Selection of an Attenuated Rifampicin Resistant Mutant of Streptococcus Iniae and Evaluation of its Immunoprotective Effect as Naturally Delivered Vaccines
Article Information
Guodong Luo1,2#, AnyuanZhang3,4#, Ling Chen3,4, Hui Wang1, Lanping Yu1*
1Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Shandong Agricultural University, 61 Daizong Street, Taian City, Shandong Province 271018, PR China
2Shandong Guoliqing Biotechnology Co., LTD., Yudong Village, Daolang Town, Daiyue District, Taian City, 271022, PR China
3Institute of verterrinary drug qulity inspection of Shandong province, jinan, 250022, PR China
4shangdong provincial key laboratory of quality safety monitoring and risk assessment for animal products, jinan, 250022, PR China
*Corresponding author: Lanping Yu, Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Shandong Agricultural University, 61 Daizong Street, Taian City, Shandong Province 271018, PR China.
Received: 14 January 2023; Accepted: 19 January 2023; Published: 25 January 2023
Citation: Guodong Luo, AnyuanZhang, Ling Chen, Hui Wang, Lanping Yu. Selection of an Attenuated Rifampicin Resistant Mutant of Streptococcus Iniae and Evaluation of its Immunoprotective Effect as Naturally Delivered Vaccines. Journal of Biotechnology and Biomedicine 6 (2023): 24-31.
View / Download Pdf Share at FacebookAbstract
Streptococcus iniae is a Gram-positive bacterium and a significant pathogen to a wide range of farmed fish. In the present study, we obtained a SF1 derivative, SF1M1, by selecting rifampicin resistance mutants. Compared to the wild type, SF1M1 (i) was slow in growth, (ii) showed less extracellular protease activity and produced a much lower amount of siderophores, and (iii) had a median lethal dose that is more than 100 times higher. In vitro and in vivo studies showed that the attenuated virulence of SF1M1 was stably maintained in the absence of selective pressure. To examine the potential of SF1M1 as a naturally delivered vaccine, Japanese flounder (Paralichthys olivaceus) were vaccinated with SF1M1 via bath immersion and oral feeding. At one and two months post-vaccination, the fish were challenged with SF1 or a heterogeneous strain S. iniae 29177, which is of serotype II. The results showed that fish vaccinated with SF1M1 exhibited relative percent survival rates of 54%-70% and produced specific serum antibodies that enhanced complement-mediated bactericidal activity against both SF1 and S. iniae 29177. Taken together, these results indicate that SF1M1 is an effective oral and immersion vaccine that induces protective immunity against serotype I and serotype II S. iniae.
Keywords
Streptococcus iniae; Attenuated vaccine; Rifampicin resistance; Vaccine delivery; Japanese flounder
Streptococcus iniae articles Streptococcus iniae Research articles Streptococcus iniae review articles Streptococcus iniae PubMed articles Streptococcus iniae PubMed Central articles Streptococcus iniae 2023 articles Streptococcus iniae 2024 articles Streptococcus iniae Scopus articles Streptococcus iniae impact factor journals Streptococcus iniae Scopus journals Streptococcus iniae PubMed journals Streptococcus iniae medical journals Streptococcus iniae free journals Streptococcus iniae best journals Streptococcus iniae top journals Streptococcus iniae free medical journals Streptococcus iniae famous journals Streptococcus iniae Google Scholar indexed journals Attenuated vaccine articles Attenuated vaccine Research articles Attenuated vaccine review articles Attenuated vaccine PubMed articles Attenuated vaccine PubMed Central articles Attenuated vaccine 2023 articles Attenuated vaccine 2024 articles Attenuated vaccine Scopus articles Attenuated vaccine impact factor journals Attenuated vaccine Scopus journals Attenuated vaccine PubMed journals Attenuated vaccine medical journals Attenuated vaccine free journals Attenuated vaccine best journals Attenuated vaccine top journals Attenuated vaccine free medical journals Attenuated vaccine famous journals Attenuated vaccine Google Scholar indexed journals Rifampicin resistance articles Rifampicin resistance Research articles Rifampicin resistance review articles Rifampicin resistance PubMed articles Rifampicin resistance PubMed Central articles Rifampicin resistance 2023 articles Rifampicin resistance 2024 articles Rifampicin resistance Scopus articles Rifampicin resistance impact factor journals Rifampicin resistance Scopus journals Rifampicin resistance PubMed journals Rifampicin resistance medical journals Rifampicin resistance free journals Rifampicin resistance best journals Rifampicin resistance top journals Rifampicin resistance free medical journals Rifampicin resistance famous journals Rifampicin resistance Google Scholar indexed journals Vaccine delivery articles Vaccine delivery Research articles Vaccine delivery review articles Vaccine delivery PubMed articles Vaccine delivery PubMed Central articles Vaccine delivery 2023 articles Vaccine delivery 2024 articles Vaccine delivery Scopus articles Vaccine delivery impact factor journals Vaccine delivery Scopus journals Vaccine delivery PubMed journals Vaccine delivery medical journals Vaccine delivery free journals Vaccine delivery best journals Vaccine delivery top journals Vaccine delivery free medical journals Vaccine delivery famous journals Vaccine delivery Google Scholar indexed journals Japanese flounder articles Japanese flounder Research articles Japanese flounder review articles Japanese flounder PubMed articles Japanese flounder PubMed Central articles Japanese flounder 2023 articles Japanese flounder 2024 articles Japanese flounder Scopus articles Japanese flounder impact factor journals Japanese flounder Scopus journals Japanese flounder PubMed journals Japanese flounder medical journals Japanese flounder free journals Japanese flounder best journals Japanese flounder top journals Japanese flounder free medical journals Japanese flounder famous journals Japanese flounder Google Scholar indexed journals bacterium articles bacterium Research articles bacterium review articles bacterium PubMed articles bacterium PubMed Central articles bacterium 2023 articles bacterium 2024 articles bacterium Scopus articles bacterium impact factor journals bacterium Scopus journals bacterium PubMed journals bacterium medical journals bacterium free journals bacterium best journals bacterium top journals bacterium free medical journals bacterium famous journals bacterium Google Scholar indexed journals pathogen articles pathogen Research articles pathogen review articles pathogen PubMed articles pathogen PubMed Central articles pathogen 2023 articles pathogen 2024 articles pathogen Scopus articles pathogen impact factor journals pathogen Scopus journals pathogen PubMed journals pathogen medical journals pathogen free journals pathogen best journals pathogen top journals pathogen free medical journals pathogen famous journals pathogen Google Scholar indexed journals cellulitis articles cellulitis Research articles cellulitis review articles cellulitis PubMed articles cellulitis PubMed Central articles cellulitis 2023 articles cellulitis 2024 articles cellulitis Scopus articles cellulitis impact factor journals cellulitis Scopus journals cellulitis PubMed journals cellulitis medical journals cellulitis free journals cellulitis best journals cellulitis top journals cellulitis free medical journals cellulitis famous journals cellulitis Google Scholar indexed journals endocarditis articles endocarditis Research articles endocarditis review articles endocarditis PubMed articles endocarditis PubMed Central articles endocarditis 2023 articles endocarditis 2024 articles endocarditis Scopus articles endocarditis impact factor journals endocarditis Scopus journals endocarditis PubMed journals endocarditis medical journals endocarditis free journals endocarditis best journals endocarditis top journals endocarditis free medical journals endocarditis famous journals endocarditis Google Scholar indexed journals meningitis articles meningitis Research articles meningitis review articles meningitis PubMed articles meningitis PubMed Central articles meningitis 2023 articles meningitis 2024 articles meningitis Scopus articles meningitis impact factor journals meningitis Scopus journals meningitis PubMed journals meningitis medical journals meningitis free journals meningitis best journals meningitis top journals meningitis free medical journals meningitis famous journals meningitis Google Scholar indexed journals
Article Details
Abbreviations:
S. iniae, Streptococcus iniae
Introduction
Streptococcus iniae (S. iniae) is a significant pathogen (a Gram-positive bacterium) to a wide range of farmed fish including rainbow trout [1]; channel catfish [2], European sea bass[3], rabbitfish [4], Adriatic Sturgeon (Acipenser naccarii) [5]. In addition, S. iniae can also infect humans and cause meningitis, cellulitis, and endocarditis [6]. As a fish pathogen, S. iniae is the etiological agent of streptococcosis, a systemic septicaemia that is known to affect both marine and freshwater fish [7, 8]. Based on their ability to react with Arginine Dihydrolase (ADH) and ribose, two distinct serotypes of S. iniae have been recognized, with serotype I isolates positive for ADH and ribose utilization, while serotype II isolates lacking arginine dihydrolase activity and unable to utilize ribose [9]. Both serotypes are known to cause disease outbreaks. Fish vaccines against S. iniae have been reported by a number of research groups; most of these vaccines are tested as injection vaccines [10-13]. Since injection is a costly process, the aquaculture application of injection vaccines is much limited in countries where economy is an important factor in disease control. Compared to injection, natural administration methods such as oral feeding and bath immersion are less expensive and cause much less stress to the animal [14-17]. For this reason, naturally delivered vaccines are more welcome to the fish farming industry. In China, outbreaks due to S. iniae infection has been reported in farmed fish such as Japanese flounder, turbot, tilapia, and channel catfish [18-20]. The aim of this study was to develop a S. iniae vaccine that could induce effective protection when administered in a non-injection fashion. In a previous study, we have reported the isolation and characterization of a virulent S. iniae isolate, SF1, that had caused an epidemic in farmed flounder [19]. In the present study, we selected a SF1 derivative, SF1M1, that exhibited attenuated virulence. We examined in a flounder model the potential of SF1M1 as a live vaccine delivered via immersion and oral routes. We found that SF1M1 induced protective immunity not only against SF1, a serotype I strain, but also against a heterogeneous serotype II strain.
Materials and Methods
Selection of SF1M1
iniae SF1 (serotype I) is a pathogenic strain that had caused an epidemic in farmed flounder [19]. S. iniae 29177 (serotype II) was purchased from ATCC (American Type Culture Collection). S. iniae SF1 was cultured in Luria-Bertani broth (LB) medium at 28°C. S. iniae 29177 was cultured in Todd-Hewitt broth (THB) at 28°C. SF1 shows its natural resistance against ampicillin but sensitive to chloramphenicol (Chl), tetracycline (Tet), and rifampicin (Rif). SF1 was analyzed for potential existence of plasmid using the procedure of Kado and Liu (1981) [21], and the results were negative. SF1M1 was obtained as described previously [22]. Briefly, SF1 was cultured in LB medium to an OD600 of 0.9, and 100 ml of the culture was plated on a LB agar plate containing 1 mg/ml rifampicin (Sangon, Shanghai, China). The plate was incubated at 28°C for 5 days. One of the colonies that had emerged on the plate were randomly selected from the plate and cultured in LB medium containing 2 mg/ml rifampicin to an OD600 of 0.8. One hundred microliters of the culture was removed and sub-cultured in LB medium containing 4 mg/ml rifampicin. The passage was repeated in LB medium containing gradually increased concentrations of rifampicin until the latter reaching 200 mg/ml. One of the colonies that grew on the LB agar plate containing 200 mg/ml rifampicin was randomly selected and named SF1M1. The genetic identity of SF1M1 was verified by sequence analysis of the 16S rRNA gene and the SF1M1-specific genes sip11 and sia10 as described previously [19, 12].
Protease and siderophore production
Protease activity was determined with a modified LB medium prepared by mixing peptone, yeast extract, ferric phosphate, gelatin, and agar in one liter aged seawater. The pH of the medium was adjusted to 7.6. The medium was autoclaved at 112.6°C for 30 min and poured into Petri dishes. SF1 and SF1M1 were cultured in standard LB medium to an OD600 of 1. The cells were washed with PBS and resuspended in PBS to 1 × 108 CFU/ml. Ten microliters of the cell suspension was added into an Oxford cup placed on an agar plate prepared above. The plate was incubated at 28°C for 36 h, and 25% trichloroacetic acid was added to the plate to induce color formation. Ten microliters of the Siderophore production was determined with the Chrome Azurol S (CAS) agar method [23] by adding 50 μl of the above bacterial cell suspension into an Oxford cup placed on a CAS agar plate, followed by incubating the plate at 28°C for 5 days.
Fish
Japanese flounder (Paralichthys olivaceus) (average 12.6±0.6 g) were purchased from local fish farms and acclimatized in the laboratory for two weeks before experimental manipulation. Fish were fed daily with commercial dry pellets and maintained at~22°C in sand-filtered and activated carbon-absorbed seawater that was changed twice daily. Before experiment, fish (5%) were randomly sampled for the examination of bacterial recovery from blood, liver, kidney, and spleen, and no bacteria could be detected from any of the examined tissues of the sampled fish. In addition, ELISA analysis showed that the randomly selected fish (5%) from the batch were negative of serum antibodies against S. iniae. For tissue examinations, fish were sacrificed with an overdose of tricaine methanesulfonate (Sigma, St. Louis, MO, USA) as described previously [24].
Virulence and stability analysis of SF1M1
The median lethal dose (LD50) was determined as described previously [24]. To examine the stability of SF1M1 under in vitro conditions, the bacterium was cultured in LB medium to an OD600 of 0.8 and diluted in fresh LB to 105 CFU/ml. The diluted culture was incubated at 28°C to OD600 0.8; one portion of the cell culture were used for LD50 examination, while another portion of the cell culture was diluted in fresh LB medium to 105 CFU/ml and incubated at 28°C to OD600 0.8 as described above. The subculture was repeated ten times. To examine the stability of SF1M1 under in vivo conditions, the bacterium was cultured in LB medium to an OD600 of 0.8, and the cells were washed with PBS and resuspended in PBS to 2 × 108 CFU/ml. One hundred microliters of the cell suspension was administered via intraperitoneal (i.p.) injection into flounder, and the fish were monitored for the appearance of disease symptom. SF1M1 was recovered from the liver, spleen, kidney, and blood of diseased fish as described above. The recovered SF1M1 from different organs were mixed together and used for next round infection and for LD50 determination. This process was repeated ten times.
Vaccination-(i) preparation of vaccine-containing microspheres
For oral vaccination, SF1M1 was encapsulated into alginate microspheres as follows. The bacterium was cultured in LB medium at 28°C to OD600 of 1. The cells were washed with and resuspended in PBS. Twenty milliliters of 3% (m/v) sodium alginate was mixed with 12 ml of bacterial suspension or PBS (control), and the mixture was emulsified by adding 800 ml of paraffin and 4 ml of Span-80. While stirring, twenty milliliters of 0.15 M CaCl2 was added to the emulsion, and microspheres were collected by centrifugation at 1000 × g for 10 min. One hundred grams of marine fish feed (purchased from Shandong Sheng-suo Fish Feed Research Center, Shandong, China) were mixed with vaccine-containing microspheres or the control microspheres. The feed was cut into small pieces of the size approximating that of the purchased fish feed. After drying at 30°C, the feed was stored at 4°C and used within 3 days.
Vaccination-(ii) Vaccination
Preliminary studies indicated that when flounder were fed for six days with feed containing SF1M1 microspheres to the amount that the daily bacterial consumption was approximately 2 × 108 CFU/fish followed by immersion for 6 h in seawater containing 2 × 108 CFU/ml SF1M1, no disease symptom or mortality was observed in the fish for a period of 3 weeks during which time the fish were monitored on a daily basis. Based on these results, the vaccination trials were conducted as follows. Flounder described above were randomly divided into two groups (N = 200) named A and B. Group A was fed for three days with feed containing SF1M1 microspheres to the amount that the daily bacterial consumption was approximately 108 CFU/fish; group B was similarly fed with feed containing control microspheres. The fish were then subjected to immersion vaccination as follows. SF1M1 was cultured in LB medium as described above and resuspended in seawater to 108 CFU/ml. Group A was immersed in SF1M1-containing seawater for 6 h, while group B was similarly treated in PBS-containing seawater. The fish were then moved to tanks containing fresh seawater and reared under normal conditions as described above. At 3-week post-vaccination, 60 fish were taken from groups A and B respectively and placed into separate tanks named groups A2 and B2. The fish in groups A2 and B2 were boosted with a second immersion vaccination as described above. At one month after the initial vaccination, 24 fish were taken from groups A and B respectively and challenged via i.p. injection with SF1; likewise, 24 fish were taken from groups A and B and challenged similarly with S. iniae 29177. At two months post-vaccination, 24 fish were taken from groups A2 and B2 respectively and challenged with SF1 as above; similarly, 24 fish were taken from groups A2 and B2 and challenged with S. iniae 29177 as above. After each challenge, the fish were monitored for mortality for 20 days, and dying fish were randomly selected for the examination of bacterial recovery from liver, kidney, and spleen as described previously [25]. Relative percent of survival (RPS) was calculated according to the following formula: RPS = {1 – (% mortality in vaccinated fish/% mortality in control fish)} × 100 (Amend 1981). All vaccination trials were conducted in duplicate.
Tissue invasion of SF1M1 in vaccinated fish
Liver, spleen, kidney, and gut were taken from SF1M1- and PBS-vaccinated fish at 1 d, 2 d, 3 d, 4 d, 6 d, 8 d, 10 d, 12 d, 14 d, 16 d, 18 d, and 20 d after vaccination (five fish per time point). Bacterial invasion into tissues was examined by determining bacterial recovery from each of the tissues as described above.
Enzyme-linked immunosorbent assay (ELISA) and serum bactericidal activity
Sera were collected from unvaccinated and vaccinated fish (five at each time point) at one and two months post-vaccination. Sera were diluted 64-fold in PBST (0.1% Tween-20 in PBS) containing 1% bovine serum albumin. Serum antibodies against SF1M1 were determined by ELISA as described previously [26]. Serum bactericidal assay was performed as follows. SF1 and S. iniae 29177 were cultured to mid-logarithmic phase and resuspended in PBS to 1 × 106 CFU/ml. Ten microliters of bacterial suspension or PBS was mixed with or without 50 ml untreated serum or heat-treated (55°C for 30 min) serum in a total volume of 110 ml. The mixture was incubated at 30°C for 1 h and, after diluting in LB, plated in LB agar plates. The plates were incubated at 30°C for 48 h, and the colonies that appeared on the plates were counted. The genetic nature of the colonies was verified as described above.
Statistical analysis
All statistical analyses were performed with analysis of variance (ANOVA) of the SPSS 17.0 package (SPSS Inc., Chicago, IL, USA). In all cases, significance was defined as P < 0.05.
Results
Selection and characterization of SF1M1
SF1M1 was obtained by repeated selection of SF1, which is naturally sensitive to rifampicin, in medium supplemented with increased concentration of rifampicin. LD50 analysis showed that when injected i.p. into flounder, the LD50 of SF1M1 was greater than 109 CFU, at which dose no mortality occurred, while the LD50 of SF1 was 106.9 CFU. When grown in LB medium, SF1M1 exhibited slower growth rates at the logarithmic and stationary phases than the wild type strain SF1 (Figure 1). Compared to SF1, SF1M1 showed less extracellular protease activity and produced a much lower amount of siderophores (Figure 2).
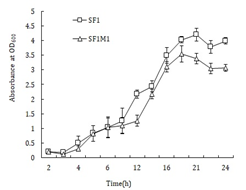
Figure 1: Growth profile of SF1M1. SF1M1 and SF1 were cultured in LB medium, and aliquots of the culture were taken at different time points for measurement of absorbance at OD600. Values are presented as means ± SE (N = 3).
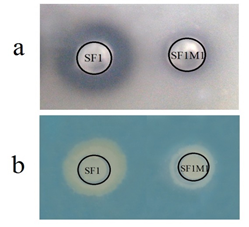
Figure 2: Analysis of the extracellular protease activity (A) and siderophore production (B) of SF1 and SF1M1. a, SF1 and SF1M1 were added into Oxford cups (indicated by black circles) placed on a LB agar plate containing gelatin, and 25% trichloroacetic acid was added to the plate after 36 h incubation. b, SF1 and SF1M1 were added into Oxford cups (indicated by black circles) placed on a chrome azurol S agar plate, and the plate was incubated at 28°C for 5 days. For both panels, halos around the bacterial spots indicate positive results.
Virulence stability of SF1M1
The ability of SF1M1 to revert to wild type was examined under in vivo and in vitro conditions. For in vitro conditions, SF1M1 was sub-cultured ten times in LB medium in the absence of the selective antibiotics rifampicin, and virulence analysis showed that the LD50 of the sub-cultures were comparable to that of SF1M1. For in vivo analysis, SF1M1 was passed ten rounds in flounder, and the LD50 of the SF1M1 recovered from fish tissues was examined. The results showed that recovered SF1M1 exhibited no significant changes in LD50.
Potential of SF1M1 as a naturally delivered vaccine
- 1 Dissemination of SF1M1 in fish tissues following vaccination
To examine the potential of SF1M1 as a naturally delivered vaccine, flounder were immunized with SF1M1 or PBS via bath immersion and oral feeding. To examine whether SF1M1 was able to invade into fish tissues following vaccination, bacterial recovery from fish tissues was examined at different time points after vaccination. The results showed that viable SF1M1 was recovered from the gut, liver, spleen, and kidney at 1 to 12 days post-vaccination (Figure 3). In all examined tissues, the recoveries peaked at 3 or 4 days post-vaccination and then declined until 10 to 12 days. In contrast, no SF1M1 was detected in the tissues of PBS-vaccinated fish.
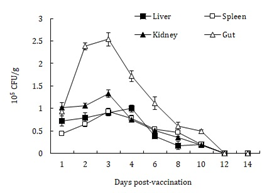
Figure 3: Invasion of SF1M1 into fish tissues following vaccination. Flounder were vaccinated via immersion and oral feeding with SF1M1. Liver, spleen, kidney, and gut were taken at different time points and determined for bacterial recovery. Data are presented as means±SE (N = 5).
- 2 Protection induced by SF1M1
Fish were challenged at one or two months post-vaccination with SF1 or the serotype II strain S. iniae 29177. Subsequent mortality monitoring indicated that following SF1 challenge at one and two-month post-vaccination, the accumulated mortality rates of SF1M1-vaccinated fish were 21% and 29% respectively, while the accumulated mortality rates of the control fish mock-vaccinated with PBS were 71% and 79% respectively. Hence, the protection rates, in terms of RPS, of SF1M1 were 70% and 63% at one and two-month post-vaccination respectively. Following S. iniae 29177 challenge at one and two-month post-vaccination, the accumulated mortality rates of SF1M1-vaccinated fish were 25% and 29% respectively, while the accumulated mortality rates of the control fish were 63%, which correspond to RPS rates of 60% and 54% in SF1M1-vaccinated fish at one and two-month post-vaccination respectively. Comparable protection rates were obtained in duplicate vaccination trials. Microbiological analysis indicated that SF1 and S. iniae 29177 were recovered from the kidney, liver, and spleen of moribund fish challenged with SF1 and S. iniae 29177 respectively, suggesting that mortality was caused by infection of the respective challenging agents.
3 Serum antibody production and bactericidal activity
ELISA analysis showed that serum antibodies against SF1M1 were detected in SF1M1-vaccinated fish at one and two months post-vaccination (Figure 4). To examine the effect of serum bactericidal activity, serum from vaccinated and unvaccinated fish were incubated with SF1 and S. iniae 29177, and bacterial survival rates were subsequently determined. The results showed that compared to the survival rates of SF1 and S. iniae 29177 in the serum of unvaccinated fish, the survival rates of SF1 and S. iniae 29177 in the serum of SF1M1-vacinated fish were reduced to 51% and 64% respectively of those in the control fish (Figure 5). When the sera were heated to inactivate complements before incubation with bacteria, the survival rates of SF1 and S. iniae 29177 in the serum of SF1M1-vacinated fish were comparable to those in the serum of control fish.
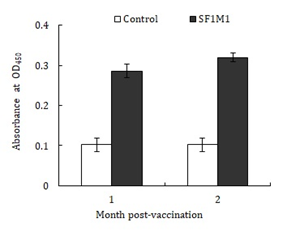
Figure 4: Serum antibody production in SF1M1-vaccinated fish. Sera were collected from the vaccinated and unvaccinated (control) flounder at one and two months post-vaccination, and serum antibodies against SF1M1 were determined by ELISA. Data are presented as means±SE (N = 5). **P < 0.01.
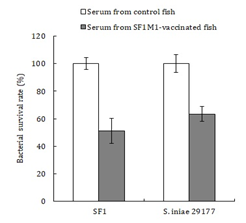
Figure 5: Serum bactericidal activity. Serum was collected from flounder vaccinated with or without (control) SF1M1, and bactericidal activity against SF1 and S. iniae 29177 was determined. The bactericidal activity of the control serum was set as 100%. Data are presented as means±SE (N = 5). **P < 0.01, *P < 0.05.
Discussion
Rifampicin is an antibiotic that blocks RNA synthesis in bacteria by inhibiting DNA-dependent RNA polymerase. Many reports have indicated that bacterial strains that undergo from a rifampicin-sensitive phenotype to a rifampicin-resistant phenotype are often reduced in pathogenicity [27]. This observation has been exploited for the selection of mutant strains with attenuated virulence [28,29,30]. In our study, we found that the rifampicin-resistant mutant SF1M1 was highly reduced in overall bacterial virulence, since the LD50 of SF1M1 was more than 100 times higher than that of the wild type SF1. Compared to SF1, SF1M1 was slower in proliferation rate and produced much less proteases and siderophores. These results suggest that the mutations that contribute to rifampicin resistance had a broad effect on the biological processes of SF1. Compared to killed vaccines and subunit vaccines, live attenuated vaccines are associated with one main disadvantage, which is the possibility of the mutants to revert back to wild type phenotype. For this reason, the vaccinate candidates have to be tested for safety. In the case of SF1M1, we found that after passing ten rounds in LB medium without rifampicin or in the host animal, SF1M1 exhibited no significant changes in LD50. These results indicate that SF1M1 was stable as far as virulence is concerned under both in vitro and in vivo conditions, which suggests a potential for SF1M1 to be used as a vaccine. Yu Liu et al (2020) [31] prepared YM011 GX005 from the parent virulent strain of S. iniae for 800 generations in vitro, and found that the strain YM011 was an ideal oral attenuated vaccine with good immunogenicity, safety and stability.
Of the live attenuated S. iniae vaccines that have been reported, only two were tested as a non-injection vaccine [10]. It was found that in a hybrid striped bass model, a S. iniae mutant defective in the M-like protein gene produced complete protection when used as an immersion vaccine [10]. The S. iniae mutant strain TBY-1ΔsrtA provided the relative percent survival value of 95.5% as an effective attenuated live vaccine candidate in Nile tilapia [32]. Yu Liu et al. (2020)[31] showed that the weak strain YM011 which after 100 successive generations with completely lost pathogenicity to tilapia, and the relative survival rates of the Oral gavage group was 76.81% and 56.69% at 15 d and 30 d, respectively. Mohammad Hayat et al.(2021)[33] found that the red hybrid tilapia were fed with oral S. iniae vaccine inactivated with different concentrations of formalin, and all fishes were experimentally infected 4 weeks after inoculation. The mortality rate in different immunized groups was 30-80% compared to 100% in the control group. In our study, we found that when delivered via oral and immersion routes, SF1M1 induced RPS rates of 70% and 63% at one and two-month post-vaccination respectively against the homologous strain SF1. For the heterologous strain S. iniae 29177, which is of a different serotype from SF1, SF1M1 induced RPS rates of 60% and 54% at one and two-month post-vaccination respectively. These results indicate that SF1M1 as an oral and immersion vaccine can confer effective protection upon flounder against two different serotypes of S. iniae. Consistent with these results, bacterial recovery analysis showed that following vaccination, SF1M1 was detected in gut, liver, spleen, and kidney from 1 to 12 days post-vaccination. These results suggest that SF1M1, though highly attenuated compared to SF1, still retains residual infectivity that enables the bacterium to disseminate into host tissues, which probably accounts for the immunoprotection induced by SF1M1 in the vaccinated fish. Mohammad Hayat et al. (2021)[33] found that the level of anti- S. iniae antibody IgM in all immune groups was significantly increased compared with the control group (p > 0.05), and all the control groups died after 5 weeks of infection. In our study, the examination of serum antibody production indicated that antibodies against SF1M1 were produced in fish vaccinated with SF1M1 at one and two months post-vaccination, suggesting that SF1M1 induced B cell-mediated humoral immune response. Serum bactericidal activity analysis showed that following treatment with the sera from SF1M1-vaccinated fish, the survival rates of SF1 and S. iniae 29177 were significantly reduced, which is consistent with the cross-serotype protective effect of SF1M1. Since the survival rates of both serotypes of S. iniae in heat-inactivated sera from SF1M1-vaccinated fish were comparable to those in heat-inactivated control sera, it is likely that the enhanced bacterium-killing activity of the serum from SF1M1-vaccinated fish was complement-dependent.
Conclusions
In conclusion, in this study we selected a rifampicin-resistant mutant S. iniae, SF1M1 that exhibits attenuated pathogenicity. When used as a live vaccine delivered via immersion and oral feeding, SF1M1 is able to elicit protective immunity in flounder at one and two months post-vaccination against both serotype I and serotype II S. iniae. These results suggest a potential application for SF1M1 in fish farming industries as a naturally delivered vaccine against streptococcosis.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
L.Y. participated in the study design. L.Y. and G.L. performed the experiments. L.Y. drafted the manuscript. A.Z., L.C., and H.W. collected the important background information. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants from the post-doctoral innovation projects of Shandong Province (201303053), Agricultural Improved Seed Project of Shandong Province, No. 2019LZGC014 and the Funds of Shandong “Double Tops” Program (SYL2017YSTD11).
Institutional Review Board Statement
The study protocol and the animal ethics were approved by the Animal Care and Use Committee of Shandong Agricultural University, Tai’an, China (Ethical Approval Number SDAUA-2019-058).
Informed Consent Statement
Not applicable.
Conflicts of Interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
References
- Ghasem Rashidian, Heba H Mahboub, Azin Fahim, et al. Mooseer (Allium hirtifolium) boosts growth, general health status, and resistance of rainbow trout (Oncorhynchus mykiss) against Streptococcus iniae Fish and Shellfish Immunology 120 (2022): 360-368.
- Shoemaker CA, Klesius PH, Evans JJ. Prevalence of Streptococcus iniae in tilapia, hybrid striped bass, and channel catfish on commercial fish farms in the United States. Am J Vet Res 62 (2001): 174-177.
- Zlotkin A, Hershko H, Eldar A. Possible transmission of Streptococcus iniae from wild fish to cultured marine fish. Environ. Microbiol 64 (1998): 4065-4067.
- Yuasa K, Kitancharoen N, Kataoka Y, et al. Streptococcus iniae, the causative agent of mass mortality in rabbitfish Siganus canaliculatus in Bahrain. Aquat. Animal Health 11 (1999): 87-93.
- Colussi S, Pastorino P, Mugetti D, et al. Isolation and Genetic Characterization of Streptococcus iniae Virulence Factors in Adriatic Sturgeon (Acipenser naccarii). Microorganisms 10 (2022): 883.
- Lau SK, Woo PC, Tse H, et al. (2003). Invasive Streptococcus iniae infections outside North America. J. Clin. Microbiol 41(3): 1004-1009.
- Low DE, Liu E, Fuller J, et al. Streptococcus iniae: an emerging pathogen in the aquaculture industry. In: Emerging Infections 3. ASM. Press, Washington, DC, USA (1999): 53-65.
- Wang Q, FuT, Li X, et al. Cross-immunity in Nile tilapia vaccinated with Streptococcus agalactiae and Streptococcus iniae Fish Shellfish Immunology 97 (2019): 382-389.
- Bachrach G, Zlotkin A, Hurvitz A, et al. Recovery of Streptococcus iniae from diseased fish previously vaccinated with a Streptococcus vaccine. Environ. Microbiol 67 (2001): 3756-3758.
- Locke JB, Vicknair MR, Ostland VE, et al. Evaluation of Streptococcus iniae killed bacterin and live attenuated vaccines in hybrid striped bass through injection and bath immersion. Dis. Aquat Organ 89 (2010): 117-123.
- Ra CH, Kim YJ, Park SJ, et al. Evaluation of optimal culture conditions for recombinant ghost bacteria vaccine production with the antigen of Streptococcus iniae GAPDH. J. Microbiol. Biotechnol 19 (2009): 982-986.
- Sun Y, Hu YH, Liu CS, et al. Construction and analysis of an experimental Streptococcus iniae DNA vaccine. Vaccine 28 (2010a): 3905-3912.
- Zou L, Wang J, Huang B, et al. MtsB, a hydrophobic membrane protein of Streptococcus iniae, is an effective subunit vaccine candidate. Vaccine 29 (2011): 391-394.
- Sommerset I, Krossøy B, Biering E, et al. Vaccines for fish in aquaculture. Expert. Rev. Vaccines 4 (2005): 89-101.
- Wang EL, Wang XL, Wang KY, et al. Preparation, characterization and evaluation of the immune effect of alginate/chitosan composite microspheres encapsulating recombinant protein of Streptococcus iniae designed for fish oral vaccination. Fish Shellfish Immunology 73 (2018): 262-271.
- Halimi M, Alishahi M, Abbaspour MR, et al. High efficacy and economical procedure of oral vaccination against Lactococcus garvieae/Streptococcus iniae in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunology 99 (2020): 505-513.
- Taylor I Heckman, Khalid Shahin, Eileen E Henderson, et al. Development and efficacy of Streptococcus iniae live-attenuated vaccines in Nile tilapia, Oreochromis niloticus. Fish and Shellfish Immunology 121 (2022): 152-162.
- Cai J, Ding Q, Wang Z, et al.. Isolation and identification of tilapia Streptococcus. Chinese J Prev Vet Med 24 (2002): 18-20.
- Cheng S, Hu YH, Jiao XD, et al. Identification and immunoprotective analysis of a Streptococcus iniae subunit vaccine candidate. Vaccine 28 (2010): 2636-2641.
- Zhou SM, Xie MQ, Zhu XQ, et al. Identification and genetic characterization of Streptococcus iniae strains isolated from diseased fish in China. J Fish Dise 31 (2008): 869-875.
- Kado CI, Liu ST. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol 145 (1981): 1365-1373.
- Sun Y, Liu CS, Sun L. Isolation and analysis of the vaccine potential of an attenuated Edwardsiella tarda strain. Vaccine 28 (2010b): 6344-6350.
- Silva-Stenico ME, Pacheco FT, Rodrigues JL, et al. Growth and siderophore production of Xylella fastidiosa under iron-limited conditions. Microbiol Res 160 (2005): 429-436.
- Wang HR, Hu YH, Zhang WW, et al. Construction of an attenuated Pseudomonas fluorescens strain and evaluation of its potential as a cross-protective vaccine. Vaccine 27 (2009): 4047-4055.
- Zhang M, Sun K, Sun L. Regulation of autoinducer 2 production and luxS expression in a pathogenic Edwardsiella tarda strain. Microbiology 154 (2008): 2060-2069.
- Sun Y, Liu CS, Sun L. A multivalent killed whole-cell vaccine induces effective protection against Edwardsiella tarda and Vibrio anguillarum. Fish. Shellfish. Immunol 31 (2011): 595-599.
- Bhatnagar N, Getachew E, Straley S, et al. Reduced virulence of rifampicin-resistant mutants of Francisella tularensis. J Infect Dis 170 (1994): 841-847.
- LaFrentz BR, LaPatra SE, Call DR, et al. Isolation of rifampicin resistant Flavobacterium psychrophilum strains and their potential as live attenuated vaccine candidates. Vaccine 26 (2008): 5582-5589.
- Pridgeon JW, Klesius PH. Development of a novobiocin-resistant Edwardsiella ictaluri as a novel vaccine in channel catfish (Ictalurus punctatus). Vaccine 29 (2011a): 5631-
- Pridgeon JW, Klesius PH. Development and efficacy of a novobiocin-resistant Streptococcus iniae as a novel vaccine in Nile tilapia (Oreochromis niloticus). Vaccine 29 (2011b): 5986-5993.
- Yu Liu, Liping Li, Fangzhao Yu, et al. Genome-wide analysis revealed the virulence attenuation mechanism of the fish-derived oral attenuated Streptococcus iniae vaccine strain YM011. Fish and Shellfish Immunology 106 (2020): 546-554.
- Wang J, Zou LL, Li AX. Construction of a Streptococcus iniae sortase A mutant and evaluation of its potential as an attenuated modified live vaccine in Nile tilapia (Oreochromis niloticus). Shellfish. Immunol 40 (2014): 392-398.
- Mohammad Hayat, Md Sabri Mohd Yusoff, Mohd Jamil Samad, et al. Efficacy of Feed-Based Formalin-Killed Vaccine of Streptococcus iniae Stimulates the Gut-Associated Lymphoid Tissues and Immune Response of Red Hybrid Tilapia. Vaccines 9 (2021): 51.
