Sentinel Lymph Node Biopsy in Early Breast Cancer Using Isosulfan Blue Dye alone in Resource-Limited Centers- Our Experience
Article Information
Huma Majeed Khan*, Bushra Rehman, Rabia Ikram, Anam Waseem
Consultant Breast Surgeon, Ittefaq Hospital (Trust) Lahore, Pakistan
*Corresponding author: Huma Majeed Khan. Consultant Breast Surgeon, Ittefaq Hospital (Trust) Lahore, Pakistan
Received: 30 October 2021; Accepted: 06 November 2021; Published: 10 November 2021
Citation: Huma Majeed Khan, Bushra Rehman, Rabia Ikram, Anam Waseem. Sentinel Lymph Node Biopsy in early Breast Cancer using Isosulfan Blue dye alone in resource-limited centers- Our experience. Journal of Surgery and Research 4 (2021): 624-634.
View / Download Pdf Share at FacebookAbstract
Objective
To evaluate the efficacy of Sentinel Lymph Node Biopsy in early Breast Cancer using Isosulfan Blue dye alone in areas where nuclear medicine facilities are not available.
Material and Methods
Data was retrospectively reviewed from July 2019 to December 2020. Only those patients were included who had clinically and radiologically negative axilla without distant metastasis. Those who needed down staging, SLNB was performed after systemic therapy completion. Decision of further axillary dissection in breast conserving surgery (BCS) was based on Z0011 protocol. Patients are followed for locoregional recurrence, disease free survival and overall survival.
Results
We studied a total of 76 patients, out of which 63 patients underwent BCS (83%) and 13 Patients underwent mastectomy (17%). SLN was successfully identified in all patients except one. Majority of patients were T2 at presentation (82%). Frozen section was negative in 51 patients (71%) and positive in 21 patients (29%). Out of 21 positive SLN, 11 underwent ALND while 10 did not. In 85% of patients total no of LNs removed were 03 or more. Follow-up was short, maximum 2 years and minimum 10 months. One patient showed local recurrence in breast at 6 months after surgery. Rest all were fine, follow-up is still going on.
Conclusion
Sentinel lymph node biopsy with Isosulfan blue dye alone is an acceptable method in resource-constrained countries, where unfortunately most patients still undergo axillary dissection irrespective of the clinical nodal status. This study may provide an incentive to such countries to adopt this simpler procedure to avoid long-term m
Keywords
Clinically negative axilla, Axillary dissection, Dual technique, Identification rate, Mapping
Clinically negative axilla articles; Axillary dissection articles; Dual technique articles; Identification rate articles; Mapping articles
Clinically negative axilla articles Clinically negative axilla Research articles Clinically negative axilla review articles Clinically negative axilla PubMed articles Clinically negative axilla PubMed Central articles Clinically negative axilla 2023 articles Clinically negative axilla 2024 articles Clinically negative axilla Scopus articles Clinically negative axilla impact factor journals Clinically negative axilla Scopus journals Clinically negative axilla PubMed journals Clinically negative axilla medical journals Clinically negative axilla free journals Clinically negative axilla best journals Clinically negative axilla top journals Clinically negative axilla free medical journals Clinically negative axilla famous journals Clinically negative axilla Google Scholar indexed journals Axillary dissection articles Axillary dissection Research articles Axillary dissection review articles Axillary dissection PubMed articles Axillary dissection PubMed Central articles Axillary dissection 2023 articles Axillary dissection 2024 articles Axillary dissection Scopus articles Axillary dissection impact factor journals Axillary dissection Scopus journals Axillary dissection PubMed journals Axillary dissection medical journals Axillary dissection free journals Axillary dissection best journals Axillary dissection top journals Axillary dissection free medical journals Axillary dissection famous journals Axillary dissection Google Scholar indexed journals Dual technique articles Dual technique Research articles Dual technique review articles Dual technique PubMed articles Dual technique PubMed Central articles Dual technique 2023 articles Dual technique 2024 articles Dual technique Scopus articles Dual technique impact factor journals Dual technique Scopus journals Dual technique PubMed journals Dual technique medical journals Dual technique free journals Dual technique best journals Dual technique top journals Dual technique free medical journals Dual technique famous journals Dual technique Google Scholar indexed journals Identification rate articles Identification rate Research articles Identification rate review articles Identification rate PubMed articles Identification rate PubMed Central articles Identification rate 2023 articles Identification rate 2024 articles Identification rate Scopus articles Identification rate impact factor journals Identification rate Scopus journals Identification rate PubMed journals Identification rate medical journals Identification rate free journals Identification rate best journals Identification rate top journals Identification rate free medical journals Identification rate famous journals Identification rate Google Scholar indexed journals Mapping articles Mapping Research articles Mapping review articles Mapping PubMed articles Mapping PubMed Central articles Mapping 2023 articles Mapping 2024 articles Mapping Scopus articles Mapping impact factor journals Mapping Scopus journals Mapping PubMed journals Mapping medical journals Mapping free journals Mapping best journals Mapping top journals Mapping free medical journals Mapping famous journals Mapping Google Scholar indexed journals Sentinel lymph node biopsy articles Sentinel lymph node biopsy Research articles Sentinel lymph node biopsy review articles Sentinel lymph node biopsy PubMed articles Sentinel lymph node biopsy PubMed Central articles Sentinel lymph node biopsy 2023 articles Sentinel lymph node biopsy 2024 articles Sentinel lymph node biopsy Scopus articles Sentinel lymph node biopsy impact factor journals Sentinel lymph node biopsy Scopus journals Sentinel lymph node biopsy PubMed journals Sentinel lymph node biopsy medical journals Sentinel lymph node biopsy free journals Sentinel lymph node biopsy best journals Sentinel lymph node biopsy top journals Sentinel lymph node biopsy free medical journals Sentinel lymph node biopsy famous journals Sentinel lymph node biopsy Google Scholar indexed journals adjuvant treatment articles adjuvant treatment Research articles adjuvant treatment review articles adjuvant treatment PubMed articles adjuvant treatment PubMed Central articles adjuvant treatment 2023 articles adjuvant treatment 2024 articles adjuvant treatment Scopus articles adjuvant treatment impact factor journals adjuvant treatment Scopus journals adjuvant treatment PubMed journals adjuvant treatment medical journals adjuvant treatment free journals adjuvant treatment best journals adjuvant treatment top journals adjuvant treatment free medical journals adjuvant treatment famous journals adjuvant treatment Google Scholar indexed journals ultrasound-guided axillary articles ultrasound-guided axillary Research articles ultrasound-guided axillary review articles ultrasound-guided axillary PubMed articles ultrasound-guided axillary PubMed Central articles ultrasound-guided axillary 2023 articles ultrasound-guided axillary 2024 articles ultrasound-guided axillary Scopus articles ultrasound-guided axillary impact factor journals ultrasound-guided axillary Scopus journals ultrasound-guided axillary PubMed journals ultrasound-guided axillary medical journals ultrasound-guided axillary free journals ultrasound-guided axillary best journals ultrasound-guided axillary top journals ultrasound-guided axillary free medical journals ultrasound-guided axillary famous journals ultrasound-guided axillary Google Scholar indexed journals lumpectomy articles lumpectomy Research articles lumpectomy review articles lumpectomy PubMed articles lumpectomy PubMed Central articles lumpectomy 2023 articles lumpectomy 2024 articles lumpectomy Scopus articles lumpectomy impact factor journals lumpectomy Scopus journals lumpectomy PubMed journals lumpectomy medical journals lumpectomy free journals lumpectomy best journals lumpectomy top journals lumpectomy free medical journals lumpectomy famous journals lumpectomy Google Scholar indexed journals
Article Details
1. Introduction
Sentinel lymph node biopsy (SLNB) has become a standard of care in the management of clinically negative axilla in breast cancer surgery. This is because the status of axillary lymph nodes is most important prognostic factor that affects adjuvant treatment decision rather being a part of therapeutic process [1]. The idea behind doing sentinel LN biopsy is; if there are no metastases in sentinel lymph nodes, it is assumed that there are no metastasis in other lymph nodes2, thus avoiding morbidity associated with complete axillary dissection. After Giuliano and colleagues first applied sentinel lymph node biopsy in breast cancer in 1994, the identification rate (of 65% in their study) 3 has increased to almost 100% in subsequent studies. In order to locate the sentinel lymph node, blue dye and radioisotopes have been used4. At some centers, dual technique is used5, while some centers use only radio-isotope technique believing dual technique is not superior to isotope alone.6 However, unlike radioactive technetium T99 sulfur colloid, which requires nuclear medicine facilities, Blue dye only requires frozen section facility besides being cost effective [7]. We used Isosulfan blue dye (Triphenylmethane dye) in our study. The purpose of this study is to validate the use of Isosulfan Blue dye alone for SLNB in early breast cancer patients and subsequent outcome in the form of loco-regional recurrence.
2. Methodology
Data was retrospectively reviewed from July 2019 to December 2020 at a single dedicated breast unit. Total 76 patients were included in the study. Demographic, clinical, operative, and histopathological data were recorded. Descriptive statistics were used to represent patient characteristics. Data was analysed using SPSS version 20. Approval from the ethical committee of the hospital was taken before collection of data.
Patient selection was important for undergoing SLN biopsy. While assessing axillary status, we recognized 3 groups as follows:
- Patients having obvious suspicious LNs both on examination and on radiology (ultrasound and mammography both) or T4 disease were directly recruited to axillary dissection in neoadjuvant setting.
- Patients having obvious benign LNs both on examination and on radiology (ultrasound and mammography both) were directly recruited to SLN biopsy.
- Patients having borderline LNs either on examination and on radiology (ultrasound and mammography both) were subjected to ultrasound-guided axillary LN FNAC. Further categorization to axillary dissection or SLNB was based on its results. We had 16 patients who underwent FNAC before undergoing SLNB (reactive LN on cytology)
One cc. of Isosulfan Blue (half vial) 2.5% dye was injected in the peri-areolar subdermal plane, 10-15 minutes before incision. Two to three minutes of breast lymphatic massage (tapping) helps in better staining and visualization.
Sentinel lymph nodes were identified via axillary skin crease incision, excised and sent for frozen section. The incision was strictly given in axillary crease (the crease at junction of breast and axilla) as sentinel LNs are most commonly located at this site. In our practice we found while dissecting the axilla, care should be taken not to vertically divide all fat down to loose tissue of the axilla, rather slowly dissect layer by layer looking for blue channels and following these. After excision, SLNs were routinely sent to the department of pathology, which would take 40min on average to notify the result. Axillary dissection if needed was done in the same setting. Decision of further axillary dissection in breast conserving surgery was based on Z0011 protocol [8] i.e. 3 positive LN in adjuvant group would undergo axillary dissection. While in neo-adjuvant setting even one positive LN would indicate axillary dissection. Patients undergoing mastectomy would undergo axillary dissection if one LN is positive irrespective of systemic therapy status. Patients are followed for loco-regional recurrence, disease free survival and overall survival. Follow-up was short, maximum 2 years and minimum 10 months because data is between July 19 to Dec. 20. Further expansion of data is planned adding 2021 results along with ongoing follow-up of all patients.
3. Results
A total of 76 patients were analyzed. Mean age was 53.3 (Range 20 - 80 years). Upfront surgery was performed in 42 patients (55%) and post-neo adjuvant surgery was performed in 34 patients (45%). Total 63 patients underwent BCS (83%) and 13 Patients underwent mastectomy (17%). Majority of patients were T2 at presentation (82%), while 14.5% were T1 at presentation. Only three patients (3.9%) were T3 at presentation. All of these three T3 patients underwent mastectomies following systemic therapy, however, only one required ALND. Multifocal/multicentric disease was present in 08 patients (11%) while bilateral cancer was present in 4 patients (5%). Almost all were invasive ductal carcinoma, except 02 patients who were ILC. DCIS in combination with IDC was observed in 17 patients (24%), while pure DCIS was present in one patient only. 51 patients were grade II (67%). ER positive patients were 79%, while 62% were PR positive. H2N positivity was present in 09 patients (12.5%) out of which 06 patients received Herceptin (Table 1). SLN identification was successful in almost all patients, only in one patient with lumpectomy done in some other center, LNs were not stained, so axillary sampling was done, removing five LNs (all were negative).
|
Characteristics |
No & Percentage |
Characteristics |
No & Percentage |
|
|
Upfront surgery: |
42 (55%) |
ER: |
Positive |
60 (79%) |
|
Post chemo surgery: |
34(45%) |
Negative |
16 (21%) |
|
|
BCS: |
63 (83%) |
PR: |
Positive |
47 (62%) |
|
Mastectomy: |
13 (17%) |
Negative |
29 (38%) |
|
|
Stage: |
T1 11 (14.5%) |
H2N: |
Positive |
9 (12%) |
|
T2 62 (82%) |
Negative |
62 (82%) |
||
|
T3 3 (3.9%) |
2+ |
5 (6.6%) |
||
|
ILC: |
2 (2.6%) |
Multifocal: |
8 (11%) |
|
|
IDC + DCIS: |
17 (24%) |
Unifocal: |
68 (89%) |
|
|
DCIS alone |
1 patient |
No. of SLN removed: |
1 SLN |
2 (2.6%) |
|
I |
02 (2.6%) |
2 SLN |
9 (11.8%) |
|
|
II |
51 (67%) |
3 SLN |
28 (36.8%) |
|
|
III |
23 (30%) |
4 SLN |
34 (44.7%) |
|
|
5 SLN |
3 (3.9%) |
Table 1: Cohort characteristics
SLNs were negative on frozen sections in 55 patients (72%) while 21 patients (28%) had one or more LN positive out of which only 11 underwent ALND (05 were upfront and 06 were post neo-adjuvant). Out of these 21(28%) positive SLN patients, 15 patients (20%) had 01 LN positive, 04 patients had 02 LNs positive and 02 patients had 03 LNs positive (Table 2 & Figure 1).
|
No of positive SLN |
Frequency |
ALND |
|
0 |
55 (72.2%) |
0 |
|
1 |
15 (19.7%) |
7 (9.2%) |
|
2 |
4 (5.3%) |
2 (2.6%) |
|
3 |
2 (2.6%) |
2 (2.6%) |
|
Total |
76 (100%) |
11 (14.4%) |
Table 2: Frozen section results and Axillary clearance
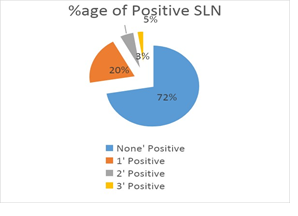
Figure 1: Percentage of Positive LN
Out of 11 Axillary clearance patients in the whole cohort (14.5%), 03 were mastectomies and 08 were BCS. All 11 patients of axillary dissection, had on average 4 LNs involved, out of average 14 LNs removed. In adjuvant setting, axillary dissection was done in only 05 patients despite 15 patients showing positive SLN biopsy results (rest of 10 were all BCS). Out of these five axillary dissections, three were BCS and two were mastectomies. While in a neo-adjuvant setting, all patients with even one positive SLN underwent axillary dissection (all 6) as shown in Table 3 and Figure 2. In the BCS group, 08 patients (05 neo-adjuvant and 03 adjuvant) underwent axillary dissection despite 18 showing one or more positive LNs (rest of 10 were all adjuvant BCS as already mentioned). Out of total 12 mastectomies in the whole group, 03 underwent axillary clearance due to one or more positive LN irrespective of chemotherapy status as shown in Table 4 and Figure 3.
|
No of positive SLN |
ALND not done |
ALND done |
Total |
|
|
Adj |
0 |
27 |
0 |
27 |
|
1 |
8 |
1 |
9 |
|
|
2 |
2 |
2 |
4 |
|
|
3 |
0 |
2 |
2 |
|
|
42 (Total) |
||||
|
Neo |
0 |
28 |
0 |
28 |
|
1 |
0 |
6 |
6 |
|
|
34 (Total) |
Table 3: SLN and consequent axillary clearance in both Adjuvant and Neo-adjuvant groups.
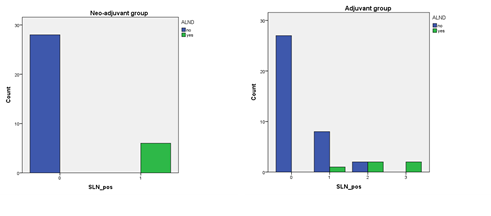
Figure 2: Bar chart showing axillary clearance acc. to chemotherapy status
|
No of positive SLN |
ALND not done |
ALND done |
Total |
|
|
BCS |
0 |
45 |
0 |
45 |
|
1 |
8 |
5 |
13 |
|
|
2 |
2 |
2 |
4 |
|
|
3 |
0 |
1 |
1 |
|
|
63 Total |
||||
|
Mastectomy |
0 |
10 |
0 |
10 |
|
1 |
0 |
2 |
2 |
|
|
3 |
0 |
1 |
1 |
|
|
13 Total |
Table 4: SLNB and consequent axillary clearance in both BCS and Mastectomy groups.
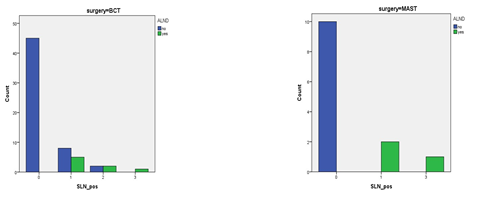
Figure 3: Bar chart showing axillary clearance acc. to type of surgery
According to Z0011 protocol that we follow in our department, for adjuvant BCS, only 3 positive LN will mandate axillary clearance, while in mastectomy (irrespective of adj /neo adj systemic therapy status) and in post neoadjuvant BCS one positive LN will mandate axillary clearance. Axillary LN assessment was done by clinical examination by one surgeon (author) and by ultrasound and mammography (single dedicated radiologist). None of the patients had any sort of allergic reaction from Isosulfan blue dye. In 85% of patients total no of LN removed were 03 or more, while in 11% 02 LNs were removed and in 1.8% (02 patients) one LN only was removed (one was BCS (1/1) with ALND and one was mastectomy (0/1) and no ALND, both remained well on 1.5 years follow-up). Follow-up was short, maximum 1.5 years and minimum 3 months; although 05 patients were lost to follow up but on tracking 4 were reached and they were following up with the oncologist. Only one patient showed local recurrence 6 months after the surgery, at tumor site excision scar, however, LNs remained clinically negative and normal looking radiologically. She is a 25- year-old young woman with TNBC and had a 48mm lump at the time of presentation; she received neoadjuvant chemotherapy with good results and underwent BCS.
4. Discussion
Staging the axilla is important in devising the treatment strategy and predicting prognosis in breast cancer. Omitting axillary dissection in carefully selected patient cohort will result in good quality of life without compromising oncological safety [9]. Clinically and radiologically negative axilla are straightaway candidates for SLNB, while those in whom LNs are borderline needs to undergo FNA (Fine Needle Aspiration) first, if that is negative too, SLNB is then performed. Conventionally, in patients who were candidates for neo-adjuvant systemic therapy to downstage T-size, axillary staging by SLNB was done before starting chemotherapy, however, now in many patients the SLNB is done post- chemotherapy [10,11]. In our study too, those needing down staging of tumor, SLNB was performed after systemic therapy completion. Axillary SLN localization is an important factor in achieving a successful and safe axillary staging. Important factors involved are expertise of a surgeon (learning curve), type of mapping technique used and blockage of actual sentinel lymphatic channels by either previous surgery or by tumor itself [12]. Dual technique is considered to have the highest identification rates compared to single agent mapping breast/axillary surgery, obese patients and when the use of radioactive colloid alone fails to produce a signal in the axilla). However, many centers are now routinely doing mapping by radio-isotope technique alone as many studies have shown no added advantage except in selected cases (negative preoperative lymphoscintigraphy) [15-17]. Commonly used radio-isotopes are 99mTc-sulfur colloid in the USA and 99m Tc-nanocolloid human serum albumin in Europe. LNs are removed following the “10% rule” (all LNs with counts >10% of ex vivo count of the most radioactive node should be removed) [18]. Although preferred but the use of RI (radio-active isotope) creates logistical challenges for hospitals, including the handling and disposal of isotopes, training of staff and legislative requirements. The 6h half-life of the isotope makes scheduling of surgery dependent on the nuclear medicine department. These factors have limited the uptake of SLNB worldwide for hospitals without access to RIs, (as is the case with our center). Krikanova et al. reported a 94.6% SLN identification rate using blue dye alone making this technique very attractive in high volume units without nuclear medicine facilities [19]. Due to above mentioned limitations, blue dye alone as a single agent, is used in many centers in resource-constrained institutions. Three types of Blue dye have been described: Isosulfan blue, Methylene blue and Patent blue. There is a strong cross-reactivity between Patent Blue V (calcium chelated dimer: CAS number: 3536-49-0) and Isosulfan Blue (CAS number: 68238-36-8). Both belong to the group of triarylmethane dyes and basically share the same formula; however, Patent Blue V has an additional hydroxyl group at position 5. Methylene blue [CAS number: 61-73-4 (anhydrous methylene blue), CAS number: 7220-79-3 (methylene blue trihydrate)] is also approved for oral or intravenous administration for the treatment of methemoglobinemia and haemolysis [20]. One of the major problems of blue dye is a possible adverse reaction up to an anaphylaxis. In case of isosulfan blue it has been reported in 0.7% to 1.1% of cases [21]. Patients should be screened for known allergies however, prophylaxis is not routinely done. Allergic reactions are reported less in methylene blue, however, intradermal and subdermal injection of methylene blue must be avoided due to the reported risk of skin necrosis [22]. In our study, no allergic reaction was reported with Isosulfan blue. Among all dyes, methylene blue is cheapest, however, we used one vial of isosulfan blue on two patients (scheduled on same day) to reduce the cost, with no effect on identification success. Many centers consider periareolar as the preferred site of injection. Chagpar et al. showed that subareolar and periareolar injection produced higher SLN identification rates than peritumoral injection [23]. Plane of injection that we followed was subdermal, in peri-areolar location to avoid staining of skin. Lymphatic massage was done by tapping with fingers from the center towards the periphery of the breast. Incision was made 10 to 15 minutes after the injection. The 1st tier sentinel axillary lymph node is most commonly present in axillary skin crease [24]. And this was the site of incision in all our patients. Clough et al found that in 98·2% of patients the axillary SLN was located medially, alongside the lateral thoracic vein, mostly below the second intercostal brachial nerve (86·8%) or in some cases above it (11·5%). In only four patients (1·8%) was the SLN located laterally in the axilla [25]. The site of incision is more important in mapping technique by dye because in radio-nucleotide mapping, the gamma probe tells us the almost exact site before even incision is made. We give incision in the skin crease, which is easily seen between breast and axilla, avoiding axillary hairline. One problem with the dye method is its lower sensitivity or lesser identification rate (IR) compared to dual method. One meta-analysis showed that SNBs mapped with dye alone result in acceptable IRs of 91% (ranged between 75 to 100% in 18 studies) but false negative rate (FNR) of 13% [26]. This is not acceptable according to standards recommended by the American Society of Breast Surgeons which says SLN should be identified in > 95% of patients undergoing SLNB with a false negative rate of 5-10% [27]. False negative rate can only be evaluated if all patients underwent axillary dissection as well, after SLNB, which was not the case in our study (axillary dissection was done in only a small number of patients where indicated). IR in our study was 98.7%, which was high compared to other studies [28]. As mentioned previously, dual technique is superior to single agent blue dye technique giving both better identification rate and false negative rates. However, in most centers in our country, nuclear medicine facilities are not available. In such a scenario, blue dye technique is a very feasible technique, albeit, at the cost of relatively higher false negative rate, which is relatively a smaller risk compared to giving avoidable morbidity of axillary dissection to a large number of patients. This would prevent unnecessary axillary dissection. Careful patient selection for the procedure is the key to better results. The false negative rate declines with more number of sentinel nodes removed.29 In our study 85.5% of patients had 3 or more SLNs removed (Table 1). Huang et al found that the FNR in patients with 1, 2, 3, and 4 SLNs was 23.53%, 15.79%, 3.85%, and 1.79%, respectively [30]. Similarly, Goyal et al. reported that the FNR in patients who had one SLN harvested was 10.1%, compared with 1.1% in those who had multiple SLNs (three or more) removed (p=0.010) [31]. We have fairly larger number of patients in 1.5 years. We intend to add year 2021 patients to it, as it has now become a common procedure at our center (on average 3 SLNB per week). One limitation of the study was its short follow-up of mean 1 year, which we intend to prolong to 3 years along with addition of more data. We used one Isosulfan blue dye vial on two patients to reduce the cost, if some centers find even this difficult due to cost issues; a simple methylene blue dye is good alternative being the cheapest dye. However, it should be kept in mind that it should not be injected superficially (skin necrosis). Frozen section facilities are relatively better available in hospitals across the country compared to scarce nuclear medicine facilities. However, even if not available at a particular set-up, it can still be successfully availed in liaison with other centers where available, in same city. In case even if frozen facility too cannot be managed, SLNB can be sent for permanent section, although not ideal but better then performing axillary dissection in a negative axilla. In neo-adjuvant setting, SLNB can be performed before starting chemotherapy, and if indicated axillary dissection can be done at second stage while doing breast surgery. In adjuvant setting, the likely hood of second axillary surgery (axillary dissection) post-chemo is less likely while following Z0011 protocol. In such scenario, patient selection is even more important, if rightly selected, only a negligible number would need second axillary surgery after completion of chemotherapy. To conclude, Isosulfan blue is an economic and feasible option for sentinel lymph node mapping in a rightly selected patients cohort. It does not need expensive equipment and bypasses surgical dependency on these. Technically, it does not require a long learning curve, though the authors strongly recommend some degree of exposure to dual technique during training years. This study aims to send a message to other centers in countries like us that by an easily available, relatively safe injection, a surgeon can easily learn this procedure and adopt in their regular practice. Widespread use will save many patients from the morbidity of axillary dissection, where it is not indicated. This practice can reduce the overall theater time, requiring short stay in the hospital, avoiding patient revisits with complications and thus cutting on the overall cost of healthcare.
Ethics Committee Approval:
Our hospital institutional review board approved this retrospective data collection and analysis
Informed Consent:
Retrospective study.
Conflict of Interest:
The authors declared no conflict of interest.
Financial Disclosure:
The authors declared that this study received no financial support
Key points
- Mapping by blue dye is acceptable alternative in term of successful identification rate
- Lack of resources does not justify staging by axillary dissection; all such centers should learn and adopt staging by sentinel LN biopsy using blue dye in clinically negative axilla.
References
- Kim H, Cho J, Kwon SY, et al. Biologic subtype is a more important prognostic factor than nodal involvement in patients with stages I and II breast carcinoma. Ann Surg Treat Res 90 (2016): 1-9.
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11(2010): 927-933.
- Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 220 (1994): 391-398.
- Krag DN, Weaver DL, Alex JC, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol 2 (1993): 335-339.
- Radovanovic Z, Golubovic A, Plzak A, et al. Blue dye versus combined blue dye-radioactive tracer technique in detection of sentinel lymph node in breast cancer. Surg Oncol 30 (2004): 913-917.
- Pei-Sheng He, Feng Li, Guan-Hua Li, et al. The combination of blue dye and radioisotope versus radioisotope alone during sentinel lymph node biopsy for breast cancer: a systematic review. BMC Cancer 16 (2016): 107.
- Takamaru T, Kutomi G, Satomi F, et al. Use of the dye guided sentinel lymph node biopsy method alone for breast cancer metastasis to avoid unnecessary axillary lymph node dissection. Experimental and Therapeutic Medicine 7 (2013): 456-460.
- Giuliano AE, McCall LM, Beitsch PD et al. ACOSOG Z0011: A randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node. Journal of Clinical Oncology 28 (2010).
- Kay Blanchard D, John H, Carol R, et al. Relapse and Morbidity in Patients Undergoing Sentinel Lymph Node Biopsy Alone or With Axillary Dissection for Breast Cancer. Arch Surg 138 (2003): 482-488.
- Franceschini G, Leone AD, Sanchez AM et al. Update on sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patient. Ann Ital Chir 91 (2020): 465-468.
- Duveken BY, Willemien van de Water, J Sven D Mieog, et al. Timing of the sentinel lymph node biopsy in breast cancer patients receiving neoadjuvant therapy - recommendations for clinical guidance. Eur J Surg Oncol 39 (2013): 417-424.
- Ted James, Alex Coffman, Anees Chagpar et al. Troubleshooting sentinel lymph node biopsy in breast cancer surgery. Ann Surg Oncol 23 (2016): 3459-3466.
- Motomura K, Inaji H, Komoike Y, et al. Combination technique is superior to dye alone in identification of the sentinel node in breast cancer. J Surg Oncol 76 (2001): 95-99.
- He PS, Li F, Li GH, et al. The combination of blue dye and radioisotope versus radioisotope alone during sentinel lymph node biopsy for breast cancer: a systematic review. BMC Cancer 16 (2016): 107
- O'Reilly EA, Prichard RS, Al Azawi D et al. The value of isosulfan blue dye in addition to isotope scanning in the identification of the sentinel lymph node in breast cancer patients with a positive lymphoscintigraphy: a randomized controlled trial (ISRCTN98849733). Ann Surg 262 (2015): 243-248.
- Bines S, Kopkash K, Ali A, et al. The use of radioisotope combined with isosulfan Blue dye is not superior to radioisotope alone for the identification of sentinel lymph nodes in patients with breast cancer. Surgery 144 (2008): 606-609.
- Derossis AM, Fey J, Yeung H, et al. A trend analysis of the relative value of blue dye and isotope localization in 2000 consecutive cases of sentinel node biopsy for breast cancer. J Am Coll Surg 193 (2001): 473-478
- Martin RC, Edwards MJ, Wong SL, et al. Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. For the University of Louisville Breast Cancer Study Group. Surgery 128 (2000): 139.
- Krikanova M, Biggar M, Moss D. Accuracy of sentinel node biopsy for breast cancer using blue dye alone. Breast J 16 (2010): 384-388.
- Andreas J. Bircher, MD Allergy Unit, Department of Dermatology Petersgraben 4, CH-4031 Basel Switzerland. Blue dyes in medicine – a confusing terminology. Contact Dermatitis 54 (2006): 231-232.
- Raut CP, Hunt KK, Akins JS, et al. Incidence of anaphylactoid reactions to isosulfan blue dye during breast carcinoma lymphatic mapping in patients treated with preoperative prophylaxis: results of a surgical prospective clinical practice protocol. Cancer 104 (2005): 692.
- Stradling B, Aranha G, Gabram S, et al: Adverse skin lesions after methylene blue injections for sentinel lymph node localization. Am J Surg 184 (2002): 350-352.
- Chagpar AB, Martin RC, Scoggins CR, et al. Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery 138 (2005): 56.
- Clough KB, Nasr R, Nos C, et al. New anatomical classification of the axilla with implications for sentinel node biopsy. The Paris Breast Centre (L’Institut du Sein). British Journal of Surgery 97 (2010): 1660-1666.
- Chiao Lo, Po-Chu Lee, Ruoh-Fang Yen, et al. Most frequent location of the sentinel lymph nodes. Asian Journal of Surgery 37 (2014), 125-129.
- Jiyu Li, Xiao Chen, Ming Qi, et al. Sentinel lymph node biopsy mapped with methylene blue dye alone in patients with breast cancer: A systematic review and meta-analysis. Plos One 13 (2018): 1-10.
- The American Society of Breast of Surgeons. Performance and practice guidelines for sentinel lymph node biopsy in breast cancer patients. 2014. Available from: https://www.breastsurgeons.org/docs/statements/Performance-and-Practice-Guidelines-for-Sen tinel-Lymph-Node-Biopsy-in-Breast-Cancer-Patients.pdf (2021).
- Brahma B, Ifandriani Putri R, Karsono R et al. The predictive value of methylene blue dye as a single technique in breast cancer sentinel node biopsy: a study from Dharmais Cancer Hospital. World J Surg Oncol 15 (2017): 41.
- Yi M, Meric-Bernstam F, Ross MI et al. How many sentinel lymph nodes are enough during sentinel lymph node dissection for breast cancer? Cancer 113 (2008): 30-37
- Huang Li, Zhang Jun, Ge Zhi-Cheng et al. Factors that affect the false negative rate of sentinel lymph node mapping with methylene blue dye alone in breast cancer. Journal of International Medical Research 47 (2019) 4841-4853 .
- Goyal A, Newcombe RG, Chhabra A, et al. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer--results of the ALMANAC validation phase. Breast Cancer Res Treat 99 (2006): 203-208.
