Selection of an Inactivated Enterococcus faecium and Evaluation of its Potential as an Immunomodulator via Intravenous Injection
Article Information
Lanping Yu1,3#, Wen Zhang1,3#, XueFeng Wang1,3#, Ke Zhang1,3, YanMin Yin1,3, JinLian Li2,3*, YouFei Shi1,3*
1College of Animal Science and Veterinary Medicine, Shandong Agricultural University, Tai’an City, Shandong Province, China, 271018
2College of Biology and Brewing Engineering, Taishan University, Tai’an 271021, China
3Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Tai’an, China, 271018
*Corresponding author: Youfei Shi, Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Shandong Agricultural University, 61 Daizong Street, Taian City, Shandong Province, 271018, China: Jinlian Li, Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Shandong Agricultural University, 61 Daizong Street, Taian City, Shandong Province, 271018, China.
#These authors contribute equally to this work and share first authorship
Received: 08 April 2022; Accepted: 16 April 2022; Published: 28 April 2022
Citation: Lanping Yu, Wen Zhang, XueFeng Wang, Ke Zhang, YanMin Yin, JinLian Li, YouFei Shi. Selection of an Inactivated Enterococcus faecium and Evaluation of its Potential as an Immunomodulator via Intravenous Injection. Journal of Biotechnology and Biomedicine 5 (2022): 42-62.
View / Download Pdf Share at FacebookAbstract
Immunosuppression of livestock has become one of the key problems troubling the healthy development of the livestock industry in China. Research on the development of novel and highly effective immune enhancers to alleviate immunosuppression in livestock is important. In this study, a dexamethasone immunosuppressed mouse model was used to investigate the possibility of inactivated Enterococcus faecium by intravenous injection as an immunomodulatory agent. The effects of intravenous injection inactivated E. faecium in normal, model and immunosuppressed mice were examined using carbon clearance index, EdU marker cell assay, real-time fluorescent quantitative PCR and flow cytometry. The results showed that the inactivated E. faecium retained its intact morphology. Intravenous injection was the most potent route of administration among the different routes. The spleen, thymus and mesenteric lymph node cells of all groups of mice showed different degrees of proliferation. The cytokine expression levels, non-specific immunity and specific immune function of normal, and DXM-immunosuppressed mice were significantly increased by intravenous injection of different doses of inactivated E. faecium. The above studies suggest that morphologically intact inactivated E. faecium can exert good immune enhancing effects by intravenous injection into mice, which has potential wide application in human medicine and veterinary clinics.
Keywords
Dexamethasone (DXM); Flow cytometry; Inactivated E. faecium; Intravenous Injection; Mice; Immunomodulator
Flow cytometry articles Flow cytometry Research articles Flow cytometry review articles Flow cytometry PubMed articles Flow cytometry PubMed Central articles Flow cytometry 2023 articles Flow cytometry 2024 articles Flow cytometry Scopus articles Flow cytometry impact factor journals Flow cytometry Scopus journals Flow cytometry PubMed journals Flow cytometry medical journals Flow cytometry free journals Flow cytometry best journals Flow cytometry top journals Flow cytometry free medical journals Flow cytometry famous journals Flow cytometry Google Scholar indexed journals Dexamethasone (DXM) articles Dexamethasone (DXM) Research articles Dexamethasone (DXM) review articles Dexamethasone (DXM) PubMed articles Dexamethasone (DXM) PubMed Central articles Dexamethasone (DXM) 2023 articles Dexamethasone (DXM) 2024 articles Dexamethasone (DXM) Scopus articles Dexamethasone (DXM) impact factor journals Dexamethasone (DXM) Scopus journals Dexamethasone (DXM) PubMed journals Dexamethasone (DXM) medical journals Dexamethasone (DXM) free journals Dexamethasone (DXM) best journals Dexamethasone (DXM) top journals Dexamethasone (DXM) free medical journals Dexamethasone (DXM) famous journals Dexamethasone (DXM) Google Scholar indexed journals Inactivated E. faecium articles Inactivated E. faecium Research articles Inactivated E. faecium review articles Inactivated E. faecium PubMed articles Inactivated E. faecium PubMed Central articles Inactivated E. faecium 2023 articles Inactivated E. faecium 2024 articles Inactivated E. faecium Scopus articles Inactivated E. faecium impact factor journals Inactivated E. faecium Scopus journals Inactivated E. faecium PubMed journals Inactivated E. faecium medical journals Inactivated E. faecium free journals Inactivated E. faecium best journals Inactivated E. faecium top journals Inactivated E. faecium free medical journals Inactivated E. faecium famous journals Inactivated E. faecium Google Scholar indexed journals Intravenous Injection articles Intravenous Injection Research articles Intravenous Injection review articles Intravenous Injection PubMed articles Intravenous Injection PubMed Central articles Intravenous Injection 2023 articles Intravenous Injection 2024 articles Intravenous Injection Scopus articles Intravenous Injection impact factor journals Intravenous Injection Scopus journals Intravenous Injection PubMed journals Intravenous Injection medical journals Intravenous Injection free journals Intravenous Injection best journals Intravenous Injection top journals Intravenous Injection free medical journals Intravenous Injection famous journals Intravenous Injection Google Scholar indexed journals Mice articles Mice Research articles Mice review articles Mice PubMed articles Mice PubMed Central articles Mice 2023 articles Mice 2024 articles Mice Scopus articles Mice impact factor journals Mice Scopus journals Mice PubMed journals Mice medical journals Mice free journals Mice best journals Mice top journals Mice free medical journals Mice famous journals Mice Google Scholar indexed journals Immunomodulator articles Immunomodulator Research articles Immunomodulator review articles Immunomodulator PubMed articles Immunomodulator PubMed Central articles Immunomodulator 2023 articles Immunomodulator 2024 articles Immunomodulator Scopus articles Immunomodulator impact factor journals Immunomodulator Scopus journals Immunomodulator PubMed journals Immunomodulator medical journals Immunomodulator free journals Immunomodulator best journals Immunomodulator top journals Immunomodulator free medical journals Immunomodulator famous journals Immunomodulator Google Scholar indexed journals biological articles biological Research articles biological review articles biological PubMed articles biological PubMed Central articles biological 2023 articles biological 2024 articles biological Scopus articles biological impact factor journals biological Scopus journals biological PubMed journals biological medical journals biological free journals biological best journals biological top journals biological free medical journals biological famous journals biological Google Scholar indexed journals bacteria articles bacteria Research articles bacteria review articles bacteria PubMed articles bacteria PubMed Central articles bacteria 2023 articles bacteria 2024 articles bacteria Scopus articles bacteria impact factor journals bacteria Scopus journals bacteria PubMed journals bacteria medical journals bacteria free journals bacteria best journals bacteria top journals bacteria free medical journals bacteria famous journals bacteria Google Scholar indexed journals electron microscopy articles electron microscopy Research articles electron microscopy review articles electron microscopy PubMed articles electron microscopy PubMed Central articles electron microscopy 2023 articles electron microscopy 2024 articles electron microscopy Scopus articles electron microscopy impact factor journals electron microscopy Scopus journals electron microscopy PubMed journals electron microscopy medical journals electron microscopy free journals electron microscopy best journals electron microscopy top journals electron microscopy free medical journals electron microscopy famous journals electron microscopy Google Scholar indexed journals mononuclear articles mononuclear Research articles mononuclear review articles mononuclear PubMed articles mononuclear PubMed Central articles mononuclear 2023 articles mononuclear 2024 articles mononuclear Scopus articles mononuclear impact factor journals mononuclear Scopus journals mononuclear PubMed journals mononuclear medical journals mononuclear free journals mononuclear best journals mononuclear top journals mononuclear free medical journals mononuclear famous journals mononuclear Google Scholar indexed journals
Article Details
1. Introduction
Enterococcus faecium (E. Faecium) has excellent biological characteristics such as heat resistance, stomach acid resistance, bile salt resistance, strong adhesion, bacteriostasis and antibiotic resistance, which is a potential new antibiotics replacement feed [1]. It can also improve the conversion efficiency of feed, enhance the immunity of animal, and reduce the rate of diarrhea and mortality. In order to improve the quality of livestock products, E. faecium micro-ecological preparations have been widely used in livestock and poultry breeding [2]. In 2005, Pollmann found that feeding E. faecium to pregnant sows and their piglets delayed the occurence of Chlamydia infection in piglets and significantly reduced the natural infection rate in piglets [3]. This may be related to the enhancement of the immune response of piglets by E. faecium. In 2012, Kreuzer found that E. faecium promoted the clearance of naturally infected rotavirus in piglets, but the study also found that the detoxification effect depended on the type of virus and might be influenced by immune status [4]. In 2016, Tian demonstrated that the anti-inflammatory effect of E. faecium, like other probiotics, relies on Toll-like receptor 2 (TLR2) and TLR-related negative regulatory molecules [5]. In 2005, Jimenez found that E. faecium was isolated and identified in cord blood samples from healthy newborns delivered by elective cesarean section and detected in amniotic fluid by Polymerase Chain Reaction (PCR) [6]. These studies suggest that E. faecium may enter the bloodstream through the GI tract and eventually reach the amniotic fluid, implying that lactic acid bacteria in the GI tract may enter the bloodstream [6]. The factors of feed processing (such as granulation and other heat treatment) often lead to loss of activity of heat-sensitive probiotics such as Lactobacillus, E. faecium and Bifidobacteria [7, 8]. Guo found that live E. faecium and heat-inactivated E. faecium had similar probiotic effects in reducing diarrhea of piglets, but live E. faecium has a better regulation effect on innate defense barrier of small intestinal mucosa of piglets, and both of them could induce an immune response dominated by tolerance [9]. Huang found that oral E. faecium EFl could promote the growth and intestinal health of piglets before and after weaning, and its probiotic effects were to improve the intestinal microecological balance, regulate the chemical and mechanical barrier functions of intestinal mucosa and innate immune response [10]. Feng confirmed the safety of weaned piglets fed with Enterococcus faecium WEI-10 by detecting the active state, diarrhea score, growth performance, bacteremia and histopathology of weaned piglets [11]. This phenomenon has aroused the keen interest of our group. Based on the above studies and the reason of immunomodulatory function of E. faecium, we boldly speculate that lactic acid bacteria entering the blood may have a stronger immunomodulatory effect. Therefore, we injected a certain amount of live E. faecium into the tail vein of mice and observed that some of the mice died over a long period of time. In this way, we concluded that injection of live bacteria is more dangerous to the body, so we injected E. faecium into the tail vein of the mice after inactivation, which could greatly improve the safety of the mice. E. faecium can effectively relieve oxidative stress damage caused by Escherichia coli infection, but high dose of E. faecium could produce side effects, causing oxidative stress response in the body and leading to biofilm damage [12]. Yi found that Salmonella attenuate could proliferate in various solid tumors after intravenous injection, but could be rapidly cleared in normal organs without obvious toxicity [13]. E. faecium had no effect on the number of lymphocyte subsets in peripheral blood of broilers challenged with Salmonella [14]. Szabó found that feeding E. faecium can promote humoral immunity of weaned piglets, and all various indexes of E. faecium treated group were superior to control group after Salmonella challenge [15]. This study benefited from the above-mentioned reports, and from the perspective of drug development, inactivated E. faecium was directly injected intravenously into various animals in its intact form, and confirmed its strong immune enhancing effect with the advantage of no drug residues in the animals.
The immune suppression of livestock in large-scale breeding has been one of the key problems in the healthy development of breeding industry. In the large-scale breeding industry, many factors could cause the immune suppression of animals, which leads to the occurrence and prevalence of infectious diseases. Therefore, it was very important to develop new and efficient immune enhancers to remove immune suppression in livestock for the prevention and control of bacterial and viral diseases and vertical transmission. Intravenous injection of complete forms of inactivated lactic acid bacteria can effectively improve immune function and improve sub-health of the body, which is very important for disease prevention and healthy breeding of livestock.
2. Results
2.1. Preparation and Morphologic Observation of Inactivated E. faecium
faecium was counted before and after inactivation using a THOMA bacterial counting plate, and the results were as follows: The bacterial counts showed no significant change in the number of bacteria before and after inactivation, and 1.0 x 109 CFU/mL was the 1st amount in subsequent trials. A small amount of inactivated E. faecium was taken for Gram staining, and the morphology of the inactivated bacteria was observed under oil microscope (Figure 1A-D). By comparison, it was found that the profiles and morphologies of inactivated bacteria and live bacteria were the same, while electron microscope observation showed that the bacteria maintained their intact morphology of the bacteria before and after inactivation (Figure 1-L1-2 / D1-2).
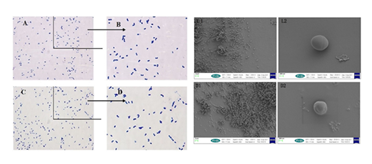
Figure 1: The results of Gram stain (A-D)and electron microscopy (L1~2/D1~2)before and after inactivation of E. faecium.
Note: Figure A: oil microscope observation results of live E. faecium; Figure B: partial magnification results of Figure A; Figure C, oil microscope observation results of inactivation E. faecium; Figure D: partial magnification results of Figure A.
L1, 2.00 KX of viable bacteria; L2,25.00KX of viable bacterial; D1,2.00KX of inactivated bacteria; L2,25.00KX of inactivated bacterial
2.2. Comparative Study on Different Administration Routes of Inactivated E. faecium
Statistical analysis of the mortality rates of mice with different routes of inactivated E. faecium administration showed that the mortality of mice in the intravenous administration group were significantly lower than those in other administration methods. The difference between the fecal inactivated E. faecium administration group and the Salmonella model group (SE) was significant (P<0.05), and the difference between the inactivated E. faecium administration group and the other administration groups was highly significant (P<0.01) (Figure 2A), so it was determined that the mice were administrated intravenously. A higher value of carbon clearance index K indicates a higher phagocytic capacity of mononuclear macrophages. Comparison of the colorimetric values of the groups showed that the intravenous (iv) injection groups was highly significant (P<0.01) compared to the control group, while the subcutaneous injection group (ih), intramuscular injection group (im) and oral administration group (po) were significantly different (P<0.05) compared to the control group (Figure 2B). Based on these results, the intravenous injection route (iv) was identified for follow-up studies.
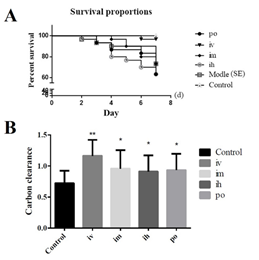
Figure 2: The results of the comparative study on the efficacy of different administration routes of inactivated E. faecium.
Note: A: Survival curves of mice with different administration routes; B: Comparison of carbon clearance in the route of administration
Note: **indicates an extremely significant difference compared with the Control (P<0.01), * indicates a significant difference compared with the Control (P<0.05).
2.3. Screening of Different Doses of Inactivated E. faecium by Intravenous Injection in Mice
After detecting the NDV antibody titers in each group, there was a significant difference between the 5-fold and 0.008-fold groups compared with the blank control group (P<0.05), and a highly significant difference between the 1-fold group and 125-fold groups compared with the blank control group (P<0.01). Numerically, the 1-fold group was significantly higher than other groups (Figure 3A). Therefore, 5 times administration (5×), 1 times administration (1×) and 0.2 times administration (0.2×) were selected as the high, medium and low doses for subsequent experiments. The comparative analysis of colorimetric values of the groups showed that the carbon clearance index K was significantly higher in the mice of the inactivated E. faecium administration group. Compared with the blank control group, there were significant differences between the 1-fold group and 0.008-fold groups (P<0.05), highly significant differences between the 5-fold group, 0.2-fold group and 0.04-fold groups (P<0.01) (Figure 3B). Therefore, 5 times administration (5×), 1 times administration (1×) and 0.2 times administration (0.2×) were selected as high, medium and low doses for subsequent experiments.
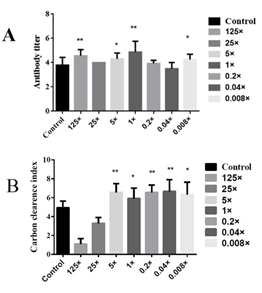
Figure 3: Screening of different doses of inactivated E. faecium by intravenous injection in mice.
Note: A, Results of NDV antibody titers obtained at different concentrations; B, Comparison of the concentration of carbon particles
Note: ** indicates an extremely significant difference compared with the Control(P<0.01), * indicates a significant difference compared with the Control(P<0.05).
2.4. Effects of Inactivated E. faecalis by Intravenous Injection on the Immune Function in Normal and Immunosuppressed Mice
As can be seen from Figure 4A, thymus pentapeptide (TP-5) did not increase the phagocytosis of mouse mononuclear macrophages as expected in the normal mouse group (Control), but significantly increased the phagocytosis of mouse macrophages (P<0.01) in the high-dose, medium-dose and low-dose groups. In the immunosuppression group, the difference between the high-dose group (is High) and the thymopentin group (is TP-5) compared with immunosuppression model group (is Model) difference was extremely significant (P < 0.01), the difference between the low-dose group (is Low) and the immunosuppression model group (is Model) was significant (P < 0.05) while there was no significant difference between the other groups compared with the immunosuppression model group (is Model), but the difference between the high-dose group (is High) and the low-dose group (is Low) was not significant compared with the normal group (is Normal). These results showed that the high dose group (is High) and low dose group (is Low) promoted phagocytosis of mouse mononuclear macrophages to normal levels (Figure 4B). The results of non-specific immune function in immunosuppressed mice showed that the carbon clearance index K value in the immunosuppressed model group (is Model) were significantly lower than those in the normal control group (is Normal). The high, middle and low doses of Gankong ® group showed highly significant or significantly higher carbon clearance index K values compared to the immunosuppressed model group (is Model). These results suggest that inactivated E. faecium intravenous injection can improve the non-specific immune function in the immunosuppressed mouse models.
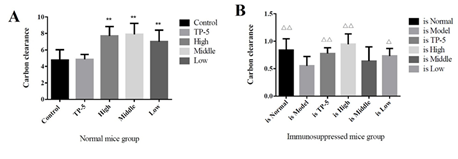
Figure 4: Mouse peritoneal macrophage phagocytosis function.
Note: A, normal mouse group; B, immunosuppressed mouse group.
**indicates an extremely significant difference compared with the Control(P<0.01)
* indicates an significant difference compared with the Control(P<0.05).
??indicates an extremely significant difference compared with the Control(p<0.01), ? indicates an significant difference compared with the Control(P<0.05).
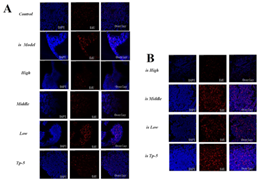
By comparing the proliferation of thymoctes in each group, it was found that the low-dose group promoted the proliferation of thymocytes in the normal mouse group (Control), while the middle-dose group (is Middle), low-dose group (is Low) and thymopentapeptide group (is TP-5) significantly increased the proliferation of thymocytes in the immunosuppressed mouse group (Supplementary Figure S1). By comparing the proliferation of splenocytes in each group, it was found that the high-dose groups significantly promoted on the proliferation of splenocytes proliferation in the normal mice group (Control), while the high-dose (is High), middle-dose (is Middle) and low-dose group (is Low) significantly promoted the proliferation of splenocytes in the immunosuppressed mice group (Supplementary Figure S2).
By comparing the proliferation of mesenteric lymph node cells in each group, it was found that the high-dose (High), middle-dose (Middle) and low-dose groups (Low) significantly promoted the proliferation of mesenteric lymph node cells in the normal mice group (Control), while the middle-dose (is Middle) and low-dose groups (is Low) significantly promoted the proliferation of mesenteric lymph node cells in the immunosuppressed mice group (Supplementary Figure S3). The thymus index of each group was analyzed and comparatively, and the results was that there was no significant difference between normal mice group (Control) and High, Middle, Low and TP-5 group, while the difference between immunosuppression model group (is Model) and control group (Control) was extremely significant (P<0.01), indicating that the immunosuppression model was successful constructed, and the difference between each different administration immunosuppression group and model group (Model) was significant for middle and low doses (P<0.05) (Figure 5).
By the comparative analysis of the spleen index of mice in each group, the difference between the normal mice in the high-dose group (High) and the control group (Control) was extremely significant (P<0.01), indicating that the spleen index of mice was significantly improved, while there was no significant difference between the other administration groups and the control group (Control) (Figure 5A). However, the differences between the high-dose group (is High) and the thymus pentapeptide groups (is TP-5) and the immunosuppression model group (Model) were extremely significant (P<0.01), indicating that the spleen index of the mice was significantly improved (Figure 5B).
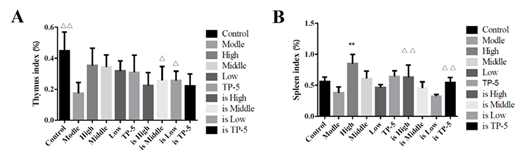
Figure 5: Effect of immune organ index in mice.
Note: A, thymus index; B, spleen index;
??indicates an extremely significant difference compared with the Control(P<0.01), ? indicates an significant difference compared with the Control(P<0.05).
2.5. Effects of Inactivated E. faecium by Intravenous Injection on the Expression of Spleen Cytokines in Normal and Immunosuppressed Mice
DXM was found to have no significant effect on IL-1β, IL-10, IL-12b, IFN-γ and TNF-α, but had an inhibitory effect on IL-12a in mice. After intravenous injection of inactivated E. faecium, the transcription levels of IL-1β and IL-10 were significantly increased in both normal mice and DXM-treated mice, and the transcription level of TNF-α in spleen of normal mice was significantly increased, but there was almost no effect on the transcription level of TNF-α in DXM-treated mice. In addition, there was no significant effect on IL-12a in normal and DXM-treated mice and no significant effect on IL-12b in normal mice, but the mRNA transcript levels of IL-12b in DXM-treated mice could be significantly or very significantly improved. Middle dose of SW-1 significantly increased the transcript level of IFN-γ in the spleen of normal mice, but had no significant effect on DXM-treated mice (Figure 6).
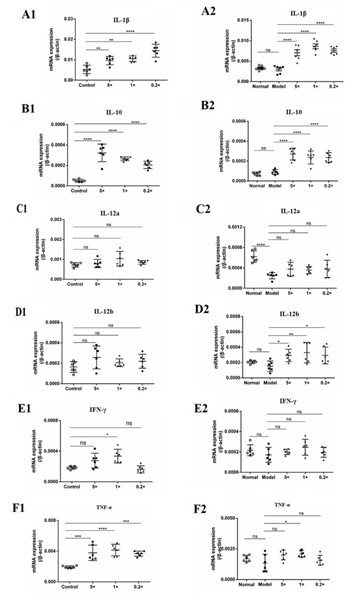
Figure 6: Inactivated E. faecium by intravenous injection induced the expression of cytokines in spleen of immunosuppressive mice.
Note: Control groups (Normal); immunosuppression groups(Model),×0.2, ×1 and ×5 dose groups
**indicates an extremely significant difference compared with the Control(P<0.01),* indicates an significant difference compared with the Control(P<0.05);
??indicates an extremely significant difference compared with the Control(P<0.01), ? indicates an significant difference compared with the Control(P<0.05).
2.6. Effects of Inactivated E. faecalis by Intravenous Administration on Spleen Cells in Normal and Immunosuppressed Mice
Inactivation of E. faecium by intravenous injection resulted in a significant increase in the number of neutrophils (Neu), monocytes (Mon), dendritic cells (DC), regulatory T cells (Tregs), and B cells in the spleen of the immunosuppressed mouse model (DXMS+SW-1) prepared by DXM and restored to normal level (PBS). CD4 and CD8 were also increased, but not significantly compared to the model group (DXMS). In addition, DXM had no effect on NK cells (Figure 7).
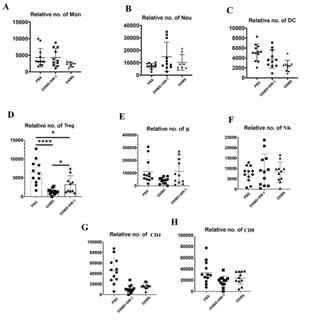
Figure 7: Effect of intravenous injection of inactivated E. faecium on spleen immune cell in immunosuppressive mice.
Effect of intravenous injection of inactivated E. faecium on the numbers of immune cells in spleen of immunosuppressive mice
Note: PBS groups; DXMS+SW-1groups; DXMS groups
**indicates an extremely significant difference compared with the Control(P<0.01),* indicates an significant difference compared with the Control(P<0.05).
2.7. Flow Cytometry was Used to Analyze the Effect on the Adaptive Immune Response of Inactivated E. faecium by Intravenous Injection in the Spleen Cells of Normal and Immunosuppressed Mice
Flow cytometric analysis showed that DXM decreased the number of CD4+ T cells compared to control mice, while tail vein injection of inactivated E. Faecium greatly increased the number of CD4+ T cells suppressed by DXM, indicating that inactivated E. faecium enhanced CD4+ T cell-mediated adaptive immunity responses. Inactivated E. Faecium increased CD4+T cells (Figure 8A). DXM significantly reduced the relative number of CD4+CD25+ Tregs compared to controls, whereas tail vein injection of inactivated E. faecium significantly increased the relative number of CD25+ Tregs inhibited by DXM. These results suggest that inactivated E. faecium enhanced the adaptive immune response mediated by CD25+ Tregs cells (Figure 8B).
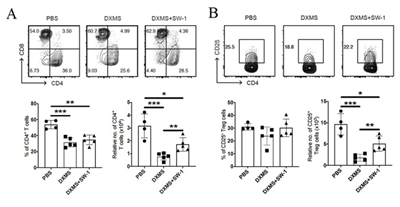
Figure 8: The results of the adaptive immunity responses of SP-CD4+ T (A)/ SP-Treg/ CD4+CD25+ T cells (B).
Note: PBS groups; DXMS+SW-1 groups,DXMS groups
** indicates an extremely significant difference compared with the Control(P<0.01),* indicates an significant difference compared with the ControlP<0.05).
2.8. Effects of Inactivated E. faecium Intravenous Injection on Humoral Immune Function in Normal and Immunosuppressed Mice
Compared with the control group (Control), the SW-1 group significantly increased the level of NDV antibodies, indicating that SW-1 intravenous injection can increase the level of NDV antibodies in normal mice, i.e., improve the specific immune function of normal mice, as shown in (Table 1).
|
Group |
Dose |
Antibody Level |
|
Control |
0 |
4.6±0.7 |
|
SW-1 |
1× |
5.4±0.7* |
Table 1: Effect of SW-1 on the level of NDV antibody in normal mice.
Compared with the control group (Control), the antibody titers of the immunosuppression model group (DXMS) decreased significantly. The antibody level of NDV in the SW-1 group was significantly increased compared with the immunosuppression model group (DXMS), and there was no significant difference between the SW-1 group and the control group (Control), indicating that the antibody level of NDV in the SW-1 group was restored to the normal level. In other words, SW-1 could improve the specific immune function of immunocompromised mice as shown in Table 2.
|
Group |
Dose |
Antibody Level |
|
Control |
0 |
4.06±0.68 |
|
DXMS |
0 |
3.31±1.01* |
|
SW-1 |
1× |
4.06±0.68? |
Table 2: Effect of SW-1 on the level of NDV antibody in immunosuppressive mice model.
3. Discussion
The research on immunomodulators has become one of the most active research fields in applied medicine at home and abroad. Most of the national and international studies on inactivated E. faecium also stop at oral administration, for example, in 2017, researchers found that inactivated E. faecium administered orally to mice could modulate the host’s immune system and alleviate viral infections [16]. In this study, it was found that among the different routes of administration, intravenous injection had the strongest effect, which was significantly different from oral, subcutaneous and intramuscular injections. In the present study, the effect of inactivated E. faecium in its intact form as an immunomodulator was initially investigated after intravenous administration. There are many methods and techniques to detect non-specific immune function. The method of carbon clearance experiment has been gradually matured and widely used for the detection of macrophage phagocytosis [17]. Zhao used carbon clearance test to determine the effect of polysaccharide (PHP) from Portulaca oleracea L. on the immune phagocytic function of tumor-bearing mice, indicating that PHP can enhance the phagocytic ability of the immune organs of mice to foreign bodies and improve the immune function of mice [18]. In this study, the carbon clearance index test was also used to explore and identify 5×109, 1×109 and 0.2×109 CFU/ mL as high, medium and low doses for administration in subsequent studies. Some studies have reported that feeding a mixture of tomato and carrot juice fermented with Lactobacillus to immunocompromised mice showed that the high and middle doses of fermentation solution significantly improved the phagocytosis of mouse macrophages compared with the immunosuppression model group, and the high dose of fermentation solution also significantly increased the conversion rate of spleenic T lymphocytes [19]. Jiang found that feeding E. faecium fermentation broth to BALB/c mice significantly increased the number of lactobacillus in the intestine of mice. Meanwhile, the concentrations of IL-6 and IFN-γ in mouse spleen lymphocyte cultures increased with the duration and concentration of increase of E. faecium feeding, and peaked at 14 days of feeding [20]. Huang showed that E. faecium could regulate the expression of cytokines in the small intestinal mucosa of newborn piglets and significantly inhibited the production of pro-inflammatory cytokines as tumor necrosis factor-α (TNF-α), γ-interferon (IFN-γ), interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-12 (IL-12) and interleukin-8 (IL-8). However, E. faecium could significantly increase the secretion of anti-inflammatory cytokines as interleukin-10 (IL-10) and transforming growth factor-β1 (TGF-β1). These results suggest that E. faecium can suppress inflammation and promote homeostasis in intestinal mucosa [10]. In this study, we found that after intravenous injection of inactivated E. faecium, both normal mice and DXM-treated mice significantly increased the transcription levels of IL-1β and IL-10, and significantly enhance the transcription level of TNF-α in the spleen of normal mice, but had little effect on the transcription of TNF-α in DXM-treated mice. Medium-dose intravenous injection of inactivated E. faecium significantly increased the transcription levels of IFN-γ transcription in the spleen, but had no significant effect on DXM-treated mice.
It is generally believed that lactobacillus can enhance immunity in two ways: first, the nonspecific immune response process of the body, with enhancing phagocytic activity of monocytes - phagocytes (monocytes and macrophages) and polymorphonuclear leukocytes, and stimulation of reactive oxygen species, lysosomal enzymes and secretion of mononuclear factor [21]. In this study, the Number of Neutrophils (NEN), Monocytes (Mon), Dendritic Cells (DC), Regulatory T Cells (Tregs) and B cells in the spleen of DXM immunosuppressed mice was significantly increased and restored to normal levels by injecting inactivated E. faecium into DXM immunosuppressed mice. New research by Seder and his team shows that intravenous BCG vaccine protects rhesus monkeys from tuberculosis and that intravenous immunization induces more antigen-responsive CD4 and CD8 T cell responses in blood, spleen, bronchial alveolar lavage, and lung lymph nodes. In addition, intravenous immunization induced a high frequency of antigen-responsive T cells in all lung parenchymal tissues [22]. Li found that oral administration of lactobacillus suppressed dust mite induced allergic airway inflammation, mainly because lactobacillus induced the proliferation of CD4+ regulatory T cell subsets in mouse spleenocytes and downregulated Th1/Th2 cytokines levels through the release ofIL-10 [21]. In this study, it was also found that IL-10 was significantly increased in both normal mice and immunosuppressed mice after the injection of inactivated E. faecium. Second, stimulation affects the specific immune response process of the organism, such as increasing the levels of IgA, IgM and IgG on the mucosal surface and in the serum, enhancing humoral immunity, promoting the proliferation of T and B lymphocytes, and improving cellular immunity [23]. Wen showed that E. faecium could improve the specific and non-specific immune functions of piglets, which is a major reason for its ability to improve the production performance of piglets [24]. In this study, intravenous injection of inactivated E. faecium increased antibody levels in NDV mice and also increased NDV antibodies back to normal levels. The results showed that intravenous administration of inactivated E. faecium improved humoral immune function in both normal mice and immunosuppressive mice. T cells are adaptive immune cells that play a central role in pathogen infection. Given that inactivated E. Faecium increased spleen induces in both normal and immunosuppressed mice, we next sought to investigate whether inactivated E. Faecium alters cell-mediated adaptive immunity responses. It has been reported that DXM treatment reduced the number of CD4+ cells, leading to immunosuppression [25]. To verify this, we established a mouse immunosuppression model by intraperitoneal injection of DXM. Next, we examined CD4+ and CD8+ in spleen cells of control mice, DXM-induced immunosuppressed mice and DXM-induced immunosuppressed mice before subsequent tail vein injection of inactivated E. Faecium. Abnormalities in the number and suppressive function of CD4+CD25+ Tregs play an important role in the pathogenesis of autoimmune and allergic diseases [26,27,28]. As an important immunosuppressive cell, CD4+CD25+ regulatory T cells (CD4+CD25+ Tregs) can amplify the suppressive effect of IL-10 by promoting IL-10 secretion by surrounding cells [29, 30]. The inflammation inhibitor IL-10 plays an important role in the development of allergic and autoimmune diseases [31]. In this study, IL-10 transcript levels were extremely significantly enhanced in both normal and DXM-treated mice after intravenous administration of inactivated E. faecium. CD4+CD25+Tregs in CD4+ T cells have the ability to induce IL-10 secretion from CD4+CD25+T cells, which may be more similar to the natural state of CD4+CD25+Tregs in vivo [31]. Such a powerful immune enhancing effect of inactivated E. faecium may be due to its direct action on Toll-like receptors of various immune cells in the blood, such as monocytes, macrophages, granulocyte, dendritic cells, NK cells, T cells, B cells, etc.), which directly activate the functions of various immune cells. The specific mechanism of action inactivated E. faecium remains to be further investigated.
4. Materials and Methods
4.1. Ethics statement
Institutional Review Board Statement: The study protocol and the poultry studies were approved by the Animal Care and Use Committee of Shandong Agricultural University, Tai’an, China (Ethical Approval Number SDAUA-2018-027).
4.1. Preparation and morphological observation of inactivated E. faecium Preparation and Morphological Observation of Inactivated E. faecium
The E. faecium strain was purchased from CICC 6049 (China Center of Industrial Culture Collection) and Laboratory preserved. E. faecium was cultured in MRS broth at 37 °C for 24 hours, and the cell culture was prepared with sterile normal saline. Bacteria count was carried out on THOMA bacteria counting board, and the suspension was diluted to 1×109 CFU/ml as 1× concentration. The suspension was inactivated at 121°C, 0.12 MPa for 15min to obtain 1× concentration inactivated E. faecium. Based on this, various concentrations of E. faecalis can be obtained by different dilution with normal saline. Gram staining, oil microscope and scanning electron microscope, respectively, were used to observe the lived and inactivated E. faecalis.
4.2. Comparative Study on Different Administration Routes of Inactivated E. faecium
In this experiment, blank control group (Control), Salmonella enteritidis (CVCC 3377 standard strain purchased from China Veterinary Microbial Species Preservation and Management Center) model group (SE)), oral administration group(po), intravenous administration group (iv), muscle administration group (im) and subcutaneous administration group (ih) were set up,each group had 30 SPF Kunming (KM) mice(purchased from Shandong Experimental Animal Center, license no. SCXK (Lu) 20140007)weighing 18-22g,half male and half female. The mice adapted to the environment for 3 days and were free to eat and drink. Then, mice in each administration group were given 1x concentration of inactivated E. faecium at 0.1mL/10g body weight one day in advance, while mice in salmonella model group (SE) and blank control group (Control) were given the same amount of normal saline. On the second day, mice were intravenously injected with 0.2 mL of Salmonella enteritidis at a concentration of 1.5×108 CFU/mL. The mice were observed for 7 consecutive days, and the death number of the mice was recorded. A total of 75 KM mice were randomly divided into 5 groups: intravenous injection group (iv), intramuscular injection group (im), subcutaneous injection group (ih), oral administration group (po) and Control group (Control). The mice were fed for 3 days and were free to eat and drink. Subsequently, 1x concentration of inactivated E. faecium was given to each administration group according to 0.1mL/10g body weight every day for 5 days, and Control group was given the same amount of normal saline. Carbon clearance index K was measured 2 hours after the last administration. The specific operation of carbon clearance index detection is as follows [18]: The mice were injected with Indian ink 0.05ml /10g through tail vein, and 20μl of blood was collected from orbital vein at 1min (T1) and 5min (T10), respectively. The blood was added to 2mL 0.1% Na2CO3 solution and shake well. The Absorbance of the blood sample was measured by spectrophotometer at 680 nm wavelength and the OD1 and OD5 were used to represent the optical density of blood samples taken in 1min and 5min respectively. Calculate the value of carbon clearance index K according to the following formula.
4.3. Screening of Different Doses of Inactivated E. faecium by Intravenous Injection in Mice
In this study, 8 intravenous administration groups with different concentrations of inactivated E. faecium were set, namely 125× group, 25× group, 5× group, 1× group, 0.2× group, 0.04× group, 0.008× group and Control group. There were 15 KM mice in each group with the body weight of 18-22g per mouse, and half male and half female. The mice were fed for 3 days and were free to eat and drink. Then, each administration group was injected inactivated E. faecium with 0.1ml /10g body weight of through tail vein, while the control group was injected with the same amount of normal saline through tail vein. 24h after administration, 0.2mL of NDV was injected into each mouse in each group through tail vein. After 3 days, blood was collected from the venous plexus of the eyeball of mice, and the antibody titer of NDV was detected by Haemagglutination test (HA) and Hemagglutination Inhibition test (HI) [32]. Then, each administration group was injected inactivated E. faecium with 0.1ml /10g body weight once a day for 5 days, while the control group was injected with the same amount of normal saline. Carbon clearance test was performed 2 hours after the last administration (same method as above).
4.4. Effects of Intravenous Injection of Inactivated E. faecalis on Immune Function in Normal and Immunosuppressed Mice
In this experiment, high dose group (5×), middle dose group (1×), low dose group (0.2×), normal control group (Control) (0.1mL normal saline per 10g body weight) and thymus pentapeptide (TP-5) group (0.02mg/mL) (0.1mL normal saline per 10g body weight) were set up. There were 15 KM mice in each group, 18-22g in body weight, half male and half female. All mice adapted to the environment and were fed for 3 days with free food and water intake. Then, the immunosuppressed model (is Model) was prepared by intraperitoneal injection of dexamethasone (DXM) at 40mg/kg body weight for 5 days [33]. On the day when the is Model groups were prepared, mice in the normal control group (is Nomal) and is Model group were immunized with normal saline by caudal vein injection respectively. The high, middle and low dose is Model groups were injected with 5×, 1× and 0.2× concentration of inactivated E. faecium into the tail vein of is Model mice, respectively.
Carbon clearance index K was measured after 2 hours at the last administration. After administration on the last day, 0.5mg/mL EDU solution was intraperitoneally injected according to 0.1ml /10g body weight of mice, and the control group and is Model group were given the same amount of normal saline. On the next day, the thymus, spleen and mesenteric lymph nodes were collected and fixed in 4% formaldehyde solution for no less than 24h for preparation of paraffin section after neck removal and death of mice. The detailed steps of section production are as follows: washing, dehydration, transparency, wax impregnation, embedding, section and patch, dyeing, section observation with Leica laser confocal microscope and photo analysis. On the 6th day, the mice sacrificed by neck removal and the thymus and spleen separated immediately and washed with PBS, and the weight of thymus and spleen was measured (mg) after absorbing excess water with absorbent paper [34]. Thymus index and spleen index were calculated by the following formula: organ index = (organ weight/mouse body weight).
4.5. Effects of Inactivated E. faecium by Intravenous Injection on the Expression of Spleen Cytokines in Normal and Immunosuppressed Mice
In each group, there were 10 Kunming mice with the body weight of 18-22g, half male and half female. In this experiment, normal control group, immunosuppressive model group and inactivated E. faecium administration dose groups at ×0.2, ×1 and ×5 concentrations were set up. Mice in normal control group and immunosuppressive model group were injected with normal saline through caudal vein. After that, each group was given intravenous injection for 5 days, once a day. Spleens were separated and stored at -80°C after dislocation. The total mRNA was extracted by Trizol, and the expression levels of IL-1β, TNF-α, IFN-γ, IL-10, IL-12a and IL-12b were detected by real-time quantitative PCR. The above gene primers and β-actin internal reference primers were designed and synthesized using Oligo 6.0 software according to GenBank gene sequence, and their primer sequences were as follows: IL-1β(F:5′-TCCAGGATGAGGACATGAGCAC–3′/R:5′-GAACGTCACACACCAGCAGGTTA–3′)(NCBI Reference Sequence: NM_008361.4), TNF-α(F:5′-GCCAGGAGGGAGAACAGAAACTC-3′/R:5′-GGCCAGTGAGTGAAAGGGACA-3′)(NCBI Reference Sequence: NM_013693.3), IFN-γ(F:5′-CGGCACAGTCATTGAAAGCCTA-3′/R:5′- GTTGCTGATGGCCTGATTGTC-3′)(NCBI Reference Sequence: NM_008337.4), IL-10(F:5′-GCCAGAGCCACATGCTCCTA–3′/R:5′- GATAAGGCTTGGCAACCCAAGTAA–3′)(NCBI Reference Sequence: NM_010548.2), IL-12a(F:5′-CCGGTCCAGCATGTGTCAA–3′/R:5′-CAGGTTTCGGGACTGGCTAAGA–3′)(NCBI Reference Sequence: NM_008351.3), IL-12b (F:5′- ACTCACATCTGCTGCTCCACAAG-3′/R:5′-CACGTGAACCGTCCGGAGTA–3′)(NCBI Reference Sequence: NM_001303244.1), β-actin(F:5′- CATCCGTAAAGACCTCTATGCCAAC3′/R:5′-ATGGAGCCACCGATCCACA–3′)(GenBank:BC138614.1). The specific operation of the above cytokine expression level detection was performed in accordance with the instructions of the SYBR Premix Ex Taq PCR Kit. The results were quantitatively analyzed by 2-??Ct method, in which ?Ct=Ct (target gene) -Ct (internal reference gene); ??Ct=?Ct (experimental group) -?Ct (control group); Relative expression rate =2-??Ct.
4.6. Effects of Inactivated E. faecalis by Intravenous Administration on Spleen Cells in Normal and Immunosuppressed Mice
In this experiment, normal control group (PBS), immunosuppression model group (DXMS) and 1× dose group (DXMS+SW-1) were set up. In each group, there were 10 Kunming mice with the body weight of 18-22g, half male and half female. Mice in PBS and DXMS were injected with normal saline through caudal vein. Administration DXMS+SW-1 was given intravenous injection for 5 days, once a day. Two hours after the last administration, the spleens were separated from each group of mice which sacrificed by dislocation, and the number of immune cells in the spleens and the effect of innate immunity were detected by flow cytometry. Splenic cells were isolated as described previously with minor modification [35]. Briefly, Spleen were minced into small pieces and digested in RPMI-1640(Gibco) containing 1 mg collagenase IV (Sigma) for 30 min at 37°C, and passed through a 100-μm cell strainer followed by lysis of erythrocytes. Spleen cells were block with CD16/CD32 (1:200, eBioscience) to eliminate of Fc receptor-mediated antibody binding. Cells were stained with FITC-conjugated anti-mouse CD3e (1:200, Cat#11-0031-85, eBioscience), Alexa Fluor 700-conjugated anti-mouse CD4 (1:400, Cat#56-0041-82, eBioscience) and PE-conjugated anti-mouse CD8a (1:1600, Cat#100708, Biolegend) for gating CD4+ or CD8+ T cells. Cells were incubated for 20 min on ice with antibodies and washed three times with PBS. Flow cytometry data collection was performed on a LSRFortessa (BD Bioscience) and analyzed using FlowJo software (Tree Star Inc.).
4.7. Effects of Inactivated E. faecium Intravenous Injection on Humoral Immune Function in Normal and Immunosuppressed Mice
Clean Kunming mice with body weight of 18-22g were divided into control group (normal mouse NDV challenge group), immunosuppression model group (DXMS) and inactivated E. faecium administration group (SW-1), with 16 mice in each group with half male and half female. The method of preparing immunosuppressed mouse model is the same as above [33]. On the day of the immunosuppression model was prepared, mice in the inactivated E. faecium administration group (SW-1) were injected with 1× concentration of inactivated E. faecium injection through tail vein. The mice in the control group (Control) and the immunosuppressive model group (DXMS) were injected with sterile normal saline through tail vein, with the volume of 0.1mL/10g body weight of mice. At 24 hours after administration, 0.2 mL of NDV with titer of 1:1024 was injected into the tail vein of mice in each group. After that, blood was collected from the venous cluster of eyeballs of mice 3 days later, and the antibody level of NDV was detected by Haemagglutination test (HA) and Hemagglutination Inhibition test (HI) [32]. The results were shown in Table 1 and Table 2.
4.8. Data analysis
SPSS22.0 statistical software was used for significance test. The experimental results were expressed as (±S), t test was used for comparison of the mean values of two samples, and F test was used for analysis of the mean values of multiple samples. P<0.05 was considered statistically significant. Graph Pad Prism 6 software was used for graph analysis and chart drawing.
5. Conclusions
In this study, the inactivated E. faecium with intact form by intravenous administration could significantly increase the thymus index and spleen index of mice, and had obvious promotion effect on the proliferation of mouse thymocytes, spleen cells and mesenteric lymph node cells. The cytokine expression levels, nonspecific immunity and specific immune function of normal, and DXM-immunosuppressed mice were significantly increased by intravenous injection of different doses of inactivated E. faecium. The above studies indicated that the inactivated E. faecium with intact morphology can exert a good immune-enhancing effect by intravenous injection in mice, and has a potential broad application prospect in human medicine and veterinary clinic.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher. No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Author Contributions
Y.S. and J.L. participated in the study design. L.Y., W.Z. and X.W. performed the experiments. L.Y. drafted the manuscript. K.Z. and Y.Y. collected the important background information. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fund of “Tai 'an Special Project for Prevention and Control of Pneumonia Epidemic in 2020” (2020FYZX05), the Fund of “School-enterprise cooperation projects” (040-380502), and the Funds of Shandong “Double Tops” Program (SYL2017YSTD11), the Fund of “Natural Science Foundation of Shandong Province” (ZR2021MC161).
Institutional Review Board Statement
The study protocol and the poultry studies were approved by the Animal Care and Use Committee of Shandong Agricultural University, Tai’an, China (Ethical Approval Number SDAUA-2018-027).
Informed Consent Statement
Not applicable.
Conflicts of Interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
References
- Xia K, Chen ZQ, Wang L. Feeding effects of Enterococcus faeciumis and its mechanism of action. Cereal & Feed Industry 5 (2016): 44-47.
- Ma FY, Jing YC, Cui X, et al. Research Progress on Enterococcus faecalis and Its Microecological Preparations. Chinese Journal of Animal Science 7 (2019): 54-57.
- Pollmann M, Nordhoff M, Pospischil A, et al. Effects of a probiotic strain of Enterococcus faecium on the rate of natural chlamydia infection in swine. Immun 73 (2005): 4346-4353.
- Kreuzer S, Machnowska P, Aßmus, et al. Feeding of the probiotic bacterium Enterococcus faecium NCIMB 10415 differentially affects shedding of enteric viruses in pigs. Vet Res (2012): 43:58.
- Tian ZY, Yang L, Li PH, et al. The inflammation regulation effects of Enterococcus faeciumHDRsEf1 on human enterocyte-like HT-29 cells. Animal Cells and Systems 2 (2016): 70-76.
- Jimenez E. Fernández L, Marin ML, et al. Isolation of Commensal Bacteria from Umbilical Cord Blood of Healthy Neonates Born by Cesarean Section. Curr Microbiol 51(2005): 270-274.
- Luo YW, Zhou YM, Wang T. Effects of feed processing technology on probiotics activity. Feed Review 3 (2006): 19-20.
- Li X, Li J, Zhang DX, et al. Effects of feed processing and storage process on probiotic activity. China Feed 7 (2011): 37-40.
- Guo GP. Regulation of tight junction proteins, cytokines and Related Toll-like receptors in small intestinal mucosa of piglets by active Enterococcus faecium and heat-inactivated Enterococcus faecium. Nanning: Guangxi University (2017).
- Huang Y, Huang Q, Cui ZW, et al. Roles of Enterococcus faecium composition of the intestinal microflora and chemical barrier in newborn piglets by oral administration. J Vet Sci 32 (2012): 1007-1010.
- Feng YY, Qiao L, Yao HM, et al. Safety Evaluation of Enterococcus faecium WEI-10 on Weaned Piglets. Chinese Journal of Animal Nutrition 29 (2017): 4588-4596.
- Hang LQ, Luo LP, Zhang YR, et al. Effects of Dietary Enterococcus faecium NCIMB11181 on Growth Performance,Intestinal Microflora and Serum Antioxidant Capacity in Broilers Challenged with Escherichia coli China Poultry 39 (2017): 17-22.
- Yi X, Zhou HL, Chao Y, et al. Bacteria-triggered tumor-specific thrombosis to enable potent photothermal immunotherapy of cancer. Sci Adv 14 (2020): e3546.
- Levkut M, Revajova V, Laukova A, et al. Leukocytic responses and intestinal mucin dynamics of broilers protected with Enterococcus faecium EF55 and challenged with Salmonella Enteritidis. Res Vet Sci 93 (2012): 195-201.
- Szabo I, Wieler LH, Tedin K, et al. Influence of a Probiotic Strain of Enterococcus faecium on Salmonella enterica Serovar Typhimurium DT104 Infection in a Porcine Animal Infection Model. Environ. Microbiol 75 (2009): 2621-2628.
- Chen MF, Weng KF, Huang SY, et al. Pretreatment with a heat-killed probiotic modulates monocyte chemoattractant protein-1 and reduces the pathogenicity of influenza and enterovirus 71 infections. Mucosal Immunol 10 (2017 ): 215-227.
- Yu HF, Zhou M, Lv XB, et al. Effects of synthetic and natural flavorins on carbon clearance rate in normal mice. Chinese journal of ethnomedicine and ethnopharmacy 21 (2012): 62-62.
- Zhao R, Gao X, Shao XY. Study on immune regulation of Portulaca oleracea polysaccharide in tumor-bearing mice. Heilongjiang Animal Science and Veterinary Medicine 11 (2014):157-160.
- Zeng XC, Liu JB, Jiang Y. Effect of Lactobacillus Fermentated juice from Tomato and Carrot on mice immune function. J Prev Med Chin PLA 23 (2005): 248-251.
- Jiang YM, Wang JQ, Deng LF, et al. Effect of artificial rumen evaluating Enterococcus faecium on ruminal fermentation. Nat Sci Ed 36 (2008): 29-33.
- Li L, Cai L, Yu BD, et al. Immunomodulatory effects of lactobacillus on splenocytes of dust mite-sensitized murine model. Immunol 3 (2011): 193-198.
- Patricia AD, Joseph JZ, Pauline M, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577 (2020): 95-102.
- Wang J. Progress of Physiological Function of Lactic Acid bacteria. Sichuan food and Fermentation 41 (2005): 43-46.
- Wen J, Sun JA, Zhou XX, et al. Effects of Enterococcus faecium on growth performance,immune and antioxidant function of Piglets. Acta Agriculturae Zhejiangensis 23(2011): 70-73.
- Chen LY, Mikael J, Konstantin Y. Regulatory effects of dexamethasone on NK and T cell immunity. Inflammopharmacology 26 (2018): 331-1338.
- Shi YH, Shi GC, Wan HY, et al. An increased ratio of Th2 Treg cells in patients with moderate to severe asthma. Chin Med J 126 (2013): 2248-2253.
- Naomi T, Hiroshi S, Chiyako O, et al. Decreases in the numbers of peripheral blood regulatory T cells and increases in the levels of memory and activated B cells in patients with active eosinophilic granulomatosis and polyangiitis. J Clin Immunol 33 (2013): 965-976.
- Marta B, Rieardo CF, Lara L, et al. Low frequency of CD4+CD25+ Treg in SLE patients a heritable trait associated with CTLA4 and TGFβ gene variants. Immunol 10 (2009): 1-14.
- Yamamoto T, Yanagimoto H, Satoi S, et al. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas 41 (2012): 409-415.
- Amit A, Yijunm C, Jean PSP, et al. A dominant function for interleukin-27 in generating interleukin 10-producing antiinflammatory T cells. Nat Immunol 8 (2007): 1380-1389.
- Xu YQ, Gao YD,Yang J, et al. A defect of CD4+CD25+ regulatory T cells in inducing interleukin-10 production from CD4+ T cells under CD46 costimulation in asthma patients. J Asthma 47 (2010): 367-373.
- Alexander DJ, Chettle NJ. Procedures for the haemagglutination and the haemagglutination inhibition tests for avian infectious bronchitis virus. Avian Pathology 6 (1977): 9-17.
- Li XL, Fing Y. Effects of Dexamethasone on Rats Macrophage Phagocytic Functions. International Medicine & Health Guidance News 20 (2005): 6-7.
- Li J, Cheng YN, Zhang XK, et al. The in vivo immunomodulatory and synergistic anti-tumor activity of thymosin α1-thymopentin fusion peptide and its binding to TLR2. Lett 337 (2013): 237-247.
- Guilliams M, Dutertre CA, Scott CL, et al. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity 20 (2016): 669-684.
Supplementary Files

Supplementary Figure 1: Effects of mouse thymocyte proliferation.
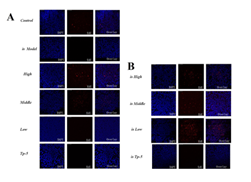
Supplementary Figure 2: Effects of mouse spleen proliferation.
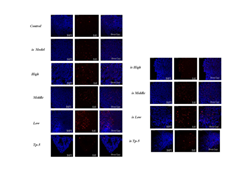
Supplementary Figure S3: Effects of mouse mesenteric lymph nodes proliferation
