Seroprevalence of SARS-CoV-2 Infection among Vaccinated Health Workers and Hospital Staff
Article Information
Claudia C Colmenares-Mejía1*, Doris C Quintero-Lesmes1, Isail Salazar Acosta1, Diana Paola Suárez1, Ligia Meneses2, Olga Lucia Sopó Rincón2, Paula K Bautista-Niño1, Norma C Serrano1
1Research Center, Fundación Cardiovascular de Colombia, Santander, Colombia
2COVID-19 Molecular Biology Laboratory, Fundación Cardiovascular de Colombia, Santander, Colombia
*Corresponding author: Claudia C Colmenares-Mejía, Research Center, Fundación Cardiovascular de Colombia, Floridablanca, Santander, 681002, Colombia
Received: 21 February 2022; Accepted: 08 March 2022; Published: 16 March 2022
Citation:
Claudia C Colmenares-Mejía, Doris C Quintero-Lesmes, Isail Salazar Acosta, Diana Paola Suárez, Ligia Meneses, Olga Lucia Sopó Rincón, Paula K Bautista-Niño, Norma C Serrano. Seroprevalence of SARS-CoV-2 Infection among Vaccinated Health Workers and Hospital Staff. Archives of Clinical and Biomedical Research 6 (2022): 290-295.
View / Download Pdf Share at FacebookAbstract
Frontline healthcare workers are critical for a timely response to SARS-CoV-2 pandemic. An observa-tional cross-sectional study, with three evaluations, was conducted. For each evaluation, RT-PCR and IgM/IgG antibodies tests were performed. 1,118 participated in round-1; 862 in round-2 and 753 in round-3 were included. Presence of infection was lower in round-1 (1.3% vs 2.8% vs 2.3%). Adjusted seropositivity decreased for round-3 [37.5% (95%CI 34.3-40.3), 36.7% (95%CI 33.5-39.8), 23.2% (95%CI 20.3-26.2)]. Participants with a previous PCR+ had a higher frequency of seropositivity [65.7% (95%CI 60.6-70.6)]. We identified asymptomatic health personnel positive for SARS-CoV-2 and low seroprevalence compared with other reports.
Keywords
Seroepidemiologic Studies; Prevalence; Coronavirus Infections; Occupational Exposure; Occupational Health
Article Details
1. Introduction
SARS-CoV-2 transmission has been reported in Colombia since March 2020, with approximately 5 million COVID-19 confirmed cases reported up to September 30th, 2021. Nevertheless, is highly probable that routine COVID-19 case reports under-estimate the total number of infections due to people with mild illness or asymptomatic infection [1]. Previous SARS-CoV-2 seroprevalence studies conducted in Colombia have focused on specific populations by geographic region or occupational groups [2]. So far, no studies have been done after SARS-CoV-2 vaccination process was started.
In Colombia, vaccination process was divided into 5 stages according to risk of exposure and age group. The first stage began on February 17th, 2021, including people >80 years (Sinovac-CoronaVac) and work force (Pfizer-BioNTech) in charge of COVID-19 patients healthcare at first line of care. Seroprevalence studies are necessary because can estimate seropositivity of people with specific antibodies against SARS-CoV-2, and can determine whether the infection is naturally occurring or it is due to vaccine-induced immunological response [3]. Therefore, the aim of this study was to estimate seroprevalence of SARS-CoV-2 in vaccinated population of healthcare workers and hospital staff during three different moments.
2. Methodology
2.1 Design and population
An observational cross-sectional study (on three different moments with prospective data collection) was developed in Bucaramanga Metropolitan Area (Santander, Colombia); this region is inhabited by 1,111,999 people according to National Population Census from 2018. It is estimated that between 2020 and 2021, the number of health professionals working in front line of care was approximately 1,500. Healthcare workers, or staff workers at first line of attention of eight healthcare institutions were invited to participate.
2.2 Sampling
A convenience sampling in each institution (public and private clinics and hospitals) was carried out.
2.3 Recruitment
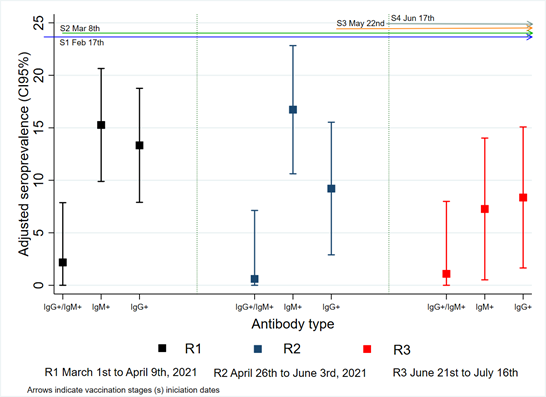
Three rounds were conducted. First round (R1) took place between March 1st and April 9th, 2021; second round (R2) between April 26th and June 3rd, 2021; and third round (R3) between June 21st to July 16th, 2021. Each round included collection of socio-demographical and clinical information and per-forming of RT-PCR and serological test to determine IgM and IgG.
2.4 Data collection and variables
All participants self-completed an online survey on socio-demographic data (age, marital status, educa-tion level, socioeconomic strata, address), occupa-tional area, cigarette smoking status, medical history (stroke, hypertension, acute myocardial infarction, dyslipidemias, diabetes mellitus, COPD/asthma, obesity, non-skin cancer, HIV/AIDS, autoimmune diseases), contact with people with suspicion or confirmed COVID-19 infection, presence of symp-toms since march 2020 (cough, fever> 38°c, chills, fatigue, myalgia, shortness of breath, wheezing, chest pain, headache, odynophagia, dizziness, rhinorrhea, diarrhea, nausea or vomiting, hemoptysis, nasal congestion, anosmia), information about possible exposition to infection such as type of transportation used to go to work or to assist medical consultations, use of personal protection elements (gloves, conventional mask, N95 masks, specific clothes or shoes to go out on the street, glasses, face mask, hat or hair up), prevention activities (Bathing when entering the home, washing hands upon arrival at destination, washing hands every two hours, use antiseptic gel, keep distance of at least two meters from other people), quarantine (if symptoms or a positive PCR test), rapid tests previously performed, COVID-19 confirmed infection by PCR after the beginning of symptoms, hospitalization or ICU.
Study data were collected and managed using REDCap electronic data capture tools hosted at Fundación Cardiovascular. Electronic informed consent was obtained from all subjects involved in the study. This consent was available to be downloaded and saved by each participant.
2.5 PCR assessment
Automated extraction of viral RNA from respiratory samples was performed using the MGIEasy Nucleic Acid Extraction Kit. This test reports a qualitative result: positive for nucleocapsid (N) and ORF1ab gene amplification and negative for no genes amplification. Participants that had positive PCR results were immediately informed through email recorded in virtual survey and were also reported to the Health and Safety at Work department in their companies and were reported to SISMUESTRAS (https://apps.ins.gov.co/sismuestras) from Instituto Nacional de Salud (INS).
2.6 IgG and IgM measurement
A peripheral blood sample (~5 cc) by venipuncture in the forearm was obtained for every participant. Immunoglobulin G (IgG) detection was done by chemiluminescence assay and Immunoglobulin M (IgM) by enzymatic fluorescence immunoassay. ARC COV2 test from Abbot® was used for immunoglobulin assessment. This test reports a qualitative result (positive/negative for each antibody).
2.7 Statistical methods
Continuous variables are reported as means with 95% CI and categorical variables as absolute and relative frequencies. Prevalence was estimated as the number of positive participants (either for IgG, IgM or both) for the numerator on the total number of participants, as the denominator. Additionally, estimated seroprevalence was adjusted by test performance (Sensitivity 85.2%, Specificity 97.3%) using the formula proposed by Sempos and Tian. Statistical analysis was done in Stata 15.
3. Results
1.118 participants were included in R1, 862 participants in R2 and 753 participants in R3. Female gender represented majority in all rounds. Most participants were residents from Bucaramanga and from low socioeconomic status (3 or below). Technicians or assistants, followed up by registered nurses, physicians and administrative staff were occupational areas with the greatest participation in every round. Vaccination rates increased for R2 and R3 as national process advanced.
3.1 Acute infection
Presence of asymptomatic infection, recorded as positive RT-PCR, was lower in R1 [1.3% (CI95% 0.8% - 2.1%)] than R2 and R3 [2.8% (CI95% 1.8% - 4.1%) vs 2.3% (CI95% 1.4% - 3.6%)]. By occupa-tional area, asymptomatic infections were present in Ambulance driver (n=1, 9.1% CI95% 0.8 – 53.7), Physical therapists (n=3, 5.6% CI95% 1.7 – 16.3), Cleaning staff (n=5, 3.7% CI95% 1.5 – 8.7), Technician/assistants (n=26, 2.9% CI95% 1.9 – 4.2), Administrative staff (n=10, 2.8% CI95% 1.5 – 5.2), Nurses (n=6, 1.1% CI95% 0.5 – 2.3), Physicians (n=1, 1.1% CI95% 0.5 – 2.6) and Lab staff (n=1, 0.6% CI95% 0.08 – 4.2).
3.2 Seroprevalence
Adjusted general seropositivity (IgM +, IgG + or both) decreased for R3 [R1 37.5% (95% CI 34.3-40.3), R2 36.7% (95% CI 33.5-39.8), R3 23.2% (95% CI 20.3-26.2)]. Some occupational areas presented higher seropositivity in each round; R1 surgical assistant [90.9% (CI95% 60.1 - 100)], technicians [44.5% (CI95% 36.5 - 52.4)], physical therapist [40.7% (CI95% 7.4 - 74)] and cleaning staff [39.0% (CI95% 19.1 - 58.9)]. In R2, cleaning staff [41.3% (CI95% 16.1 - 66.6) and R3, physical therapist [40.0% (CI95% 0 – 82.0)], pharmacy [31.3% (CI95% 0 – 93.8)] and administrative [29.6% (CI95% 13.4-45.8)] staff. According to antibody type, seropositivity for only IgM was higher in R1 and R2 [R1 15.3% (CI95% 9.8 – 20.6); R2 16.7% (CI95% 10.6 – 22.8); R3 7.3% (6.9 – 10.9)] than seropositivity for only IgG [R1 13.3% (CI95% 7.9 – 18.7); R2 10.3% (CI95% 2.8 – 15.5) and R3 9.6% (CI95% 1.6 – 15.0)]. Seropositivity for both antibodies decreased after R1 [R1 2.2% (CI95% 0 – 7.9), R2 0.6% (CI95% 0 – 7.1), R3 1.1% (CI95% 0 – 7.9)] (Figure 1). Participants with a previous positive PCR had a higher frequency of seropositivity [65.7% (95% CI 60.6-70.6)].
4. Discussion
Presence of infection (positive RT-PCR) was higher in round 2 and 3. This may correspond due to the occurrence of peak presented at the end of April and later in the middle of the year in Colombia. SARS-CoV-2 infection was more frequent in occupations in direct contact with the patient, such as ambulance drivers and physical therapists. Recent studies suggest that occupational exposure has been reduced in healthcare personnel due to better infection prevention in healthcare settings [4]. Antibody asse-ssment in frontline healthcare workers and hospital staff from eight institutions from Bucaramanga and its metropolitan area showed evidence of immuno-logical response against SARS-CoV-2, mainly due to previous viral exposition. Seropositivity for first and second rounds were almost half from proportions reported in a similar occupational population [2] (10.3%, 9.2% versus 20% seropositivity), except for the third round which was further reduced (6% vs. 20%). For health personnel in general in the national study conducted in several cities, where Bucara-manga was included, it reached 32% seropositivity [5].
Although for rounds 2 and 3, vaccination process presented high coverage in the population evaluated, seroprevalence decreased over time. Some possible reasons for this may be: 1. Due to the time elapsed between previous peak (2020) and the antibody measurement in the last rounds of the study; 2. Less than four weeks between the second dose of the vaccine and the evaluation of the antibodies, so the immune response would not be complete (especially for IgM); 3. The IgG evaluated corresponds to the antibody against the virus's nucleocapside, which is not stimulated by vaccination. An advantage of this study was sample size, being able to capture same individuals in rounds two and three, and with positive test results being able to isolate first-line health personnel on time. A limitation of this study is that assessed workers in Bucaramanga and its metro-politan area, it is possible that findings are not generalizable to other cities. It is noteworthy that 33% of the study participants were technicians (including administrative and healthcare areas) in the three rounds. The distribution by sex in the three rounds showed that women are the majority (75%) of the personnel in the first line of care.
Acknowledgments
The authors are grateful for Government of Santander and the Órgano Colegiado de Administración y Decisión (OCAD) that evaluates, makes feasible, approves and prioritizes the programs and projects that will be financed with resources from the Science, Technology and Innovation Fund (FCTeI) of Sistema General de Regalías.
Funding
This research received funding from the Sistema General de Regalias of the Department of Santander, Colombia with BPIN code: 2020000100082, with the supervision of the Ministerio de Ciencia, Tecnología e Innovación (Minciencias).
Ethical Considerations
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Fundación Cardiovascular de Colombia (protocol code CEI-2020-01858, December 15th, 2020).
Conflicts of Interest
none.
References
- la Hoz-Restrepo F de, Alvis-Zakzuk NJ, la Hoz-Gomez JF De, et al. Is Colombia an example of successful containment of the COVID-19 2020 pandemic? A critical analysis of the epidemiological data. Int J Infect Dis (2020).
- Colmenares-Mejía CC, Serrano-Díaz N, Quintero-Lesmes DC, et al. Seroprevalence of sars-cov-2 infection among occupational groups from the bucaramanga metropolitan area, Colombia. Int J Environ Res Public Health 18 (2021): 4172.
- Krammer F, Srivastava K, Alshammary H, et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med 384 (2021): 1372-1374.
- Jacob JT, Baker JM, Fridkin SK, et al. Risk Factors Associated with SARS-CoV-2 Seropositivity among US Health Care Personnel. JAMA Netw Open 4 (2021): e211283-e211283.
- Estudio Nacional: Seroprevalencia (2021).
https://www.ins.gov.co/estudio-nacional-de-seroprevalencia/reporte.html