Sclerosing Mesenteritis Presenting with Small Bowel Obstruction in a Patient with Compensated Cirrhosis: A Case Report
Article Information
Georgios Zacharioudakis1, Christina Liava2*, Apostolos Andronikou1, Eleni Syndouka3, Apostolos Kambaroudis1, Evangelos Akriviadis2
1Department of General Surgery, Aristotle University of Thessaloniki, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece
2Department of Internal Medicine, Aristotle University of Thessaloniki, Hipporation General Hospital, Thessaloniki, Greece
3Department of Radiology, Theageneio General Hospital of Thessaloniki, Thessaloniki, Greece
*Corresponding Author: Christina Liava, Resident of Internal Medicine-Gastroenterology, Department of Internal Medicine, Aristotle University of Thessaloniki, Hippokration General Hospital, Thessaloniki, Greece
Received: 04 October 2021; Accepted: 18 October 2021; Published: 29 October 2021
Citation: Georgios Zacharioudakis, Christina Liava, Apostolos Andronikou, Eleni Syndouka, Apostolos Kambaroudis, Evangelos Akriviadis. Sclerosing Mesenteritis Presenting with Small Bowel Obstruction in a Patient with Compensated Cirrhosis: A Case Report. Archives of Clinical and Medical Case Reports 5 (2021): 748-758.
View / Download Pdf Share at FacebookAbstract
Sclerosing Mesenteritis (SM) is a rare fibroinflammatory disease of unknown etiology in which intestinal obstruction results from encasement of variable lengths of bowel by a dense fibrocollagenous membrane that gives the appearance of a cocoon. To our knowledge no cases of SM have been reported in patients with compensated cirrhosis without ascites. We report a case with SM diagnosed at laparotomy due to rapidly worsening small bowel obstruction, in a patient with compensated alcoholic cirrhosis. A 50-yearsold Caucasian male was evaluated because of cramping abdominal pain and discomfort associated with diarrhea, occasional post-prandial vomiting, fatigue and a significant weight loss. Compensated alcoholic cirrhosis had been diagnosed, two months before current presentation. Physical examination revealed abdominal distention with highpitched metallic sounds and bilateral peripheral pedal edema. Computed tomography of the abdomen demonstrated severe dilated small bowel loops as well as fluid into the peritoneal cavity. Based on the imaging findings, a surgical procedure was carried out. During laparotomy, an extra fibrous layer fully surrounding the small intestine was divided and a side-to-side functional ileo-cecum anastomosis was performed. Histopathological examination revealed fibroconnective tissue proliferation and inflammatory infiltration. Combined with the characteristic surgical findings a diagnosis of SM was made in a patient with compensated alcoholic cirrhosis. The patient recovered uneventfully and at 6-month follow-up he had no symptoms or signs of SM recurrence. The insidious onset of bowel obstruction symptoms in patients with even compensated cirrhosis, should include SM in the differential diagnosis.
Keywords
Sclerosing Mesenteritis; Mesenteric Lipodystrophy; Mesenteric Panniculitis; Compensated Cirrhosis; Bowel Obstruction; Case Report
Sclerosing Mesenteritis articles; Mesenteric Lipodystrophy articles; Mesenteric Panniculitis articles; Compensated Cirrhosis articles; Bowel Obstruction articles; Case Report articles
Sclerosing Mesenteritis articles Sclerosing Mesenteritis Research articles Sclerosing Mesenteritis review articles Sclerosing Mesenteritis PubMed articles Sclerosing Mesenteritis PubMed Central articles Sclerosing Mesenteritis 2023 articles Sclerosing Mesenteritis 2024 articles Sclerosing Mesenteritis Scopus articles Sclerosing Mesenteritis impact factor journals Sclerosing Mesenteritis Scopus journals Sclerosing Mesenteritis PubMed journals Sclerosing Mesenteritis medical journals Sclerosing Mesenteritis free journals Sclerosing Mesenteritis best journals Sclerosing Mesenteritis top journals Sclerosing Mesenteritis free medical journals Sclerosing Mesenteritis famous journals Sclerosing Mesenteritis Google Scholar indexed journals Mesenteric Lipodystrophy articles Mesenteric Lipodystrophy Research articles Mesenteric Lipodystrophy review articles Mesenteric Lipodystrophy PubMed articles Mesenteric Lipodystrophy PubMed Central articles Mesenteric Lipodystrophy 2023 articles Mesenteric Lipodystrophy 2024 articles Mesenteric Lipodystrophy Scopus articles Mesenteric Lipodystrophy impact factor journals Mesenteric Lipodystrophy Scopus journals Mesenteric Lipodystrophy PubMed journals Mesenteric Lipodystrophy medical journals Mesenteric Lipodystrophy free journals Mesenteric Lipodystrophy best journals Mesenteric Lipodystrophy top journals Mesenteric Lipodystrophy free medical journals Mesenteric Lipodystrophy famous journals Mesenteric Lipodystrophy Google Scholar indexed journals Lipodystrophy articles Lipodystrophy Research articles Lipodystrophy review articles Lipodystrophy PubMed articles Lipodystrophy PubMed Central articles Lipodystrophy 2023 articles Lipodystrophy 2024 articles Lipodystrophy Scopus articles Lipodystrophy impact factor journals Lipodystrophy Scopus journals Lipodystrophy PubMed journals Lipodystrophy medical journals Lipodystrophy free journals Lipodystrophy best journals Lipodystrophy top journals Lipodystrophy free medical journals Lipodystrophy famous journals Lipodystrophy Google Scholar indexed journals Mesenteric Panniculitis articles Mesenteric Panniculitis Research articles Mesenteric Panniculitis review articles Mesenteric Panniculitis PubMed articles Mesenteric Panniculitis PubMed Central articles Mesenteric Panniculitis 2023 articles Mesenteric Panniculitis 2024 articles Mesenteric Panniculitis Scopus articles Mesenteric Panniculitis impact factor journals Mesenteric Panniculitis Scopus journals Mesenteric Panniculitis PubMed journals Mesenteric Panniculitis medical journals Mesenteric Panniculitis free journals Mesenteric Panniculitis best journals Mesenteric Panniculitis top journals Mesenteric Panniculitis free medical journals Mesenteric Panniculitis famous journals Mesenteric Panniculitis Google Scholar indexed journals Compensated Cirrhosis articles Compensated Cirrhosis Research articles Compensated Cirrhosis review articles Compensated Cirrhosis PubMed articles Compensated Cirrhosis PubMed Central articles Compensated Cirrhosis 2023 articles Compensated Cirrhosis 2024 articles Compensated Cirrhosis Scopus articles Compensated Cirrhosis impact factor journals Compensated Cirrhosis Scopus journals Compensated Cirrhosis PubMed journals Compensated Cirrhosis medical journals Compensated Cirrhosis free journals Compensated Cirrhosis best journals Compensated Cirrhosis top journals Compensated Cirrhosis free medical journals Compensated Cirrhosis famous journals Compensated Cirrhosis Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Bowel Obstruction articles Bowel Obstruction Research articles Bowel Obstruction review articles Bowel Obstruction PubMed articles Bowel Obstruction PubMed Central articles Bowel Obstruction 2023 articles Bowel Obstruction 2024 articles Bowel Obstruction Scopus articles Bowel Obstruction impact factor journals Bowel Obstruction Scopus journals Bowel Obstruction PubMed journals Bowel Obstruction medical journals Bowel Obstruction free journals Bowel Obstruction best journals Bowel Obstruction top journals Bowel Obstruction free medical journals Bowel Obstruction famous journals Bowel Obstruction Google Scholar indexed journals Radioembolization articles Radioembolization Research articles Radioembolization review articles Radioembolization PubMed articles Radioembolization PubMed Central articles Radioembolization 2023 articles Radioembolization 2024 articles Radioembolization Scopus articles Radioembolization impact factor journals Radioembolization Scopus journals Radioembolization PubMed journals Radioembolization medical journals Radioembolization free journals Radioembolization best journals Radioembolization top journals Radioembolization free medical journals Radioembolization famous journals Radioembolization Google Scholar indexed journals Case Report articles Case Report Research articles Case Report review articles Case Report PubMed articles Case Report PubMed Central articles Case Report 2023 articles Case Report 2024 articles Case Report Scopus articles Case Report impact factor journals Case Report Scopus journals Case Report PubMed journals Case Report medical journals Case Report free journals Case Report best journals Case Report top journals Case Report free medical journals Case Report famous journals Case Report Google Scholar indexed journals
Article Details
Abbreviations:
SM: Sclerosing Mesenteritis; PD: Peritoneal Dialysis; PVS: Peritoneovenous Shunting; SBP: Spontaneous Bacterial Peritonitis; CT: Computed Tomography; PPI: Proton Pump Inhibitor; MR: Magnetic Resonance
1. Introduction
Sclerosing Mesenteritis (SM) is a rare fibroinflammatory disease of unknown etiology in which intestinal obstruction results from encasement of variable lengths of bowel by a dense fibrocollagenous membrane that gives the appearance of a cocoon. This membrane encases partially or completely predominately the small bowel, but can also extend to involve other intraperitoneal organs including colon, liver, stomach, appendix and ovaries among others. In the majority of cases, SM involves the root of the small bowel mesentery, but occasionally it may also involve the sigmoid mesentery, the mesocolon, the peripancreatic space, parts of the omentum, the retroperitoneum or the pelvic cavity [1, 2]. Depending on the etiopathogenesis and the pathological characteristics of the encasing membrane, SM can be classified as primary (idiopathic), secondary or congenital [3]. Peritoneal dialysis (PD) is the most common cause of secondary SM [4, 5]. Other causes include prior abdominal surgery, beta-blocker treatment, peritoneovenous shunting (PVS) and decompensated cirrhosis complicated by spontaneous bacterial peritonitis (SBP) [3-11]. To our knowledge no cases of SM have been reported in patients with compensated cirrhosis without ascites. We report a case with SM diagnosed at laparotomy due to rapidly worsening small bowel obstruction, in a patient with compensated alcoholic cirrhosis.
2. Case Presentation
A 50-years-old Caucasian male was evaluated in the outpatient clinic because of cramping abdominal pain and discomfort associated with diarrhea, occasional post-prandial vomiting, fatigue and a significant weight loss of 9 kg over the previous two months. Compensated alcoholic cirrhosis had been diagnosed [based on abdominal computed tomography (CT)], two months before current presentation. He had no history of abdominal or pelvic surgery. The patient had a 15-year history of daily alcohol consumption and a 40-pack year smoking history. Medications included magnesium, vitamin tablets, rifaximin and proton pump inhibitor (PPI). He lived with his wife and daughter in an urban area of Greece. Physical examination revealed abdominal distention with high-pitched metallic sounds and bilateral peripheral pedal edema. A large right inguinal hernia was also present. Patient’s vital signs were normal. Laboratory test results are summarized in Table 1. Significantly elevated C-reactive protein, normocytic norm-ochromic anemia, electrolyte disorders and hypoalbum-inemia were the most significant laboratory abnormalities.
Diagnostic evaluation and cirrhosis staging was performed; esophagogastroduodenoscopy revealed severe erosive esophagitis but no evidence of esophageal or gastric varices. Histologic examination and serologic test results were negative for Helicobacter pylori infection or celiac disease. Skin biopsy specimen was also negative for amyloidosis. An x-ray abdominal radiograph showed findings suggestive of small bowel obstruction (Figure 1).
A contrast-enhanced CT of the abdomen demonstrated severe dilated small bowel loops as well as fluid into the peritoneal cavity (Figure 2). Magnetic resonance (MR) enterography revealed dilated loops of the small intestine without an obvious obstructive cause. The patient was immediately referred to the Surgery Service and a surgical procedure was carried out. Exploration through a midline incision revealed marked dilatation of the small intestine (Figure 3) and the presence of intra-abdominal fluid. The last 10 cm of the terminal ileum appeared collapsed, but no evident cause of obstruction (such as tumor or adhesions) was identified, according to the CT findings (Figure 4). While inspecting carefully the area, an extra fibrous layer was identified which fully surrounded the small intestine. Initially, it was thought to be the intestinal serosa.
The division of the cocoon was performed with Metzenbaum scissors and electrocautery. The encapsulated intestine follows a tortuous course within the cocoon, with some bowel segments being stenotic while others being dilated. The surgical team decided to resect the collapsed segment of the terminal ileum. On attempting to mobilize the ascending colon, it was noted that it was also encapsulated by a similar fibrous layer (Figure 5). This cocoon-like membrane was divided at its anterior surface and a side-to-side functional ileocecal anastomosis was subsequently performed.
Histopathological examination of the colonic cocoon revealed fibroconnective tissue proliferation and inflammatory infiltration. Although the patient was diagnosed with alcoholic cirrhosis, his postoperative course was uncomplicated. The patient was discharged from the hospital after 7 days. At a 6-month follow-up, he had no symptoms or signs of SM recurrence.
|
Laboratory tests |
Reference Range |
|
|
White Blood Cells |
11.300 |
3.800 - 10.500 103 K/μL |
|
Neutrofils |
82,2 |
45 - 75 % |
|
Lymphocytes |
7,0 |
20 - 51 % |
|
Monocytes |
8,9 |
2 - 11 % |
|
Eosinophils |
1,0 |
0,5 - 10 % |
|
Hemoglobin |
10,2 |
14 -18 g/dL |
|
Hematocrit |
29,9 |
40 - 52 % |
|
Mean Corpuscular Volume |
87,3 |
80 - 99 fL |
|
Mean Corpuscular Hemoglobin |
29,7 |
27 - 32 pg |
|
Mean Corpuscular Hemoglobin Concentration |
34,1 |
32 - 35 g/dL |
|
Red Cell Distribution Width |
15,1 |
11,5 - 14,5 % |
|
Platelets |
189 |
150 - 450 103/μL |
|
Aspartate aminotransferase |
26 |
10 - 37 U/L |
|
Alanine aminotransferase |
20 |
10 -45 U/L |
|
Glucose |
108 |
< 100 mg/dL |
|
Urea |
41 |
10 - 43 mg/dL |
|
Creatinine |
0,86 |
0,84 - 1,25 mg/dL |
|
Sodium |
132 |
136 - 145 mmol/L |
|
Potassium |
3,9 |
3,5 - 5,1 mmol/L |
|
Calcium |
8,4 |
8,8 - 10,6 mg/dL |
|
Gamma-Glutamyl Transpeptidase (GGT) |
125 |
< 55 U/L |
|
Total Proteins |
6,0 |
6,6 - 8,3 g/dL |
|
Albumin |
2,7 |
3,5 - 5,2 g/dL |
|
Total Bilirubin |
2,39 |
0,3 - 1,2 mg/dL |
|
Direct Bilirubin |
1,43 |
0 - 0,2 mg/dL |
|
Magnesium |
1,05 |
1,8 - 2,6 mg/dL |
|
C-Reactive Protein (CRP) |
205,8 |
< 6 mg/L |
Table 1: Patient’s Laboratory Test Results.
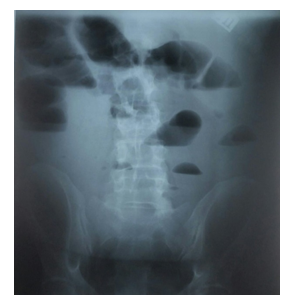
Figure 1: X-ray Abdominal Radiograph Findings: Dilated small bowel loops in the upper abdomen with multiple air-fluid levels suggestive of obstructive ileus of the small bowel.
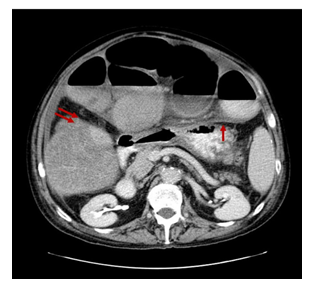
Figure 2: Axial Contrast-Enhanced Computed Tomography Image: Dilated small bowel loops enveloped by a dense fibro-collagenous membrane (single red arrow) as well as small amount of fluid into the peritoneal cavity. Thickening and enhancement of the peritoneal surface is also recognised (double red arrows).
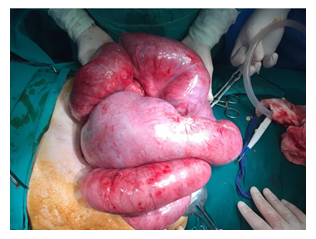
Figure 3: Intraoperative View during Laparotomy: Intraoperative view of the dilated small bowel loops encased by a thick fibrocollagenous membrane with a cocoon-like appearance. At first sight this membrane was thought to be the intestinal serosa of the dilated small bowel loops but after incision it was noted that these loops were actually entrapped by the fibrotic membrane.
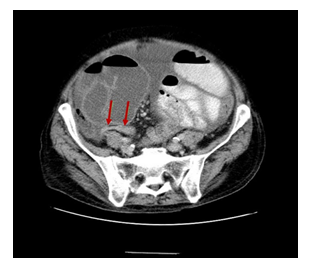
Figure 4: Axial Contrast-Enhanced Computed Tomography Image: Dilated small bowel loops encased by a thick membrane and the collapsed distal part of the terminal ileum (red arrows).
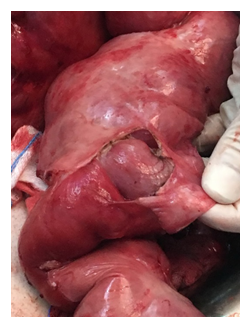
Figure 5: Intraoperative View During Laparotomy After Division of the Membrane: The appearance of the encased dilated small bowel loops and the thick grayish-white fibrotic membrane.
3. Discussion
This rare cause of abdominal obstruction was first reported by Owtschinnikow in 1907 and was coined the term “peritonitis chronic fibrosa incapsulata” [12]. The idiopathic form is also known as abdominal cocoon which was first described by Foo et al [13] in 1978. On the basis of the predominant histology and in order to better describe this clinicopathologic entity the following terms have also been used: mesenteric lipodystrophy (predominantly fatty degeneration and necrosis), mesenteric panniculitis (marked chronic inflammation) and retractile mesenteritis (predominant fibrosis). Many authors have proposed the umbrella term sclerosing mesenteritis (SM) which can be used for all sub-categories of this entity [14].
In primary SM, no demonstrable cause is identified; the role of cytokines and fibroblasts in the development of peritoneal fibrosis and neoangiogenesis has been hypothesized [1, 3]. Idiopathic SM classically presents in young adolescent girls in tropical and subtropical countries [1-3], although cases of idiopathic SM in adult males in temperate zones have also been reported [2, 15, 16]. A systematic review of 192 cases of SM conducted by Sharma et al [14] showed that men were more commonly affected than women (70% vs 30%) and the majority of patients were Caucasians (60%). The median age at diagnosis was 61 years. Similar results were reported in a retrospective/ prospective study of 92 patients diagnosed at Mayo Clinic [17] and also in a retrospective study of 65 Chinese patients with abdominal cocoon [18].
Secondary SM is more common than idiopathic. In secondary SM, a local or systemic factor triggers the inflammatory process in the peritoneum [1, 2, 3]. PD is the most common cause. Studies have shown a direct relationship between prolonged PD and the development of secondary SM [1-5]. Other rare causes include prior abdominal surgery, beta-blocker treatment, subclinical primary peritonitis, recurrent peritonitis, chemotherapy (methotrexate), PVS, and more rarely abdominal tuberculosis, sarcoidosis, familial Mediterranean fever, systemic lupus erythematous, intraperitoneal chemotherapy, cirrhosis, liver transplantation, gastrointestinal malignancy, endometriosis, protrein S deficiency and fibrogenic foreign material [1-3].
In cirrhotic patients SM has been described after PVS and in those treated with beta-blockers [7-10]. It should also be included in the differential diagnosis in cirrhotic patients with recurrent intraabdominal infection, such as SBP, who present with symptoms of intestinal obstruction [11]. Moreover, it is known that SM can occur as a consequence of persistent low-grade peritonitis and may complicate the postoperative course after liver transplantation [20, 21]. However our patient was diagnosed with compensated alcoholic cirrhosis (Child-Pugh A score) and had neither of these conditions. The early clinical features of SM are non-specific and often not recognized. Clinically it presents with recurrent episodes of acute, subacute or chronic gastrointestinal obstruction (complete or incomplete), nausea, vomiting, fever, anorexia, appetite loss, weight loss and malnutrition [1-3, 14, 17, 18, 22, 23]. These symptoms were also present in our patient. A painless and soft abdominal mass can also be palpated in some patients, while others (10%) may be asymptomatic [1-3, 14, 25, 26]. In some cases, abdominal distension was secondary to ascites [17, 27]. Gastrointestinal perforation is quite rare in patients with SM [27]. However, a few case reports describe patients with protein losing enteropathy [28, 29] and also with IgG4-related disease suggesting that SM [30, 31] may have severe clinical manifestations.
Additionally, in a case series of patients with SM, the authors described a possible relationship between SM and disturbance of glucose metabolism [32]. Notably, our patient experienced symptoms of partial bowel obstruction for a relatively short period of time (approximatelly 2 months). Retrospective assessment of the abdominal CT showed that the cocoon was already present at the time of diagnosis of cirrhosis (Figure 6). However pre-operative diagnosis of SM is a real diagnostic challenge even for experienced clinicians. During laparotomy, it is difficult to distinguish the fibrotic membrane from the intestinal serosa, as in our case (Figure 3 and 5).
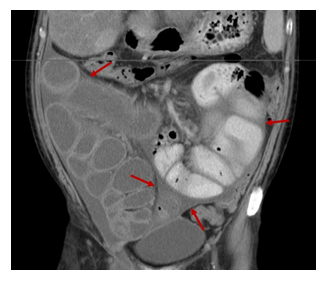
Figure 6: Coronal Contrast-EnhancedComputed Tomography Image: Severe dilated small bowel loops encased by a thin membrane (red arrows) and fluid collections entrapped between the membrane and the bowel wall.
4. Conclusion
Diagnosis of SM is a diagnostic challenge and requires a high index of clinical suspicion. It can be facilitated by the patient’s history, existing predisposing factors, various biochemical parameters and radiological imaging. A better awareness of this rare clinical entity and the imaging findings may facilitate pre-operative diagnosis, which is critical for selecting the appropriate surgical intervention or even avoid the need for surgery and instead apply immunosuppressive drug therapy. There are case reports in the literature in which treatment with corticosteroids led to alleviation of the obstruction symptoms and improvement of the disease course. Our case suggests that the insidious onset of bowel obstruction symptoms in a cirrhotic patient, even if cirrhosis is compensated and uncomplicated, should include SM in the differential diagnosis.
Conflict of Interest
No potential conflict of interest
Funding
No funding source
References
- Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol 21 (2015): 675-687.
- Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: Abdominal cocoon. World J Gastroenterol 18 (2012): 1999-2004.
- Machado NO. Sclerosing Encapsulating Peritonitis. Sultan Qaboos Univ Med J 16 (2016): e142-e151.
- Nomoto Y, Kawaquchi Y, Kubo H, et al. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Group. Am J Kidney Dis 28 (1996): 420-427.
- Kawanishi H. Encapsulating peritoneal sclerosis. Nephrology (Carlton) 10 (2005): 249-255.
- Suh WN, Lee SK, Chang H, et al. Sclerosing Encapsulating Peritonitis (Abdominal Cocoon) after Abdominal Hysterectomy. Korean J Intern Med 22 (2007): 125-129.
- Harty FR. Sclerosing Peritonitis and Propranolol. Arch Intern Med 138 (1978): 1424-1426.
- Brown P, Baddeley H, Read AE, et al. Sclerosing peritonitis, an unusual reaction to a beta-adrenergic-blocking drug (practolol). Lancet 2 (1974): 1477-1481.
- Cudazzo E, Lucchini A, Puviani PP, et al. Sclerosing peritonitis. A complication of LeVeen peritoneovenous shunt. Minerva Chir 54 (1999): 809-812.
- Stanley MM, Reyes CV, Greenlee HB, et al. Peritoneal fibrosis in cirrhotics treated with peritoneovenous shunting for ascites. An autopsy study with clinical correlations. Dig Dis Sci 41 (1996): 571-577.
- Yamamoto S, Sato Y, Takeishi T, et al. Sclerosing encapsulating peritonitis in two patients with liver cirrhosis. J Gastroenterol 39 (2004): 172-175.
- Owtschinnikow PJ. Peritonitis chronic fibrosa incapsulata. Arch Klin Chir 83 (1907): 623-634.
- Foo KT, Ng KC, Rauff A, et al. Unusual small intestinal obstruction in adolescent girls: The abdominal cocoon. Br J Surg 65 (1978): 427-430.
- Sharma P, Yadav S, Needham CH, et al. Sclerosing mesenteritis: a systematic review of 192 cases. Clin J Gastroenterol 10 (2017): 103-111.
- Xu P, Chen LH, Li YM. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon): A report of 5 cases. World J Gastroenterol 13 (2007): 3649-3651.
- Al Ani AH, Al Zayani N, Najmeddine M, et al. Idiopathic sclerosing encapsulating peritonitis (abdominal cocoon) in adult male. A case report. Int J Surg Case Rep 5 (2014): 735-738.
- Akram S, Pardi DS, Schaffner JA, et al. Sclerosing Mesenteritis: Clinical Features, Treatment, and Outcome in Ninety-Two Patients. Clin J Gastroenterol Hepatol 5 (2007): 589-596.
- Li N, Zhu W, Li Y, et al. Surgical Treatment and Preoperative Management of Idiopathic Abdominal Cocoon: Single-Center Review of 65 Cases. World J Surg 38 (2014): 1860-1867.
- Maguire D, Srinivasan P, O’ Grady J, et al. Sclerosing encapsulating peritonitis after orthotopic liver transplantation. Am J Surg 182 (2001): 151-154.
- Lin CH, Yu JC, Chen TW, et al. Sclerosing encapsulating peritonitis in a liver transplant patient: A case report. World J Gastroenterol 11 (2005): 5412-5413.
- Mekeel K, Moss A, Reddy KS, et al. Sclerosing peritonitis and mortality after liver transplantation. Liver Transpl 15 (2009): 435-439.
- Green MS, Chhabra R, Goyal H. Sclerosing mesenteritis: a comprehensive clinical review. Ann Transl Med 6 (2018): 336.
- Vlachos K, Archontovasilis F, Falidas E, et al. Sclerosing Mesenteritis: Diverse clinical presentations and dissimilar treatment options. A case series and review of the literature. Int Arch Med 4 (2011): 17.
- Avelino-Silva VI, Leal FE, Coelho-Netto C, et al. Sclerosing mesenteritis as an unusual cause of fever of unknown origin: a case report and review. Clinics (Sao Paulo) 67 (2012): 293-295.
- Gu GL, Wang SL, Wei XM, et al. Sclerosing mesenteritis as a rare cause of abdominal pain and intraabdominal mass: a cases report and review of the literature. Cases J 1 (2008): 242.
- McDermott RL, Hutchinson B, Ryan C, et al. Mesenteric lipodystrophy-An unusual intraabdominal mass. Int J Surg Case Rep 4 (2013): 232-234.
- Lim HW, Sultan KS. Sclerosing Mesenteritis Causing Chylous Ascites and Small Bowel Perforation. Am J Case Rep 18 (2017): 696-699.
- Saito Y, Hiramatsu K, Nosaka T, et al. A case of protein-losing enteropathy caused by sclerosing mesenteritis diagnosed with capsule endoscopy and double-ballon endoscopy. Clin J Gastroenterol 10 (2017): 351-356.
- Rajendran B, Duerksen DR. Retractile mesenteritis presenting as protein-losing gastroenteropathy. Can J Gastroenterol 20 (2006): 787-789.
- Kitagawa S, Okamura K. IgG4-Related Sclerosing Mesenteritis. Am J Gastroenterol 112 (2017): 834.
- Nomura Y, Naito Y, Eriguchi N, et al. A case of IgG4-related sclerosing mesenteritis. Pathol Res Pract 207 (2011): 518-521.
- Tavares Pereira JP, Romão V, Eulálio M, et al. Sclerosing Mesenteritis and Disturbance of Glucose Metabolism: A New Relationship? A Case Series. Am J Case Rep 17 (2016): 55-59.
