Severe Immune-Related Cutaneous Vasculitis Induced by Osimertinib
Article Information
Benoit Calderon, Léa Vazquez*
Sainte Catherine Institut du Cancer Avignon-Provence, Avignon, France
*Corresponding Author: Léa Vazquez, Sainte Catherine Institut du Cancer Avignon-Provence, 250 chemin des baigne-pieds, 84918 Avignon Cedex 9, France
Received: 19 May 2022; Accepted: 14 June 2022; Published: 12 July 2022
Citation: Benoit Calderon, Léa Vazquez. Severe Immune-Related Cutaneous Vasculitis Induced by Osimertinib. Archives of Clinical and Medical Case Reports 6 (2022): 524-527
View / Download Pdf Share at FacebookAbstract
Osimertinib is a third-generation epidermal growth factor receptor (EGFR)- tyrosine kinase inhibitors (TKIs) which is the current standard of care for first-line treatment of advanced EGFR+ Non-Small Cell Lung Cancer (NSCLC). The most common Osimertinib-related adverse events (AEs) are hematologic toxicity, rash, dry skin, and paronychia which are usually tolerable and manageable. Serious AEs occur infrequently and the etiopathogenesis of some remains unclear. We present a case of grade 3 cutaneous vasculitis induced by osimertinib in a Caucasian woman that required immunosuppressive therapy.
Keywords
Osimertinib; Epidermal Growth Factor Receptor; Immunosup pressive Therapy; Tyrosine Kinase Inhibitors
Osimertinib articles; Epidermal Growth Factor Receptor articles; Immunosuppressive Therapy articles; Tyrosine Kinase Inhibitors articles
Osimertinib articles Osimertinib Research articles Osimertinib review articles Osimertinib PubMed articles Osimertinib PubMed Central articles Osimertinib 2023 articles Osimertinib 2024 articles Osimertinib Scopus articles Osimertinib impact factor journals Osimertinib Scopus journals Osimertinib PubMed journals Osimertinib medical journals Osimertinib free journals Osimertinib best journals Osimertinib top journals Osimertinib free medical journals Osimertinib famous journals Osimertinib Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Syndrome articles Syndrome Research articles Syndrome review articles Syndrome PubMed articles Syndrome PubMed Central articles Syndrome 2023 articles Syndrome 2024 articles Syndrome Scopus articles Syndrome impact factor journals Syndrome Scopus journals Syndrome PubMed journals Syndrome medical journals Syndrome free journals Syndrome best journals Syndrome top journals Syndrome free medical journals Syndrome famous journals Syndrome Google Scholar indexed journals Immunosup pressive Therapy articles Immunosup pressive Therapy Research articles Immunosup pressive Therapy review articles Immunosup pressive Therapy PubMed articles Immunosup pressive Therapy PubMed Central articles Immunosup pressive Therapy 2023 articles Immunosup pressive Therapy 2024 articles Immunosup pressive Therapy Scopus articles Immunosup pressive Therapy impact factor journals Immunosup pressive Therapy Scopus journals Immunosup pressive Therapy PubMed journals Immunosup pressive Therapy medical journals Immunosup pressive Therapy free journals Immunosup pressive Therapy best journals Immunosup pressive Therapy top journals Immunosup pressive Therapy free medical journals Immunosup pressive Therapy famous journals Immunosup pressive Therapy Google Scholar indexed journals Lymphoangio matous Proliferation articles Lymphoangio matous Proliferation Research articles Lymphoangio matous Proliferation review articles Lymphoangio matous Proliferation PubMed articles Lymphoangio matous Proliferation PubMed Central articles Lymphoangio matous Proliferation 2023 articles Lymphoangio matous Proliferation 2024 articles Lymphoangio matous Proliferation Scopus articles Lymphoangio matous Proliferation impact factor journals Lymphoangio matous Proliferation Scopus journals Lymphoangio matous Proliferation PubMed journals Lymphoangio matous Proliferation medical journals Lymphoangio matous Proliferation free journals Lymphoangio matous Proliferation best journals Lymphoangio matous Proliferation top journals Lymphoangio matous Proliferation free medical journals Lymphoangio matous Proliferation famous journals Lymphoangio matous Proliferation Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Liver Trauma articles Liver Trauma Research articles Liver Trauma review articles Liver Trauma PubMed articles Liver Trauma PubMed Central articles Liver Trauma 2023 articles Liver Trauma 2024 articles Liver Trauma Scopus articles Liver Trauma impact factor journals Liver Trauma Scopus journals Liver Trauma PubMed journals Liver Trauma medical journals Liver Trauma free journals Liver Trauma best journals Liver Trauma top journals Liver Trauma free medical journals Liver Trauma famous journals Liver Trauma Google Scholar indexed journals Debridement articles Debridement Research articles Debridement review articles Debridement PubMed articles Debridement PubMed Central articles Debridement 2023 articles Debridement 2024 articles Debridement Scopus articles Debridement impact factor journals Debridement Scopus journals Debridement PubMed journals Debridement medical journals Debridement free journals Debridement best journals Debridement top journals Debridement free medical journals Debridement famous journals Debridement Google Scholar indexed journals Tyrosine Kinase Inhibitors articles Tyrosine Kinase Inhibitors Research articles Tyrosine Kinase Inhibitors review articles Tyrosine Kinase Inhibitors PubMed articles Tyrosine Kinase Inhibitors PubMed Central articles Tyrosine Kinase Inhibitors 2023 articles Tyrosine Kinase Inhibitors 2024 articles Tyrosine Kinase Inhibitors Scopus articles Tyrosine Kinase Inhibitors impact factor journals Tyrosine Kinase Inhibitors Scopus journals Tyrosine Kinase Inhibitors PubMed journals Tyrosine Kinase Inhibitors medical journals Tyrosine Kinase Inhibitors free journals Tyrosine Kinase Inhibitors best journals Tyrosine Kinase Inhibitors top journals Tyrosine Kinase Inhibitors free medical journals Tyrosine Kinase Inhibitors famous journals Tyrosine Kinase Inhibitors Google Scholar indexed journals
Article Details
1. Introduction
The 2018 National Comprehensive Cancer Network (NCCN) Clinical Practice Guideline for NSCLC recommend EGFR-TKIs as first-line treatment for unresectable, EGFR mutation-positive, advanced NSCLC [1]. Osimertinib, a third-generation EGFR-TKI that targets T790M mutation, showed a longer Progression-Free Survival (PFS) than first generation EGFR-TKIs (gefitinib or erlotinib) when it was administered as first-line treatment, regardless of T790M mutation status [2, 3]. Moreover, the incidence of adverse events of grade 3 or higher among patients who received Osimertinib was similar to that among patients who received gefitinib or erlotinib, despite longer treatment exposure [2, 3]. All generations of EGFR-TKIs have similar side-effects profiles, although the frequency and severity of AEs vary by the respective drugs. Indeed, EGFR plays an essential role in epithelial maintenance and, therefore, EGFR-TKIs might impair epithelial cell growth and migration and alter cytokine expression, leading to the recruitment of inflammatory cells and consequent tissue injury. Rash, paronychia, and diarrhoea were the most common AEs reported with first and second-generation EGFR [4].
Infrequently, serious AEs, mainly drug-induced lung injury, occur with EGFR-TKIs and even call for dose reduction, treatment discontinuation, or pharmacotherapeutic intervention and hospitalisation. Although some studies seemed to suggest that cutaneous reactions may predict treatment efficacy, cutaneous reactions have a profound influence on patients’ quality of life and adherence to the treatment, and can, therefore, represent a barrier to oncologic treatment [5, 6]. Due to the higher selectivity to the mutated receptor, Osimertinib is associated with less severe gastrointestinal and skin toxicity compared with first and second-generation EGFR TKIs [7]. Rarely, dermatologic serious AEs can occur such as necrolytic migratory erythema, toxic dermal epidermolysis, transient acanthotic dermatosis, anaphylaxis, or vasculitis [8-10]. Drug-induced vasculitis reactions are defined as inflammatory vasculitis in which a specific drug is established as the causal agent. While rashes are common with EGFR-TKIs, vasculitis is very different from other hypersensitivity reactions. Vasculitis is characterized by histological evidence of blood vessel inflammation, often defined by a perivascular lymphocytic infiltration, which can lead to the thinning or rupture of the blood vessel wall [11]. We present a case of Osimertinib-induced grade 3 cutaneous vasculitis in a Caucasian woman.
2. Case Presentation
We describe the case of an 86-year-old non-smoker European
woman referred to our institution for a 60mm-tumoral mass in the right upper lobe of the lung associated with enlarged mediastinal lymph nodes, and bone, hepatic and adrenal metastases, revealed by a thoracic computed tomography (CT). There was no family history of cancer. She was never-smoker, but she relates second-hand smoke exposure. Patient physical examination revealed lumbar pain, a unilateral and diffuse increase in vesicular breath sounds over the right lung tissue and an ECOG Performance Status of 1. Bronchoscopy confirmed the tumor in the right upper bronchus, and a biopsy revealed lung adenocarcinoma with EGFR exon 19 mutation. Brain CT reveals no cerebral metastases but Thoracic-Abdominal-Pelvic CT shows bone metastases with a fracture of 7th thoracic vertebrae. The diagnosis of lung adenocarcinoma T3N2M1c clinical stage IVb was confirmed. As a first step, pain management is initiated with analgesic radiation therapy (8Gy 1fraction) on 7th thoracic vertebrae and algology consultation for the follow-up.
First-line treatment with Osimertinib (monotherapy 80mg/d) was initiated the day after radiation therapy. Nineteen days later, the patient is admitted in emergency for numerous skin lesions (necrotic purpura) suitable with cutaneous vasculitis (Figure 1a and 1b) with neither pain nor pruritus. Lesions are located on the nose, ears, face, tongue, palate, soles of the feet and hands, fingers of the feet and hands, knees, elbows, and shins. The lesions appeared successively for about a week. The patient reports chest pressure sensation and epistaxis, but no other signs of bleeding. At physical examination, blood pressure is normal, no fever, no bone, thoracic or abdominal pain at palpation, no dyspnoea, no abnormalities on neurologic examination. However, there is an important weight loss (>10%) and an asthenia which could be associated with systemic vasculitis.
Laboratory tests revealed slight lymphocytopenia and thrombocytopenia. Renal and liver function were slightly disrupted as well as plasma ionogram. C-Reactive Protein was increased. The coagulation factors (fibrinogen, prothrombin, factor V, tissue thromboplastin) were normal except an increase of D-dimer (>12 000µg/L). (Table 1) Pulmonary embolism was excluded by normal CT pulmonary angiography. This examen revealed a good response to Osimertinib: from 60mm to 48mm for the lung mass, from 25mm to 14mm for the mediastinal lymph node, from 48mm to 23 mm for the hepatic metastasis, from 28mm to 16mm for adrenal metastasis, and no apparent bone lesion. No autoimmune disorders were found. Antinuclear antibody, antiphospholipid antibodies, cryoglobulin, proteinase-3, myeloperoxidase, antineutrophilic cytoplasmatic antibody were all negative. Serology tests (hepatitis B and C, herpes zoster, chicken pox, HIV, Epstein-Barr Virus) were also negative. IgA, IgM, IgG and C3 were not detected on direct immunofluorescence of skin biopsy. Osimertinib was discontinued and corticosteroids therapy was initiated (120mg/d for 6 days and 60mg/d) associated with cyclophosphamide (500mg every 2 weeks for 3 injections). After 8 days, the skin lesions regressed (Figure 2a and 2b) and after 33 days the skin lesions almost disappeared (Figure 3a and 3b).
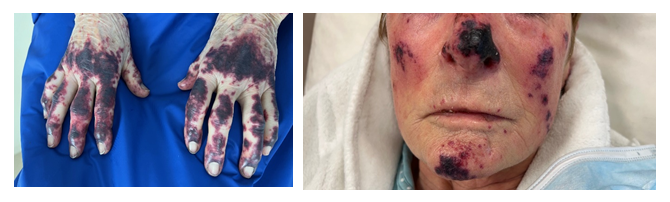
Figure 1a and 1b: Hands (a) and face (b) skin lesions at the admission (19 days after Osimertinib initiation).
|
Abnormal parameters |
Normal Range |
Results |
|
Lymphocytes |
1070-3900 |
693/µL |
|
Platelets |
177-379 |
174 000/µL |
|
CRP |
0-5 |
69.6 mg/L |
|
Urea |
2.5-7.2 |
16.7 mmol/L |
|
Sodium |
136-145 |
133 mmol/L |
|
Potassium |
3.4-4.5 |
4.7 mmol/L |
|
GGT |
8-33 |
72 UI/I |
|
CPK |
29-68 |
15 U/I |
|
PT |
64-83 |
59g/L |
|
Albumin |
34-48 |
24.5g/L |
|
D-dimer |
<500 |
12 518 ng/mL |
Table 1: Laboratory tests.
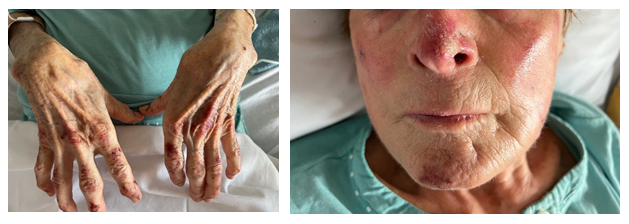
Figure 2a and 2b: Hands (a) and face (b) skin lesions 8 days after Osimertinib discontinuation and immunosuppressive therapy initiation.
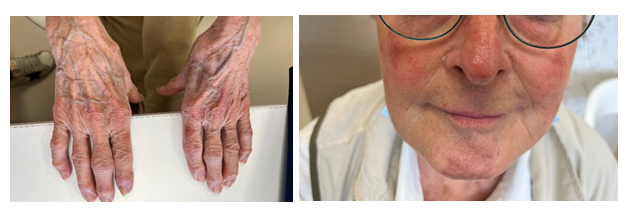
Figure 3a and 3b: Hands (a) and face (b) skin lesions 33 days after Osimertinib discontinuation and immunosuppressive therapy initiation.
3. Discussion
Osimertinib is a third-generation inhibitor of the intracellular tyrosine kinase domain of epidermal growth factor receptor, nowadays prescribed in first line treatment for lung carcinoma with EGFR mutation. The most common cutaneous AEs of Osimertinib, mostly grade 1-2, are rash or acne (58%), dry skin (36%) and paronychia (35%) [2]. Clinically, the cutaneous vasculitis also known as necrotizing vasculitis or leukocystoclastic vasculitis, is an unusual toxicity. EGFR-TKIs-induced cutaneous vasculitis can manifest as ecchymosis, petechia, and purpuric plaques. These lesions occurred in the first month of treatment [12]. To our knowledge, we report the second case of cutaneous vasculitis induced by Osimertinib [13], and the first one such early after treatment initiation. Nevertheless, few authors had already reported cutaneous vasculitis induced by first-generation EGFR-TKIs [14-17]. Most of cutaneous AEs induced by EGFR-TKIs can be attributed to the specific inhibition of EGFR but it is also possible that EGFR-TKIs exert a direct or indirect effect on the capillaries [18] or that these lesions originate from immunologic reactions induced by deposition of immunocomplexes [14]. In our case, the absence of IgA, IgM, IgG or C3 deposits in the vessel wall does not consolidate this hypothesis. The exact pathogenesis of cutaneous vasculitis induced by Osimertinib (or other generation of EGFR-TKIs) has not yet been clarified.
Treatment discontinuation and corticosteroids therapy have shown appropriate efficacy in the management of cutaneous vasculitis induced by EGFR-TKIs. On the advice of medical internist, we decided to add cyclophosphamide to corticosteroids in the management of our patient because of the severity of local (necrotic lesions) and general symptoms (weight loss, fatigue, high level of C-reactive protein). In absence of any high-quality data to guide management, more aggressive immunosuppressive drugs than corticosteroids could be considered [19]. As some studies suggest, cutaneous reactions may predict treatment efficacy. For the patient of this case report, the Osimertinib treatment duration was precisely 19 days. The CT pulmonary angiography carried out to exclude pulmonary embolism revealed a very good response to the treatment despite its short duration (Photo 4). Some international studies have found a correlation between clinically graded skin toxicity and patient-reported outcome, with longer PFS and OS. The development and the severity of skin lesion could be an important predictor for efficacy of EGFR-TKIs treatment [20-22]. While the results of the previous studies seem to correlate specifically skin rash with EGFR-TKIs efficacy (gefitinib or erlotinib), others skin toxicities could also be an efficient clinical marker for predicting the response of patients with NSCLC to EGFR-TKIs. In summary, cutaneous vasculitis is an uncommon complication of EGFR-TKIs. Grade 1-2 cutaneous vasculitis is reported in 0.3% of cases in the summary of product characteristics against 0% of cases for Grade 3. However, two cases have already been published. Clinicians need to be alert to this side effect for manage it effectively.
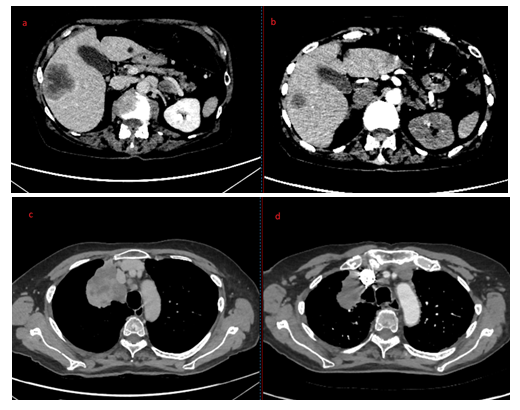
Figure 4a, 4b, 4c and 4d: Hepatic metastasis before (a) and after (b) Osimertinib treatment; Pulmonary mass before (c) and after (d) Osimertinib treatment.
Acknowledgments
We thank all physicians who participate in data collection and we thank the patient for accepting the publication
Funding
None
References
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 16 (2018): 807-821.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med 378 (2018): 113-125.
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med 382 (2020): 41-50.
- Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation positive non-small cell lung cancer. Lung Cancer 88 (2015): 74-79.
- Pastore S, Lulli D, Girolomoni G. Epidermal growth factor receptor signalling in keratinocyte biology: implications for skin toxicity of tyrosine kinase inhibitors. Arch Toxicol 88 (2014): 1189-203.
- Perez-Soler R. Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer 8 (2006): S7-S14.
- Finlay MR, Anderton M, Ashton S, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem 57 (2014): 8249-8267.
- Balagula Y, Garbe C, Myskowski PL, et al. Clinical presentation and management of dermatological toxicities of epidermal growth factor receptor inhibitors. Int J Dermatol 50 (2011): 129-146.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol 56 (2007): 317-326.
- Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugsand targeted therapies for cancer Part II. Targeted therapies. J Am Acad Dermatol 71 (2014): 217.e1-217.
- Langford, C.A. Vasculitis.Journal of Allergy and ClinicalImmunology 111 (2003): S602-S612.
- Agero AL, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 55 (2006): 657-670.
- Hamada K, Oishi K, Okita T, et al. Cutaneous Vasculitis Induced by Osimertinib. J Thorac Oncol 14 (2019): 2188-2189.
- Fernandez-Guarino M, Ryan AM, Perez-Garcia B, et al. Necrotizing vasculitis due to gefitinib (Iressa). Int J Dermatol 46 (2007): 890-891.
- Ko JH, Shih YC, Hui RC, et al. Necrotizing vasculitis triggered by gefitinib: an unusual clinical presentation. J Clin Oncol 29 (2011): e169-e170.
- Boeck S, Wollenberg A, Heinemann V. Leukocytoclastic vasculitis during treatment with the oral EGFR tyrosine kinase inhibitor erlotinib. Ann Oncol 18 (2007): 1582-1583.
- Su BA, Shen ST, Chang LY, et al. Successful rechallenge with reduced dose of erlotinib in a patient with lung adenocarcinoma who developed erlotinib-associated leukocytoclastic vasculitis: a case report. Oncol Lett 3 (2012): 1280-1282.
- Kurokawa I, Endo K, Hirabayashi M. Purpuric drug eruption possibly due to gefinitib (Iressa). Int J Dermatol 44 (2005): 167-168.
- Chen KR, Carlson JA. Clinical approach to cutaneous vasculitis. Am J Clin Dermatol 9 (2008): 71-92.
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 358 (2008): 1160-1174.
- Liu HB, Wu Y, Lv TF, et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 8 (2013): e55128.
- Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 13 (2007): 3913-3921.
