Serological and PCR evidence of Infection in 105 Patients with SPPT
Article Information
Alexis Lacout1*, Marie Mas4, Michel Franck2, Véronique Perronne3, Julie Pajaud2, Pierre Yves Marcy5, Christian Perronne3
1Centre de diagnostic ELSAN, Centre Médico–Chirurgical, 83 avenue Charles de Gaulle, 15000, Aurillac, France
2ADNucleis, 3 route des Pierres Blanches, 69290, Grézieu-la-Varenne, France
3Hôpital Universitaire Raymond Poincaré (Assistance Publique – Hôpitaux de Paris), Département d’Infectiologie. Université de Versailles – Saint Quentin, Paris-Saclay
4Clinique Convert, Médecine Générale, Service des Urgences, 62 avenue de Jasseron, 01000, Bourg en Bresse, France
5Polyclinique Les Fleurs Service Imagerie Médicale, 332 Avenue Frederic Mistral, 83190, Ollioules, France
*Corresponding Author: Alexis Lacout, Centre de diagnostic ELSAN, Centre Médico–Chirurgical, 83 avenue Charles de Gaulle, 15000, Aurillac, France
Received: 11 December 2020; Accepted: 22 December 2020; Published: 05 January 2021
Citation: Alexis Lacout, Marie Mas, Michel Franck, Véronique Perronne, Julie Pajaud, Pierre Yves Marcy, Christian Perronne. Serological and PCR evidence of Infection in 105 Patients with SPPT. Archives of Microbiology & Immunology 5 (2021): 139-150.
View / Download Pdf Share at FacebookAbstract
Introduction: The main aim of this study is to determine the nature of the exposure of patients presenting with polymorphic signs and symptoms to the parasite Babesia, through the study of serology. The secondary aim is to report the different serological or PCR results observed in these patients.
Material and methods: The following serologies were performed in all patients looking for: Babesia divergens, Borrelia, Bartonella, Coxiella burnetii, Anaplasma phagocytophilum. The following PCRs were performed looking for: Borrelia spp, Babesia spp, Bartonella (Bartonella spp, B. quintana, B. Henselae,) Coxiella spp, Anaplasma spp, Ehrlichia spp, Rickettsia spp, most often on several matrices (venous blood, capillary blood, urine and saliva).
Results: In this study, 105 patients were included, 62 females and 43 males, sex ratio F/M was 62/43 = 1.44; mean age was 45.5 year old (range; 5 years, 79 years old). Of the 105 serologies for B. divergens, 41 % were found to be positive. Of the 104 serologies for Borrelia, 19.2 % were found to be positive. Of the 104 serologies for Borrelia, 19.2 % were found to be positive. Of the 95 serologies for Anaplasma, 27,3 % were found to be positive. Borrelia spp, Babesia spp, Bartonella spp, Coxiella spp, Anaplasma spp, Ehrlichia spp, Rickettsia spp were found by using rtPCR.
Conclusion: Our study has shown that patients with SPPT/PTLDS, a syndrome close to fibromyalgia, could harbor several tick borne microorganisms. Microbiologic analyses should thus not be merely limited to Borrelia's research alone.
Keywords
Lyme; Borrelia; Babesia; PCR; PTLDS; SPPT; Serology; PCR
Lyme articles; Borrelia articles; Babesia articles; PCR articles; PTLDS articles; SPPT articles; Serology articles; PCR articles
Article Details
Introduction
Lyme disease is a tick-borne infectious disease caused by the bacterium Borrelia burgdorferi. The prevalence seems to be increasing in many countries around the world, particularly in France. Interestingly, ticks transmit many other pathogenic bacteria (Bartonella spp, Ehrlichia spp, Anaplasma spp, Rickettsia spp...), parasites (Babesia spp) and viruses, the so-called co-infections [1-4]. The main aim of this study is to determine the nature of the exposure of patients presenting with polymorphic signs and symptoms to the parasite Babesia, through the study of serology. The secondary aim is to report the different serological or PCR results observed in these patients.
Patients and Methods
This is a retrospective observational study including patients with persistent polymorphic syndrome possibly due to a tick bite (SPPT). SPPT is a clinical syndrome close to post-treatment Lyme disease syndrome (PTLDS), which is officially recognized by the French High Authority for Health (HAS).
The SPPT is defined by a clinical triad persisting for a continuous period of at least 6 months, associating several times a week, a polyalgic syndrome (musculoskeletal pain and/or neuropathic pain and/or headaches); persistent fatigue with reduced physical capacities; cognitive complaints. The difference between SPPT and PTLDS is that the diagnosis of Lyme disease has not to be proven and patients may have not been treated [5, 6].
1. Patients:
Over a reference period from October 2016 to April 2020, patient’s inclusion criteria clustered SPPT patients of all gender who were tested positive for Babesia serology, who presented with the following clinical symptoms persisting for a continuous period of at least 6 months, without prior diagnosis:
1) Cognitive disorders
2) More than two of the following chronic symptoms including : myalgia, arthritis or arthralgia, facial paralysis, central or peripheral involvement, myelitis, root pain, paresthesias, dysesthesias, radiculopathy
3) Abnormal asthenia
2. microorganisms that were sought
The following serologies were performed in all patients looking for: Babesia divergens, Borrelia, Bartonella, (B. quintana and B. henselae) Coxiella burnetii, Anaplasma phagocytophilum. The following PCRs were performed looking for: Borrelia spp, Babesia spp, Bartonella (Bartonella spp, B. quintana, B. Henselae,) Coxiella spp, Anaplasma spp, Ehrlichia spp, Rickettsia spp, most often on several matrices (venous blood, capillary blood, urine and saliva). Most of the PCRs (in 51 out of 59 patients) were performed in the Adnucleis laboratory using rt-PCR (Table 1, 2).
This retrospective observational study was approved by the "Comité de protection des personnes" CPP SUD 9EST VI Clermont Ferrand, France. All patients and control persons signed an informed consent in accordance with the Declaration of Helsinki.
Results
In this study, 105 patients were included, 62 females and 43 males, sex ratio F/M was 62/43 = 1.44; mean age was 45.5 year old (range; 5 years, 79 years old). Results are summarized in (Table 3).
Table 3: PCR and serological results in 105 patients presenting with a persistent polymorphic syndrome after a possible tick-bite (SPPT)
|
Number of patients tested |
Positive |
Negative |
|
|
Babesia serology* |
105 |
43 (41%) |
62 (59%) |
|
PCR |
50 |
6 (12%) 3/6: negative serology |
44 (88%) |
|
Borrelia serology |
104 |
20 (19.2%) |
84 (80.8%) |
|
PCR |
55 |
9 (16.4%) 8/9: negative serology |
46 (54%) |
|
Bartonella serology |
97 |
3 (3.1%) |
94 (96.9%) |
|
PCR |
47 |
9 (19.1%) 9/9: negative serology |
38 (80.9%) |
|
Coxiella serology |
93 |
3 (3.2%) |
91 (96.8%) |
|
PCR |
39 |
2 (5.1%) 2/2: negative serology |
37 (94.9%) |
|
Anaplasma serology |
95 |
26 (27.3%) |
69 (72.7%) |
|
PCR |
39 |
3 (7.7%) 2/3: negative serology |
36 (92.3%) |
|
Ehrlichia PCR |
39 |
4 (10.3%) |
35 (89.7%) |
|
Rickettsia PCR |
38 |
19 (50%) |
19 (50%) |
*Babesia divergens
1. Babesia divergens serology
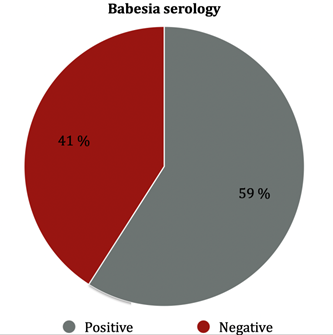
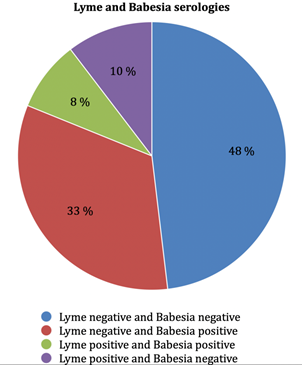
Of the 105 serologies for B. divergens, 92 were performed in the Biomnis laboratory, 6 at the Strasbourg laboratory (National Reference Center for Borreliosis), 6 at the Institut hospitalo-universitaire (IHU) laboratory in Marseille, and one at Sylab laboratory. Biomnis laboratory gave two levels of results, weakly positive or strongly positive. Results were the following (Figure 1). (i) At the Biomnis laboratory, 39 out of 92 (42.4%) blood tests were found to be positive, 30 (32.6%) weakly positive and 9 (9.8%) strongly positive. (ii) At the Strasbourg laboratory, 2 out of 6 were positive (both quantified at 1/120). (iii) At the IHU of Marseille, 2 out of 6 were positive (respectively quantified at 1/60 and 1/30). (iv) At Sylab laboratory, the only patient was negative. Among the patients who presented with positive results for B. divergens serology, 4 had had previous negative serology (2 patients two months before and 2 patients 12 months before). Among patients who were tested positive for B. divergens serology, 3 out of 31 (9.7%) were Babesia positive by PCR, 5 out of 30 (16.7%) were Borrelia positive by PCR, 9 out of 43 (20.9%) had a positive Borrelia serology (Figure 2), 5 patients had Borrelia, B. divergens and Anaplasma phagocytophilum positive serology.
2. Babesia spp PCR
Babesia spp PCRs were performed in 50 patients (venous blood sampling, n = 19, venous blood and urine sampling; n =3, venous blood, urine and saliva; n = 23, venous blood, urine, saliva and capillary blood sampling; n = 5). Babesia PCRs were found to be positive in 6 (12%) patients, (4 in venous blood, 1 in capillary blood and 1 in saliva samples). Among patients presenting with a positive Babesia PCR, 3 out of 6 (50%) had a Babesia positive serology (3 strongly positive and 1 weakly positive), none out of 5 tested had a Borrelia positive serology, one out of 6 (16.7%) tested patients had a positive Borrelia PCR result.
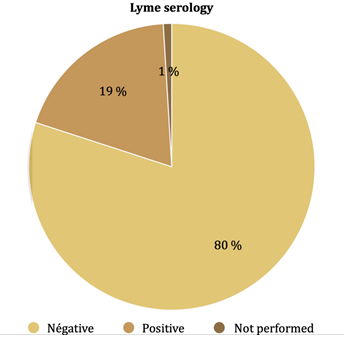
3. Borrelia serology
Among the 20 (19.2%) patients who had a positive Borrelia serology, 5 were positive in IgM and 15 in IgG (Figure 3). Among the patients presenting with a positive Borrelia serology, 1 out of 9 had a positive Borrelia PCR , 9 out of 20 (45 %) had a positive Babesia serology, none out of 10 had a positive Babesia PCR (Figure 2).
4. Borrelia spp PCR
Borrelia spp PCRs were performed in 55 patients (venous blood sample; n = 23, blood/urine mixture sample; n = 1, 3 venous blood and urine sample; n = 3, venous blood, urine and saliva, sample; n = 23; venous blood, urine, saliva and capillary blood; n = 5 samples). Nine patients were found to be positive with (16.4%) Borrelia PCRs (venous blood sample; n = 5, urine sample; n = 2, blood/urine mixture sample; n =1, saliva sample; n = 1).
Among the 9 patients who were tested positive for Borrelia by PCR, 1 patient had a positive Borrelia serology (in IgG), 5 patients had a positive Babesia serology (3 strongly positive, 2 weakly positive), 1 patient out of 6 tested was tested positive for Babesia by PCR. Among the 46 patients presenting with a negative Borrelia result by PCR, 8 patients out of 45 tested (17.8%) had a positive Borrelia serology (3 specific IgM and 5 IgG), 37 patients out of 45 tested (82.2%) had a negative Borrelia serology.
5. Bartonella serology
Among the 3 (3.1%) patients with a positive serology for Bartonella, one had a positive serology for Babesia and Anaplasma phagocytophilum (weakly IgG positive result), and one had a positive serology for Anaplasma phagocytophilum (weakly IgM positive result).
6. Bartonella PCR
Nine (19.1%) patients had a positive PCR for Bartonella: Bartonella spp in 3, B. quintana in 2, and B. henselae in 4. Serologies for Bartonella were negative in all these cases.
7. Coxiella burnetii serology
Three (3.2%) patients had a positive serology for C. burnetii. One patient was serologically positive to Borrelia, and one patient was positive to Borrelia and Babesia.
8. Coxiella spp PCR
Two (5.1%) patients had a positive PCR for C. burnetii. Serologies for C. burnetii were negative in these cases.
9. Anaplasma phagocytophilum serology
Among the 26 patients with a serology positive for Anaplasma phagocytophilum, 18 were IgM positive (of which 16 were weakly positive), 3 were IgG and IgM positive (of which 2 were weakly positive in IgM and strongly positive in IgG, and 1 was weakly positive in IgM and IgG), 5 were IgG weakly positive. Among them, 13 patients had positive Babesia diverens serology, 7 patients had positive Borrelia serology and 5 patients had positive Borrelia and Babesia divergens serology.
10. Anaplasma spp PCR
Among the 3 (7.7%) patients with a positive PCR for Anaplasma spp, one had a low positive IgM serology level.
11. Some patients had a positive PCR for Ehrlichia spp or Rickettsia spp (Table 1)
Table 1: Real Time Multiplex PCR (ADNucleis laboratory)
|
Samples |
Urine and saliva were collected in dry bottles, five milliliters of blood were collected by venous puncture and around 500µl of capillary blood were collected by finger prick in tubes with EDTA as anti-coagulant, before any antibiotic treatment and were sent in Vacutainer® K2 tubes. Samples (venous blood, urine, saliva, capillary blood) were drawn twice at Day 0 (D0) and Day 2 (D2). |
|
Selection of Primers |
To allow the detection of bacteria and parasites, primers targeting specific genes of each microorganism were used to amplify DNA by qPCR. Details of qPCR kits used is listed in Table 2. |
| Robustness of PCR Mixes |
The portion of target genes were synthesized and introduced into a plasmid to obtain a control DNA and facilitate its multiplication. This control DNA was used to validate the amplification mixes. Serial dilution of the plasmid was performed and amplified to determine the robustness parameters of each PCR kit: the limit of detection (LOD), the limit of quantification (LOQ), the repeatability and the reproducibility. |
| DNA Extraction and Purification |
The DNA was extracted without any prior treatment using 300 μl of whole blood with an equal volume of ADNucleis extraction buffer (5 M guanidium thiocyanate, 500 mM TrisHCL, 50 mM EDTA, 20% Tween 20, 20% Triton X-100, 750 μg proteinase K). After incubation for 20 min at 56°C and 15 min at 80°C, the extracted DNA was purified by means of silica magnetic beads and eluted in 250 μl of elution buffer (10 mM TrisHCl, pH 8.5). |
| Control of the Extraction |
Human glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) was used as a housekeeping gene as an internal control for PCR extraction and inhibition. The extracted samples were first checked with a PCR targeting the GAPDH gene. If the results of this PCR were consistent (Ct of GAPDH below 32), the samples were then analyzed for the other pathogens. The sequence of interest of GAPDH was inserted into a plasmid and this plasmid was used as a positive DNA for the validation of GAPDH primers and PCR mix as well as a positive control for subsequent PCRs. The primers used for GAPDH are described in Table 2. |
| Real-Time PCR (rt PCR) |
Real-time PCR was carried out in a total volume of 50 μl with a PCR mix containing ADNucleis PCR buffer (20 mM Tris-HCl, 10 mM NH4SO4, 10 mM KCl, 2 mM Mg2+, 0.1% TritonX-100, pH 8.8), 2 mM of each dNTP, 600 nM of each primer, 1 μl of Evagreen and 5 units of Taq polymerase ADNucleis. Twelve μl of extracted samples were amplified. An initial denaturation step of 5 min at 95°C was followed by 42 cycles of 15 s at 95°C and 40 s at 60°C (hybridization-elongation). The dissociation curves were generated by a last step of 10 min with temperature increments from 75 to 95°C for qPCR kits using Sybr green technology. |
| Quantification |
Positive samples were quantified using a standard curve obtained by amplifying known and calibrated concentrations of control DNA of the desired targets. Quantification was obtained using the standard curve equation (Ct = a (Log10 [DNA]) + b) where “a” is the slope and “b” the intercept of the curve. The results were expressed in genome units (UG) per ml of sample. |
Table 2: List of desired targets and details of PCR kits (ADNucleis laboratory)
12. All serology-negative patients:
All serologies (Borrelia, Babesia divergens, Bartonella, Coxiella and Anaplasma phagocytophilum) were performed in 87 patients. Among them, 32 (36.8%) were negative for all these serologies. Of these 32 patients, one had a positive PCR for Babesia by PCR (capillary blood), one for Borrelia (venous blood), and one for both Bartonella quintana (saliva) and Ehrlichia (saliva).
Discussion
The hypothesis of our study is that Lyme disease could not be the only causative factor to explain the persistent polymorphic syndrome possibly due to a tick bite (SPPT), a syndrome close to the post-treatment Lyme disease syndrome (PTLDS) and fibromyalgia syndromes [5-8]. Indeed, A large number of bacteria (other than Borrelia), parasites (Babesia) and viruses are transmitted by tick bites and could cause different signs and symptoms in patients [5]. Indeed the clinical triptych associates a disabling fatigue, neuro-psychic disorders (memory, sleep, concentration disorders) and various somatic signs including in the first place pain (articular, muscular, tendinous, neurological) [6]. Our retrospective study shows the frequent presence of various different tick-borne infections, first and foremost Babesia.
Babesiosis is mainly described as an acute and severe disease in immunocompromised subjects or those with splenectomy. Babesiosis is in fact poorly known, and it is possible that the frequency of infection by these parasites could be underestimated [9]. Some articles report authentic Babesia spp infections, as recurrent types in immunocompetent patients, or as a torpid, chronic presentation [10-12]. Diagnosis of babesial infection is usually made by identification of typical intraerythrocytic parasites on a blood smear, Babesia DNA by using PCR, and serology test. In our study, exposure to Babesia divergens was found in 41% of patients with SPPT and 45% of patients with Borrelia positive serology, this result is higher than those reported in previous seroprevalence studies. Studies by Svenson et al performed in patients with a positive serology for Borrelia, showed a seroprevalence of respectively: 26.9% for Babesia microti (healthy control group: 6.7%) [13] , 16.3% for B. microti and B. divergens (healthy control group: 2.5%) [14]. Hunfeld et al, found a seroprevalence of 11.5% for B. microti and B. divergens after tick bite exposures (control group: 1.7%) [15]. Pancewics et al, found a positive B. microti serology in 5 out of 144 foresters (4.4%), from the forest inspectorate in Poland. All were also IgG-seropositive for B. burgdorferi [16]. In Rigaud et al study, 1 of 810 (0.1%) and 20 of 810 (2.5%) forest workers in France were seropositive for Babesia. divergens and Babesia microti respectively [17]. While the positivity of a serology test merely discloses a previous exposure, the positivity of Babesia by PCR test states that piroplasma was indeed present in 12% of the patients. It could thus be important to diagnose such infections, which can be responsible for the symptomatology and require anti-infectious treatments with anti-malaria drugs. According to the large number of coinfections that were depicted in our study patients, Lyme disease turns out to be the tip of an iceberg, as testing was indeed positive in 19.2% by serology and in 16.4% by PCR of all the tested patients.
No studies have estimated the seroprevalence of Borrelia in patients with SPPT yet. In De Kekeureire, et al study, sixty-seven of 310 (21.6%) forest workers were seropositive for Borrelia (18). In Rigaud et al study, 419 of 2975 (14.1%) forest workers were seropositive for Borrelia [17]. In Finland, seroprevalence in the general population was estimated at 3.9% [19]. We thus postulate that the patients’clinical symptoms are partly related to the presence of other concomitant microorganisms than Borrelia. The sensitivity of Borrelia serology is controversial and some meta-analyses show insufficient sensitivity. In our study, serology was negative in 8 of the 9 patients with a positive PCR for Borrelia. However, PCR is still an imperfect technique: PCR sensitivities and specificities are heterogeneous and/or under-evaluated. Some studies used PCR to identify Borrelia burgdorferi in early Lyme disease (at a early stage of the disease, thus different from the one in our study) . In Eshoo et al's study, the sensitivity was 62% and the specificity was 100% [20]. Liveris, et al reported a sensitivity of 40.6% [21]. In these studies on early Lyme disease, the direct detection sensitivity was lower than that of the two-tier serology. However, in Bil-Lula's study, 3% negative ELISA IgM results, 2.8% negative results of Line blot IgM, 3.1% and 2.7% of negative ELISA IgG and Line blot IgG results, respectively, were positive in rt PCR [22]. Few studies looked for Borrelia in urine by PCR. In one study, detection rate was 91% in patients with Lyme disease skin lesions [23]. In another study, results were disappointing [24]. Our study thus may have underestimated the number of patients infected by Borrelia.
Anaplasma phagocytophilum is the second most frequent germ found in serology (27.3%) ahead of Borrelia (19.2%); this microorganism was found in 3 out of 39 PCRs (10.3%). Together with Ehrlichia, found in PCR in 2 cases out of 39 (5.1%), these bacteria are responsible for summer pseudo-flu syndromes, including hepatitis, leukopenia and thrombocytopenia. However, this infection may frequently be subclinical. Indeed, cross-sectional seroprevalence studies have demonstrated that up to 15% of the population in northwest Wisconsin, 1% Connecticut habitants and US military personnel, 17% of Slovenians, and 12% of the population of Sweden's Koster Islands have positive antibodies for Anaplasma phagocytophilum without a history of clinical manifestations [25].
Bartonella and Coxiella were poorly detected by serology tests during our study. Bartonella serology only looks for B. quintana and B. henselae whereas many other species are described; and Bartonellae usually infecting animals have already been observed in humans [26-28]. This study showed that most patients with a combination of signs and symptoms that are consistent with the diagnosis of SPPT have a history of exposure (demonstrated by serology test) or presence of microorganisms (bacteria and parasites) (demonstrated by PCR). In our study, only 36.8% of patients were serologically negative for all micro-organisms tested, and of these, some were PCR positive. Some patients were serologically positive for Borrelia and Babesia divergens, or for Borrelia, Babesia divergens and Anaplasma phagocytophilum. The clinical signs observed in SPPT may thus have an infectious origin (even if dysimmunitary phenomenons can play a role), and not a psychiatric origin as it has been previously hypothetized [29].
This makes sense, given the number of bacteria and parasites transmitted by ticks, the possibility of multiple tick bites and the fact that ticks themselves can be poly-infected. In addition, some microorganisms are not transmitted by ticks. This study shows a clear limitation of different serologies, which should be put into perspective and the sensitivity reevaluated. Indeed, among patients with positive PCR, serologies were frequently negative: especially for Borrelia, Babesia divergens, Anaplasma phagocytophilum, Bartonella or Coxiella. Overall 83% of these serologies were negative when the PCR showed the presence of the micro-organism. One explanation could be that the serologies only look for some species (e.g. only Babesia divergens while there are other Babesia species as B. microti for example [13]. All those results need to be confirmed and further evaluated in larger studies. This study did not look for viruses, which are potentially transmitted by ticks. As all patients suffered from signs and symptoms, it is probable that the isolated microorganisms were actually responsible for the disease. However, further studies on larger populations, including healthy control persons, should look at the possibility of asymptomatic carriage.
In conclusion, our study has shown that patients with SPPT/PTLDS, a syndrome close to fibromyalgia, could harbor several tick borne microorganisms. Microbiologic analyses should thus not be merely limited to Borrelia's research alone. The other concomitant pathogens were found by serology and/or PCR. Babesia seems to be the most frequent, followed by Anaplasma phagocytophilum and Borrelia. Future prospective studies are needed by using systematic serology and PCR testings in all patients.
Disclosure:
Michel Franck is CEO of ADNucleis; The others authors do not declare any conflict of interest
References
- Cisak E, Wójcik-Fatla A, Zajac V, et al. Prevalence of tick-borne pathogens at various workplaces in forest exploitation environment. Med Pr 65 (2014): 575-581.
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, et al. Polymicrobial nature of tick-borne diseases. MBio 10 (2019): e02055-e02019.
- Michelet L, Delannoy S, Devillers E, et al. High-throughput screening of tick-borne pathogens in Europe. Frontiers in Cellular and Infection Microbiology 4 (2014): 103.
- Nelder MP, Russell CB, Sheehan NJ, et al. Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasites & Vectors 9 (2016): 265.
- Aucott JN. Posttreatment Lyme disease syndrome. Infectious Disease Clinics 29 (2015): 309-323.
- de Santé HA. Recommandation de bonne pratique. Borréliose de Lyme et autres maladies vectorielles à tiques (MVT) (2018).
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care & Research 62 (2010): 600-610.
- Rebman AW, Bechtold KT, Yang T, et al. The clinical, symptom, and quality-of-life characterization of a well-defined group of patients with posttreatment Lyme disease syndrome. Frontiers in Medicine 4 (2017): 224.
- Martinot M, Paleau A, Greigert V, et al. Babésiose en France et en Europe: une pathologie à redéfinir. Médecine et Maladies Infectieuses 48 (2018): S112.
- Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infectious Disease Clinics of North America 22 (2008): 469-488.
- Krause PJ. Human babesiosis. International Journal for Parasitology 49 (2019): 165-174.
- Krause PJ, Spielman A, Telford SR, et al. Persistent parasitemia after acute babesiosis. New England Journal of Medicine 339 (1998): 160-165.
- Curcio SR, Tria LP, Gucwa AL. Seroprevalence of Babesia microti in individuals with Lyme disease. Vector-Borne and Zoonotic Diseases 16 (2016): 737-743.
- Svensson J, Hunfeld KP, Persson KE. High seroprevalence of Babesia antibodies among Borrelia burgdorferi-infected humans in Sweden. Ticks and Tick-Borne Diseases 10 (2019): 186-190.
- Hunfeld KP, Lambert A, Kampen H, et al. Seroprevalence of Babesia infections in humans exposed to ticks in midwestern Germany. Journal of Clinical Microbiology 40 (2002): 2431-2436.
- Pancewicz S, Moniuszko A, Bieniarz E, et al. Anti-Babesia microti antibodies in foresters highly exposed to tick bites in Poland. Scandinavian Journal of Infectious Diseases 43 (2011): 197-201.
- Rigaud E, Jaulhac B, Garcia-Bonnet N, et al. Seroprevalence of seven pathogens transmitted by the Ixodes ricinus tick in forestry workers in France. Clinical Microbiology and Infection 22 (2016): 735-e1.
- De Keukeleire M, Robert A, Luyasu V, et al. Seroprevalence of Borrelia burgdorferi in Belgian forestry workers and associated risk factors. Parasites & Vectors 11 (2018): 277.
- van Beek J, Sajanti E, Helve O, et al. Population-based Borrelia burgdorferi sensu lato seroprevalence and associated risk factors in Finland. Ticks and Tick-Borne Diseases 9 (2018): 275-280.
- Eshoo MW, Crowder CC, Rebman AW, et al. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PloS One 7 (2012): e36825.
- Liveris D, Schwartz I, McKenna D, et al. Comparison of five diagnostic modalities for direct detection of Borrelia burgdorferi in patients with early Lyme disease. Diagnostic Microbiology and Infectious Disease 73 (2012): 243-245.
- Bil-Lula I, Matuszek P, Pfeiffer T, et al. Lyme borreliosis–the utility of improved real-time PCR assay in the detection of Borrelia burgdorferi infections. Advances in Clinical and Experimental Medicine 24 (2015): 663-670.
- Schmidt BL, Aberer E, Stockenhuber C, et al. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in the urine and breast milk of patients with Lyme borreliosis. Diagnostic Microbiology and Infectious Disease 21 (1995): 121-128.
- Rauter C, Mueller M, Diterich I, et al. Critical evaluation of urine-based PCR assay for diagnosis of Lyme borreliosis. Clinical and Diagnostic Laboratory Immunology 12 (2005): 910-917.
- Guzman N, Beidas SO. Anaplasma phagocytophilum (anaplasmosis). InStatPearls [Internet] (2019).
- Maggi RG, Mozayeni BR, Pultorak EL, et al. Bartonella spp. bacteremia and rheumatic symptoms in patients from Lyme disease–endemic region. Emerging Infectious Diseases 18 (2012): 783.
- Berghoff W. Suppl 1: Chronic Lyme Disease and Co-infections: Differential Diagnosis. The Open Neurology Journal 6 (2012): 158.
- Breitschwerdt EB, Maggi RG, Nicholson WL, et al. Bartonella sp. bacteremia in patients with neurological and neurocognitive dysfunction. Journal of Clinical Microbiology 46 (2008): 2856-2861.
- Kennedy AG. Differential diagnosis and the suspension of judgment. Journal of Medicine and Philosophy 38 (2013): 487-500.