Mahamid, Ehud Rozner, Rony Hakim, Yoav Turgeman. Should An Early Hospital Discharge Strategy Be Implemented Following A Successful Primary PCI For Acute ST Elevation Myocardial Infarction?. Cardiology and Cardiovascular Medicine 3 (2019): 403-421.
View / Download Pdf Share at FacebookAbstract
Background: Early hospital discharge following ST elevation myocardial infarction and primary PCI is reasonable after 72 hours for selective low risk patients. Yet, these recommendations, which were mainly based on data from, are not widely implemented. We present a single center experience regarding efficacy and safety of early hospital discharge.
Method: We conducted a retrospective study based on data from 2014 to 2015. The patients were classified into three groups based on the duration of hospitalization; within 48 h, 48–72 h, and >72 h. The primary endpoints were all-cause mortality and major cardiovascular events (MACE) within 30 days and at 1 year. Secondary endpoint was a diagnosis of acute kidney injury.
Results: 178 patients were included; 60 patients (33.7%) were discharged within 48 hours, 75 patients (42.1%) were discharged after 72 hours, and 43 patients (24.2%) were discharged between 48 and 72 h. Patients discharged >72 h were significantly older (p <0.001), had extensive myocardial damage (p < 0.001) and a significant reduction in left ventricular systolic function (p < 0.02). Most common catheterization approach in this group was the femoral artery (p < 0.02). No statistically significant difference was observed between the three regarding primary and secondary.
Conclusion: Early hospital discharge, within 48 to 72 hours after successful primary PCI for Acute STEMI was not associated with a higher rate of all-cause mortality or MACE up to one year after discharge for selected patients. In an era where the incidence of and mechanical complications are low an early hospital discharge strategy should be widely and routinely implemented.
Keywords
Myocardial Infarction; PCI
Article Details
Introduction
Length of hospitalization is a result of a delicate balance between efficacy and safety of medical treatment and the costs and resources of health services [1, 2]. Over the past decade, several studies have examined this balance in acute myocardial infarction, in particular, ST segment elevation myocardial infarction (STEMI). The treatment of these patients during hospitalization was influenced by various factors such as success rate, complication rate, patient's education, secondary prevention, and community rehabilitation program [3–8].
The past decades have been characterized by enormous innovations in interventional cardiology. Fibrinolytic therapy has been replaced by primary PCI in most centers, radial approach was widely used, new and improved drug eluting stents introduced into daily practice. As a result, a decrease in mortality following acute STEMI was observed [9]. In 2012, the European and American Cardiology Associations published recommendations regarding the duration of hospitalization after primary percutaneous coronary intervention (PPCI) following STEMI [10]. These recommendations were not changed in the subsequent updates in 2015; thus, patients who underwent successful coronary catheterization without complications were to be monitored for at least 24 h in the ICU and, in the absence of complications (especially life-threatening arrhythmias), could be monitored in the non-ICU ward for an additional 48 h (recommendation IC) [11-12]. Early hospital discharge (72 h after admission) is possible and recommended in patients at low risk. Prior discharge, patients should be provided with a scheduled follow-up plan (recommendation IIB). These recommendations were based on the PAMI-II study, published in 1998, which reported that early discharge does not increase the risk of adverse cardiovascular events (e.g. arrhythmia, recurrent infarction, stroke, congestive heart failure or death) during hospitalization and up to six months thereafter [13].
In order to identify patients with low risk of cardiovascular complications, several predictive models such as CADILLAC (Table 1s – supplementary) and ZWOLLE (Table 2s – supplementary) have been proposed [14-16]. Numerous studies have succeeded in validating these models supporting the assumption that in selected group, early discharge (within 72 h) does not increase the risk of major adverse cardiovascular events (MACE) [17-19].
However, only few studies have examined whether patients can be discharged earlier than 72 h. The most prominent study published by Swaminathan et al. in 2015, retrospectively examined 33,920 patients who were admitted due to STEMI. Patients were divided into three groups according to the duration of hospitalization (less than 3 days, 3–5 days, and more than 5 days). The rates of cardiovascular events were higher among patients hospitalized for more than 5 days (2.9 %) compared to the patients in the other two groups (1.7%). Moreover, very early discharge (less than 48 h) was accompanied by a high percentage (approximately 2.9%) of adverse cardiovascular events, similar to that seen in patients who were discharged after 5 days [20, 21].
Study Rationale
In this study, we assessed the outcome of early discharge of patients who were hospitalized following STEMI and primary PCI. The primary endpoints were all-cause mortality and MACE within 30 days and 1 year after hospital discharge.
Materials and methods
Study design
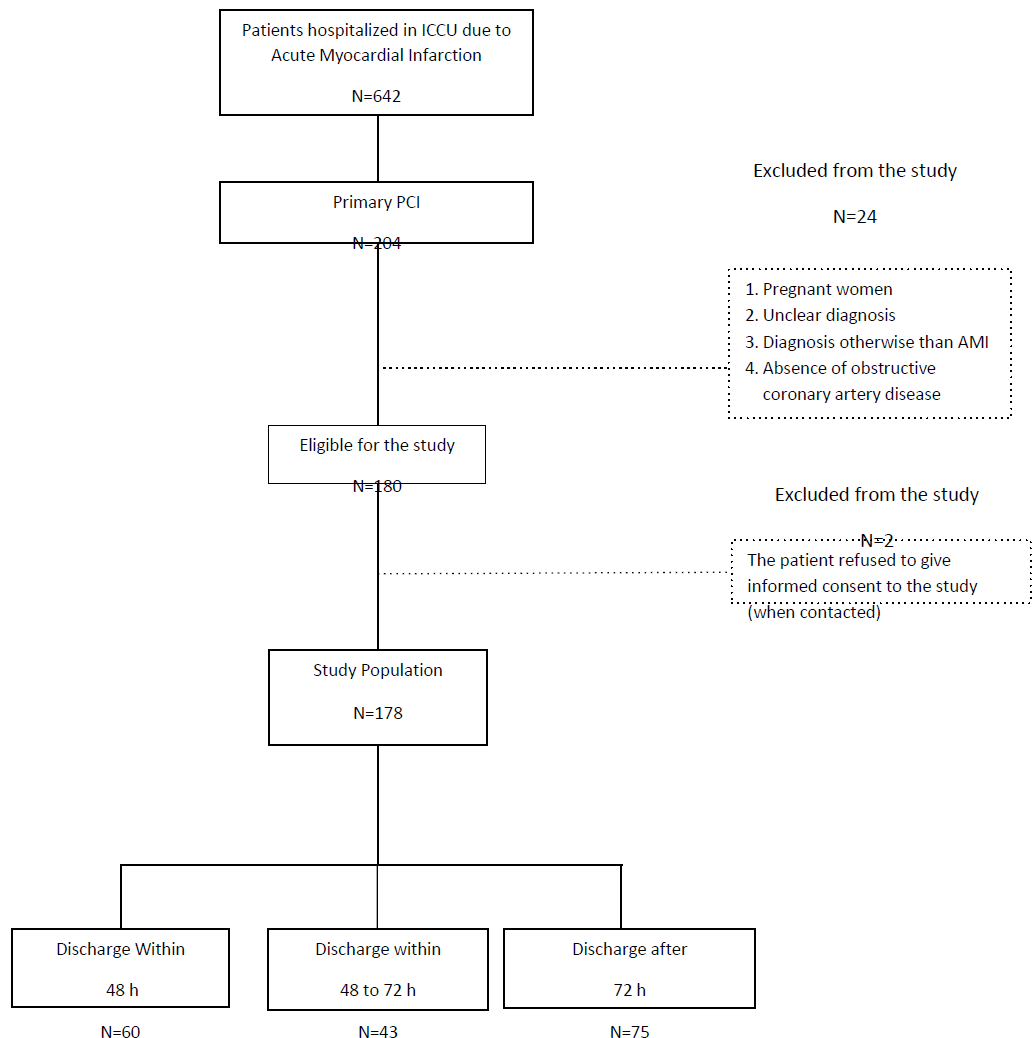
This retrospective cohort study enrolled patients who were hospitalized in an intensive cardiac care unit following STEMI and Primary PCI from January 2014 to December 2015. Pregnant women, unclear diagnosis, diagnosis other than myocardial infarction, urgent catheterization due to a non-ST elevation AMI and the absence of obstructive coronary artery disease on primary PCI were the main exclusion criteria (Table 3s-supplemntary). Additionally, patients who refused to participate in the study when contacted, or in case of significant lack of adequate information in the medical records.
Patients were classified into three groups based on duration of hospitalization: 48 hours, 48 to 72 hours, and more than 72 hours. Primary end points were all-cause mortality and major adverse cardiovascular events (MACE) defined as recurrent myocardial infarction, recurrent unplanned catheterization and hospitalization due to heart failure, stroke or arrhythmia. Secondary end point was acute renal failure during hospitalization and one week following discharge. Median Follow-up period was 1 year.
Sample Size
Minimum Sample size was based on the estimation rate of mortality and MACE rate and within group difference of approximately 5%. Thus, data from 170 patients were collected to demonstrate a statistically significant power of 80% with a two-tailed p-value of p≤.05. Random sampling was done using a predefined arbitrary law for data mining of eligible patients (We used the first patient in each 4-consecutive patient).
Statistics
Categorical variables are presented as frequencies and percentages, and continuous variables are presented using standard distribution indices (e.g., mean, standard deviation, and median). The study groups were compared using the t-test (or the Wilcoxon test). For categorical variables, the Chi-square test (or the Fisher test) was used. Statistical processing was performed using SAS software 9.4. A result was considered statistically significant if the p-value was <0.05.
Ethics
Study was approved by Internal Ethics Committee (IEC). Informed consent was not required due to confidentiality of patient data.
Results
Research population included 921 patients who underwent primary PCI due to acute STEMI and during study period. Twelve patients were excluded. A total number of 909 patients were eligible for the study. We randomly selected 180 patients to meet minimum sample size. Data from two patients was excluded from the study analysis due to their refusal to give informed consent. The remaining 178 patients, who formed the study group, were classified into three groups according to the duration of hospitalization: 60 patients (33.7%) were discharged within 48 h; 43 patients (24.1%) were discharged between 48–72 h; and 75 patients (42.1%) were discharged after 72 h (Figure 1s – supplementary).
The patients in the >72 h group were significantly older than those in the other two groups, while the lowest mean age was noted in the 48–72 h group; the average age of the patients was 62.1 ± 12.3 years (p < 0.001). The number of males was highest in the <48 h group and the lowest in the >72 h group (p < 0.02). Patients in the >72 h group were mainly catheterized via a femoral approach, while those in the <48 h group were catheterized using mainly radial approach (p < 0.02).
No significant differences in co-morbidities such as smoking, obesity (BMI >30), hyperlipidemia, diabetes mellitus, previous incidence of stroke (TIA or CVA), chronic renal failure, ischemic heart disease, peripheral vascular disease or congestive heart failure were noted among the three groups. The average levels of hemoglobin in patients in the >72 h group were significantly lower than those in the <48 h group (13.72 ± 1.58 and 14.55 ± 1.63, respectively; p < 0.01). Serum creatine phosphokinase (CPK) levels were highest in the >72 h and lowest in the <48 h groups (2807 ± 2799 and 1516 ± 1439, respectively). The left ventricular systolic function after catheterization was lowest in the >72 h group when compared with the other groups (p < 0.02) (Table 1).
No statistically significant differences in the following parameters were noted among the three groups: KILLIP class, infarct wall territory, presence of multiple vascular disease, ischemic time or success of catheterization defined by opened IRA (infarct related artery). Likewise, no significant differences in the incidence of mechanical complications related to PCI including coronary artery perfusion, free wall rupture, and tamponade were noted among the groups in this study (p < 0.19)(Table 4s – supplementary).
No differences in infarct related complications such as arrhythmias or conduction disorders, pericarditis, cardiogenic shock, infarct extension, re-infarction or ischemic mitral insufficiency were observed between the three groups ((Table 5s – supplementary).
|
<48 hours (n=60) |
48–72 hours (n=43) |
>72 hours (n=75) |
p-Value |
|
|
Age |
57.0±11.2 (55; 36-88) |
52.2±11.3 (49; 28-79) |
62.1±12.3 (63; 39-92) |
.001 |
|
Gender (male) |
55 (91.7) |
38 (88.4) |
56 (74.7) |
.02 |
|
Smoking |
36 (62.1) |
27 (62.8) |
38 (50.7) |
.30 |
|
Obesity (BMI >30) |
17 (28.8) |
10 (23.3) |
10 (13.3) |
.08 |
|
Chronic Renal Failure |
2 (3.4) |
1 (2.3) |
5 (6.7) |
.48 |
|
Ischemic heart disease |
13 (21.7) |
6 (14.0) |
13 (17.3) |
.59 |
|
High Blood Pressure |
26 (44.1) |
12 (27.9) |
33 (44.6) |
.16 |
|
Hyperlipidemia |
29 (49.2) |
16 (37.2) |
41 (54.7) |
.19 |
|
Diabetes (type 1 or type 2) |
16 (27.1) |
5 (11.7) |
22 (29.3) |
.08 |
|
Stroke (TIA / CVA) |
3 (5.1) |
3 (7.0) |
6 (8.0) |
.81 |
|
Peripheral vascular disease |
2 (3.4) |
0 (0.0) |
1 (1.3) |
.40 |
|
Heart Failure |
0 (0.0) |
2 (4.7) |
3 (5.1) |
.15 |
|
Chronic anticoagulant treatment |
2 (3.4) |
0 (0.0) |
4 (5.3) |
.30 |
|
Chronic antiaggregant treatment |
22 (37.3) |
12 (27.9) |
28 (37.3) |
.53 |
|
Creatinine level (mg/dL) |
0.870±0.203 |
0.824±0.194 |
0.982±0.422 |
.09 |
|
Hemoglobin (% g) |
14.55±1.63 |
14.53±1.85 |
13.72±1.58 |
.01 |
|
Troponin T Peak (Ng / L) |
2906.78±3088 |
3346.07±3165 |
4614.34±3972 |
.08 |
|
CPK Peak (Mg / DL) |
1439.30±1516 |
2279.74±1643 |
2807.17±2799 |
.001 |
Table 1: Baseline patients' characteristics among study groups.
The incidence of acute renal failure (secondary end point), defined as a >25% increase in creatinine levels pre-catheterization or an absolute creatinine value >1.5 mg/dL, was not different between the three groups (p < 0.12).
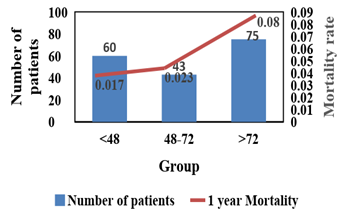
Overall all-cause mortality rate was not differed significantly among the three groups (log Rank χ2 = 0.78; p > 35). while patient discharge within 48 h seems to have higher 30 days mortality rate, patient discharge after 72 h have higher 1-year mortality rate. Patient discharge within 48 to 72 h has the lowest mortality risk (Table 2).
|
<48 Hours (n=60) |
48-72 Hours (n=43) |
>72 Hours (n=75) |
P-Value |
|
|
Acute kidney failure¥ |
0 (0.0) |
0 (0.0) |
3 (4.0) |
.12 |
|
Mortality |
||||
|
30 days |
1 (1.7) |
0 (0.0) |
3 (4.0) |
.35 |
|
1 Year |
1 (1.7) |
1 (2.3) |
6 (8.0) |
.28 |
|
Major adverse cardiovascular events (MACE) |
||||
|
30 days rate (95% CI) |
1(1.7) 1.67 (0.29-8.85) |
0 (0) 0.00 (0.00-8.20) |
1 (1.3) 1.34 (0.23-7.17) |
>.99 |
|
1 Year rate (95% CI) |
6 (10.0) (4.66-20.15) |
2 (4.6) 4.65 (1.28-15.45) |
3 (4.0) 4.00 (1.37-11.11) |
.35 |
Table 2: Primary and Secondary end points
¥Follow-up period of 30 days after discharge
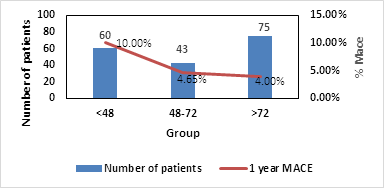
No statistically significant difference regarding MACE was observed between the three groups yet it seems that patient discharge within 48 h had higher 30 days and 1-year MACE rate, mainly due to recurrent myocardial infarction, compared to patient discharge at 48 to 72h who had the lowest rate (Table 3).
|
<48 hours (n=60) |
48-72 hours (n=43) |
>72 hours (n=75) |
P-Value |
|
|
30-days MACE |
||||
|
No events |
59 (98.3) |
43 (100.0) |
74 (98.7) |
>.99 |
|
Recurrent myocardial infarction |
0 (0.0) |
0 (0.0) |
0 (0.0) |
-- |
|
Unplanned Revascularization |
0 (0.0) |
0 (0.0) |
0 (0.0) |
.-- |
|
Stroke / transient ischemic attack |
1(1.7) |
0 (0.0) |
0 (0.0) |
>.99 |
|
Heart arrhythmia |
0 (0.0) |
0 (0.0) |
0 (0.0) |
-- |
|
Heart Failure |
0 (0.0) |
0 (0.0) |
1 (1.3) |
.58 |
|
1-year MACE |
||||
|
No events |
54 (90.0) |
41 (95.3) |
72 (96.0) |
.35 |
|
Recurrent myocardial infarction |
3 (5.0) |
2 (4.3) |
2 (2.7) |
.70 |
|
Unplanned Revascularization |
2 (3.3) |
0 (0.0) |
1 (1.3) |
.61 |
|
Stroke / transient ischemic attack |
1 (1.7) |
0 (0.0) |
0 (0.0) |
>.99 |
|
Heart arrhythmia |
0 (0.0) |
0 (0.0) |
0 (0.0) |
-- |
|
Heart Failure |
0 (0.0) |
0 (0.0) |
1 (1.3) |
.58 |
Table 3: MACE among study group within 30-days of discharge
Discussion
The recommended length of stay in medical facility balances the effectiveness and safety of medical care provided and the likelihood of unnecessary complications that endanger the patient and lead to high costs of resources and funds. Efficacy and safety of medical treatment highly depend on technology, medical innovation, efficient and accessible professional medical service, and patient awareness.
During the past decade, medical therapy for acute myocardial infarction was remarkably changed. Awareness of myocardial infarction symptoms had reached all corners of the population. Emergency Medical Service can make a fast and proper diagnosis onsite and transfer patients directly to catheterization laboratory. As a result, the prevalence of complications related to severe longstanding myocardial ischemia has dramatically decreased. Success rate increased and mortality rates decreased. Current technology available for cardiac catheterization was changed considerably. The use of radial artery approach significantly reduced vascular and port of entry complications. Additional technologies such as: OCT, FFR, IVUS etc. help reaching a proper diagnosis and highly accurate target lesion treatment. Physicians feel safe to discharge patient as soon as possible to strong community health care services that provide continued supporting treatment.
Our study examined the clinical significance of changes related to early patient discharge (within 72 h) with regard to mortality, acute adverse cardiovascular events, and acute renal failure. The patients were followed up for one year after discharge.
Our study demonstrates that old age; extensive myocardial infarction estimated by low systolic ejection fraction and the use of femoral approach were predictors against early hospital discharge. This subgroup of patients characterized by higher mortality rate and major cardiovascular events if discharge prior to 72 hours from admission.
At the same time, our study indicates that low-risk patients may benefit from early hospital discharge within 48 to 72 hours. This group of patients had significant lower mortality rate (Figure 1) and MACE rate up to one year (Figure 2). Patient discharged within 48 hours had significant higher mortality rate and MACE event which were mainly due to recurrent myocardial infarction (Figures 2s & Figure 3s – supplementary).
To isolate the possibility that high-risk patients were discharged earlier than expected, existing models such as CADILLAC and ZWOLLE were used to assess the risk in this study group. 69 patients with a score of 3 or lower according to the ZWOLLE model and 113 patients with a score of 5 or lower according to the CADILLAC index were identified as low risk patients, which according to current guidelines an early discharge will be reasonable and a safe option. We classified this group based on actual discharge time. No significant differences in the incidence of adverse cardiovascular events or death up to one year after discharge were noted among the three groups (Tables 6s and 7s – supplementary).
Early hospital discharge, to our experience, influences the patient's ability to cope with the disease, understanding the need to change existing lifestyle, and affect their adherence to new medical therapy. We believe that a decision on early hospital discharge should not be based solely on inpatient data but also on the community health services supporting the same area and the likelihood that the patient will use these services.
Limitations of the research
Follow-up was based on computerized medical records although these data are updated regularly; the possibility that vital information has been lost remains. However, attempts were made to gather complete information from the patients where the information in the medical records was lacking. Our sample size was based on a prevalence rate of 5.5% of MACE in patient discharge after 72 h and a difference of 5% among groups. Our MACE incidence rate and inter-group difference were lower than expected. Thus, these findings should be examined in a larger random sample. In this study, we examined the objective findings that could be assessed or measured but did not relate to the degree of fragility or frailty of the patient. The duration of hospitalization may be influenced by these factors.
Conclusion
Early hospital discharge within 48 to 72 hours, was not accompanied by a high mortality rate or adverse cardiovascular events compared to patients discharged within 48 hours or after 72 hours. Additionally, early discharge before 72 hours is not associated with high incidence rate of acute renal failure. Age and left ventricular systolic function were the most influential variable in selecting low-risk patients. Hospital discharge within 48 hours was associated with higher 30-days mortality rate and higher 30-days and 1-year MACE event, despite the lack of statistical significance.
Acknowledgements
We would like to thank Dr. Regina Koren for her tremendous help in editing and proofreading the article
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Conflicts of interest
The authors report no relationships that could be construed as a conflict of interest
References
- Newby LK, Eisenstein EL, Califf RM, et al. Cost effectiveness of early discharge after uncomplicated acute myocardial infarction. N Engl J Med 342 (2000): 749-755.
- Desideri A, Fioretti PM, Cortigiani L, et al. Cost of strategies after myocardial infarction (COSTAMI): a multicentre, international, randomized trial for cost-effective discharge after uncomplicated myocardial infarction. Eur Heart J 24 (2003): 1630-1639.
- Barchielli A, Balzi D, Marchionni N, et al. Early discharge after acute myocardial infarction in the current clinical practice. Community data from the AMI-Florence Registry, Italy. Int J Cardiol 114 (2007): 57-63.
- Chin CT, Weintraub WS, Dai D, et al. Trends and predictors of length of stay after primary percutaneous coronary intervention: a report from the CathPCI registry. Am Heart J 162 (2011): 1052-1061.
- Branca G, Capodanno D, Capranzano P, et al. Early discharge in acute myocardial infarction after clinical and angiographic risk assessment. J Cardiovasc Med (Hagerstown) 9 (2008): 858-861.
- Bogaty P, Dumont S, O'Hara GE, et al. Randomized trial of a noninvasive strategy to reduce hospital stay for patients with low-risk myocardial infarction. J Am Coll Cardiol 37 (2001): 1289-1296.
- Kaul P, Newby LK, Fu Y, et al. International differences in evolution of early discharge after acute myocardial infarction. Lancet 363 (2004): 511-517.
- Karabulut A, Cakmak M, Uzunlar B, Bilici A. What is the optimal length of stay in hospital for ST elevation myocardial infarction treated with primary percutaneous coronary intervention? Cardiol J 18 (2011): 378-384.
- Newby LK, Califf RM, Guerci A, et al. Early discharge in the thrombolytic era: an analysis of criteria for uncomplicated infarction from the Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO) trial. J Am Coll Cardiol 27 (1996): 625-632.
- Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33 (2012): 2569-2619.
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol 44 (2004): 671-719.
- Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol 67 (2016): 1235-1250
- Topol EJ, Burek K, O'Neill WW, et al. A randomized controlled trial of hospital discharge three days after myocardial infarction in the era of reperfusion. N Engl J Med 318 (1988): 1083-1088.
- Kinjo K, Sato H, Sakata Y, et al. Identification of uncomplicated patients with acute myocardial infarction undergoing percutaneous coronary intervention: are these patients suitable for early discharge? Circ J 69 (2005): 1163-1169.
- Tralhao A, Ferreira AM, Madeira S, et al. Applicability of the Zwolle risk score for safe early discharge after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. Rev Port Cardiol 34 (2015): 535-541.
- Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 45 (2005): 1397-1405.
- Schellings DAAM, Adiyaman A, Giannitsis E, et al. Early discharge after primary percutaneous coronary intervention: the added value of N-terminal pro-brain natriuretic peptide to the Zwolle Risk Score. J Am Heart Assoc 3 (2014): e001089.
- Kotowycz MA, Syal RP, Afzal R, Natarajan MK. Can we improve length of hospitalization in ST elevation myocardial infarction patients treated with primary percutaneous coronary intervention? The Canadian Journal of Cardiology 25 (2008): 585-588.
- Luca G de, Suryapranata H, van 't Hof AWJ, et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation 109 (2004): 2737-2743.
- Swaminathan RV, Rao SV, McCoy LA, et al. Hospital length of stay and clinical outcomes in older STEMI patients after primary PCI: a report from the National Cardiovascular Data Registry. J Am Coll Cardiol 65 (2015): 1161-1171.
- Novobílský K, Kryza R, ?erný P, Kau?ák V, Mrózek J, Horák I. Early discharge (within 72 h) in low risk patients after acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Single centre experience. Cor et Vasa 57 (2015): e45-e49.
Supplementary
Table 1s: CADILLAC index for the prediction of low-risk patients (2005)18.
|
Index |
Score |
Significance |
|
Baseline LV Ejection Fraction ≤ 40% |
4 |
High-risk-6 points or more Moderate risk-3–5 points Low-risk-0–2 points |
|
Chronic renal failure |
3 |
|
|
KILLIP class 2,3 |
3 |
|
|
Age over 65 |
2 |
|
|
Final TIMI flow (0-2) |
2 |
|
|
Anemia (hemoglobin 12 g/dL) |
2 |
Table 2s: ZWOLLE index for the prediction of low-risk patients (2004)15.
|
Index |
Score |
Meaning |
|
|
KILLIP class |
1 |
0 |
Low-Risk ≥3 High-Risk <3 |
|
2 |
4 |
||
|
3-4 |
9 |
||
|
TIMI flow post PCI |
3 |
0 |
|
|
2 |
1 |
||
|
0-1 |
2 |
||
|
Age |
>60 |
0 |
|
|
≤60 |
2 |
||
|
Triple Vessel Disease |
Yes |
0 |
|
|
No |
1 |
||
|
Anterior wall infarction |
No |
0 |
|
|
Yes |
1 |
||
|
Ischemia time >4 hours |
No |
0 |
|
|
Yes |
1 |
Table 3s: Criteria for inclusion and exclusion from the study
|
Criteria for Inclusion |
Criteria for Exclusion |
|
1. Patients over 18 years of age 2. Patients who underwent primary PCI due to ST elevation myocardial infarction |
3. Pregnant women 4. Unclear diagnosis 5. Diagnosis otherwise acute myocardial infarction 6. urgent catheterization due to an acute non-ST elevation myocardial infarction 7. Absence of obstructive coronary artery disease on coronary angiography 8. The patient refused to give informed consent to the study (when contacted) |
Table 4s: Preliminary admission data among study groups
|
<48 hours (n=60) |
48-72 hours (n=43) |
>72 hours (n=75) |
p-Value |
|
|
Infarct wall territory |
0.25 |
|||
|
Anterior wall Infarct |
45 (76.3) |
37 (86.0) |
66 (88.0) |
|
|
Inferior wall infarct |
6 (10.2) |
1 (2.3) |
5 (6.7) |
|
|
Lateral wall Infarct |
8 (13.6) |
5 (11.6) |
4 (5.3) |
|
|
KILLIP class |
0.46 |
|||
|
KILLIP 1 KILLIP 2 KILLIP 3 |
58 (96.7) 1(1.7) 0 (0.0) |
40 (93.0) 3 (7.0) 0 (0.0) |
72 (96.0) 2 (2.7) 1 (1.3) |
|
|
LV Ejection Fraction prior PPCI (%) |
47.6±10.8 (48; 30-65) |
45.3±10.4 (40; 30-65) |
41.7±8.7 (39; 28-65) |
0.02 |
|
LV Ejection Fraction post PCI (%) |
58.8±5.0 |
57.4±6.5 |
57.5±7.7 |
0.53 |
|
Catheterization approach |
0.02 |
|||
|
Radial Artery |
58 (98.3) |
40 (93.0) |
67 (90.5) |
|
|
Femoral Artery |
1 (1.7) |
0 (0.0) |
6 (8.1) |
|
|
Ulnar Artery |
0 (0.0) |
3 (7.0) |
1 (1.4) |
|
|
Number of blood vessels involved |
0.56 |
|||
|
1 |
42 (72.4) |
29 (70.7) |
44 (59.5) |
|
|
2 |
10 (17.2) |
8 (19.5) |
20 (27.0) |
|
|
3 |
6 (10.3) |
4 (9.8) |
10 (13.5) |
|
|
>3 |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
|
Catheterization Failure († IRA Closed) |
0 (0.0) |
0 (0.0) |
1 (1.3) |
0.42 |
|
Ischemic time (h) |
4.67 |
6.90 |
5.23 |
0.84 |
† IRA – Infarct Related Artery
Table 5s: Complications during hospitalization among study groups.
|
<48 Hours (n=60) |
48-72 Hours (n=43) |
>72 Hours (n=75) |
P-Value |
|
|
Mechanical complications |
0.19 |
|||
|
Coronary artery perforation |
1 (1.7) |
1 (2.3) |
0 (0.0) |
0.46 |
|
Left ventricular rupture |
0 (0.0) |
0 (0.0) |
0 (0.0) |
-- |
|
Pericardial Effusion/Tamponade |
0 (0.0) |
0 (0.0) |
0 (0.0) |
-- |
|
Bleeding in the vascular access area |
0 (0.0) |
0 (0.0) |
0 (0.0) |
-- |
|
Complications of myocardial infarction |
26.0 |
|||
|
Cardiogenic shock |
0 (0.0) |
0 (0.0) |
0 (0.0) |
-- |
|
Arrhythmias or conduction disorders |
2 (3.3) |
0 (0.0) |
3 (4.0) |
0.43 |
|
Pericarditis |
0 (0.0) |
0 (0.0) |
1 (1.3) |
0.50 |
|
Infarct extension or re-infarction |
0 (0.0) |
0 (0.0) |
0 (0.0) |
-- |
|
Acute Mitral insufficiency |
21 (36.2) |
14 (32.6) |
37 (49.3) |
0.14 |
|
Stroke |
0 (0.0) |
1 (2.3) |
2 (2.7) |
0.46 |
|
Use of IABP |
0 (0.0) |
2 (4.7) |
0 (0.0) |
0.07 |
Figure 2s: Mortality rate among study group within 30-days of discharge
Figure 3s: MACE among study group within 30-days of discharge
Table 6s: MACE events and mortality in low-risk patients according to the ZWOLLE model.
|
ZWOLLE SCORE ≤3 |
||||
|
<48 hours (n=22) |
48-72 hours (n=13) |
>72 hours (n=34) |
p-Value |
|
|
30 days MACE |
0 (0.0) |
0 (0.0) |
0 (0.0) |
----- |
|
30 Days Mortality |
0 (0.0) |
0 (0.0) |
1 (2.9) |
>0.99 |
|
1 Year MACE |
3 (13.6) |
4 (30.8) |
2 (5.9) |
0.08 |
|
1 Year Mortality |
0 (0.0) |
0 (0.0) |
1 (2.9) |
>0.99 |
Table 7s: MACE events and mortality among low-risk patients according to the CADILLAC model.
|
CADILLAC SCORE <6 |
||||
|
<48 hours (n=37) |
48-72 hours (n=25) |
>72 hours (n=51) |
p-Value |
|
|
30 days MACE |
1 (2.7) |
0 (0.0) |
0 (0.0) |
0.55 |
|
30 Days Mortality |
0 (0.0) |
0 (0.0) |
1 (2.0) |
>0.99 |
|
1 Year MACE |
3 (8.1) |
3 (12.0) |
2 (3.9) |
0.40 |
|
1 Year Mortality |
0 (0.0) |
0 (0.0) |
2 (3.9) |
0.70 |