Short Report: Saturation Rate after Vaccination against COVID-19
Article Information
Arno Mohr1, Florian Hitzenbichler2, Michael Pfeifer1, 3, Thilo Hinterberger4, Thomas H Loew4*, Beate Leinberger4
1Center for Pneumology, Donaustauf Hospital, Donaustauf, Germany
2Department of Infection Prevention and Infectious Diseases, University Hospital Regensburg, Regensburg, Germany
3Department of Internal Medicine, University Hospital Regensburg, Regensburg, Germany
4Department of Psychosomatic Medicine, University Hospital Regensburg, Regensburg, Germany
*Corresponding author: Prof. Dr. Thomas Loew, Klinik und Poliklinik für Innere Medizin II, Universitätsklinikum Regensburg, Franz-Josef-Strauß Allee 11, D-93053 Regensburg, Germany
Received: 14 March 2022; Accepted: 21 March 2022; Published: 25 April 2022
Citation: Arno Mohr, Florian Hitzenbichler, Michael Pfeifer, Thilo Hinterberger, Thomas H Loew, Beate Leinberger. Short Report: Saturation Rate after Vaccination against COVID-19. Archives of Clinical and Biomedical Research 6 (2022): 402-407.
View / Download Pdf Share at FacebookAbstract
Introduction: Cormirnaty® was the first available vaccine in Europe that contains mRNA encoding the spike protein of SARS-CoV2. An enormous peptide commonality and thus molecular mimicry between SARS-CoV2 spike protein and human proteins could be shown. Molecular mimicry could be attributable to SARS-CoV2 associated illness.
Objectives: So far saturation rate was not examined in patients who received Cormirnaty®. A decline in saturation rate would be suggestive that main reason for respiratory failure in patients with COVID-19 is the immune response to the spike protein.
Method: The study was conducted at the Centre of Pneumology in Donaustauf, Germany. Employees that qualified for vaccination against COVID-19 and who were willing to be vaccinated with Cormirnaty® (second dose) were asked to participate in this study. Participants were asked to measure saturation rate three times a day for seven days after vaccination.
Results: 106 participants (84 female, 22 male) were included into the analysis. Mean oxygenation satur-ation measured directly after vaccination was 97.4% (max 99%, min 93%. During days two to five, mean oxygen saturation dropped to 97.2% (max 99%, min 83%). This difference was significant in a one-tailed signed rank test with z=-1.88 (p=0.030).
Conclusion: A mild but significant decline in satu-ration could be detected. As Cormirnaty® comprises mRNA for encoding peptides of the spike protein the decline in saturation rate might hint that molecular mimicry is an important element of COVID-19.
Keywords
COVID-19; Dyspnoea; Saturation; Vaccination
Article Details
1. Introduction
Until now COVID-19 led to more than 100 million confirmed cases and 2.5 Million deaths. Infection with SARS-CoV2 is in many aspects different from classical respiratory infections. Pathogenesis is poorly understood, but the host immune response is thought to play a major role in the pathophysiological effects of organ failure in COVID-19 patients [1]. Previous corticosteroid use is no important risk factor for a poor outcome [2]; in hypoxemia, dexamethasone even showed to be beneficial [1]. Infection control in health care settings was broadened by the availability of different vaccinations lately [3-5]. Vaccination against SARS-CoV2 plays an important role amid pandemic control. Cormirnaty® was the first available vaccine in Europe that contains mRNA encoding the spike protein of SARS-CoV2 [3].
Interestingly, lately an enormous peptide commonality and thus molecular mimicry between SARS-CoV2 spike protein and human proteins could be demon-strated [6]. Molecular mimicry could be attributable to the SARS-CoV2 associated illness. In the pandemic H1N1 influenza vaccination campaign in 2009-2010 an increased incidence of narcolepsy was seen in many countries [7]. Molecular mimicry between the H1N1 influenza virus and endogenous proteins respectively Pandemrix® and endogenous proteins is suspected to be the reason of this increased incidence [7, 8]. So far saturation rate was not examined in patients who received Cormirnaty® [3, 9]. A decline in saturation rate would be suggestive that main reason for respir-atory failure in patients with COVID-19 is the immune response to the spike protein. To our knowledge no decline of saturation rate was detected after applic-ation of other vaccines. This study was performed to document real-life adverse effects and to examine whether an impact of the vaccination on the respire-tory system occurs.
2. Methods
2.1 Study population, study design and data collection
The study was conducted at the Centre of Pneumology in Donaustauf, Germany. The hospital is a quartiary care provider for pneumology, where patients from the eastern region of Bavaria are seen for specialized care. Employees that qualified for vaccination against COVID-19 and who were willing to be vaccinated were asked to participate in this study. Participants had to fill out a questionnaire about adverse events after the second vaccination with Cormirnaty®, general health information was also obtained. Employees were asked to measure saturation rate three times a day at rest after second vaccination. According to guideline of our local ethics committee at the University of Regensburg no approvement for these kind of observation studies is necessary. The study was cond-ucted between 25.01.2021 and 10.02.2021
2.2 Participants
The following eligibility criteria were applied: Employee at the hospital, 18 years of age or older, eligible for vaccination with Cormirnaty® and one performed vaccination with Cormirnaty®, willingness to be vaccinated, willingness to fill out the question-nnaire and to measure saturation rate three times a day at rest for seven days after vaccination.
2.3 Statistics
Summary statistics of continuous variables are presen-ted as mean ± standard deviation. Data were analysed using Microsoft Excel (version 2016, Redmond, USA) and IBM SPSS (version 24.0, IBM, Armonk, USA). O2 saturation was analysed using Matlab 2020 (Mathworks Inc.). For testing of a vaccination-related drop of O2 saturation, mean O2 saturation values in the participants during the first day (one to three measures at the day of vaccination) and the last day (three measures six days after vaccination) served as subject-specific baseline. Mean O2 saturation during day two to five was related to this baseline value and statis-tically analysed using a one-tailed nonparametric signrank test.
3. Results
3.1 Baseline characteristics
149 employees received the second dose of Cormir-naty® during the study period, of these 73.2% (n=109) were willing participate and did submit the results of the questionnaire and the saturation rate. Due to missing values, n=106 participants (84 female, 22 male) were included into the final analysis. Mean age was 49.6 ± 9.1 years, 22 were smokers, pulmonary disease was reported by twelve, eight of them reported to have asthma bronchiale. 61 did receive the seasonal influenza vaccination before.
3.2 Oxygenation saturation
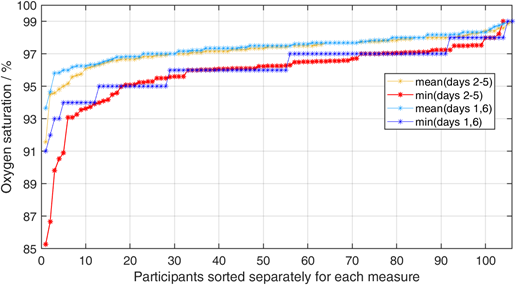
Mean oxygenation saturation measured directly after vaccination was 97.4% (max 99%, min 93%). Mean oxygenation saturation during the first day was also 97.4% (max 99%, min 92%). During the final baseline at day six after vaccination, mean O2 saturation was again 97.4% (max 99%, min 91%). During days two and five, mean oxygen saturation dropped to 97.2% (max 99%, min 83%). This difference was significant in a one-tailed signed rank test with z=-1.88 (p=0.030). To illustrate the post vaccination effect, the minima of oxygen saturation within the two phases were plotted for each participant after sorting them by the minima of days two to five. However, as the four days post vaccination phase contains twice the number of measurements compared to the two days baseline, lower minimum values might be expected depending on the stochastic variance. We accounted for this effect by averaging the minima resulting from 1000 random selections of six samples out of the 12 sampling times during the days two to five. Oxygen levels dropped in 12 participants below 94% in the time period between day two and five after vaccination (Figure 1). In contrast, during the baseline period, only four participants showed such a strong decrease. This effect remains stable even after removal of the two participants with the most extraordinary values. Interestingly, while the minimum O2 only decreases in 44 participants, it increases in 56 participants. Looking at the average O2 levels during the phases, O2 increases in the post vaccination phase in 36 participants and decreases in 70 participants.
4. Discussion
To our knowledge this is the first study examining saturation rate in a real-life collective after vaccination with Cormirnaty®. A significant decline in saturation can be detected. Importantly the decline in saturation was only detected for a short period after vaccination and saturation reached base level values after seven days. As Cormirnaty® comprises mRNA for encoding peptides of the spike protein the decline in saturation rate might show that molecular mimicry is an important element of COVID-19. This could be one of the reasons why therapy with steroids are beneficial in patients with respiratory failure. As the decline of saturation occurs on the fifth day (and not initially) after vaccination is an additional point that could show that parts of adaptive immune system are involved. Five days after vaccination stress-or vaccination-induced hyperventilation and thus shift of the oxygen dissociation curve to the left is not a relevant reason for the decline in saturation rate. While the majority of participants show a decrease in O2 levels during two to five days after vaccination, a strong drop below 94% was only seen in about 10% of the participants.
4.1 Limitations
Our study has many limitations. First of all, we had no control group without vaccination and we did only measure saturation rate and not oxygen partial press-ure; furthermore, it is a single center study and the number of employees we examined is rather small; on the other hand, this is the first study at all analysing saturation rate after vaccination with Cormirnaty®.
5. Conclusion
Low saturation rate is not described in the Phase 1 study of Cormirnaty® and not known as adverse event of other vaccinations. The mild, but obvious decline in saturation rate might be no typical side effect but give a hint to the reason of hypoxemia and pathophysiology in ARDS in SARS-CoV2. Further prospective studies are needed.
Declaration
Ethics approval
According to guideline of our local ethics committee at the University of Regensburg no approvement prior the beginning of this kind of observation study was necessary.
Consent for publication
Not applicable.
Availability of data and material
The dataset analyzed during the current study is avail-able from the corresponding author upon reasonnable request.
Competing interests
Dr. Mohr reports grants from Gilead Sciences, outside the submitted work.
Dr. Hitzenbichler reports grants from Gilead Sciences and MSD, outside the submitted work.
Dr. Pfeifer, Dr. Loew and Dr. Hinterberger have nothing to disclose.
Funding
Scanderra GmbH, Suisse sponsored pulse oximeters
Authors‘ contributions
All authors made a substantive intellectual contribu-tion, have read and approved the final version of the manuscript and agreed to be accountable for all aspects of the manuscript.
Acknowledgements
No specific acknowledgments.
References
- Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 384 (2021): 693-704.
- Tlayjeh H, Mhish OH, Enani MA, et al. Association of corticosteroids use and out-comes in COVID-19 patients: A systematic review and meta-analysis. J Infect Public Health13 (2020): 1652-1663.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 383 (2020): 2603-2615.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 384 (2021): 403-416.
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397 (2021): 99-111.
- Kanduc D, Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res 68 (2020): 310-313.
- Sarkanen TO, Alakuijala APE, Dauvilliers YA, et al. Incidence of narcolepsy after H1N1 influenza and vaccinations: Syste-matic review and meta-analysis. Sleep Med Rev 38 (2018): 177-186.
- Tesoriero C, Codita A, Zhang M-D, et al. H1N1 influenza virus induces narcolepsy-like sleep disruption and targets sleep-wake regulatory neurons in mice. Proc Natl Acad Sci U S A 113 (2016): E368-E377.
- Walsh EE, Frenck RW, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med 383 (2020): 2439-2450.