SARS-CoV-2 and Exposure to Pollution of the Population near an Industrial Area in the Metropolitan Region in São Paulo State, Brazil
Article Information
Maria Angela Zaccarelli-Marino1,*, Thalles Zaccarelli Balderi2, Felipe Mingorance Crepaldi3, Rudá Alessi4, Marco Martins5
1Internal Medicine Department, Endocrinology Service, ABC Medical School Foundation, Santo André, SP, Brazil
2 Internal Medicine Service, Santa Paula Hospital, São Paulo, SP, Brazil
3ABC Medical School Foundation, Santo André, SP, Brazil
4Internal Medicine Department, ABC Medical School Foundation, Santo André, SP, Brazil
5Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, USA 12
Maria Angela Zaccarelli-Marino
*Corresponding author: Maria Angela Zaccarelli-Marino, Internal Medicine Department, Endocrinology Service, ABC Medical School Foundation, Santo André, SP, Brazil
Received: 08 February 2022; Accepted: 15 February 2022; Published: 03 March 2022
Citation: Maria Angela Zaccarelli-Marino, Thalles Zaccarelli Balderi, Felipe Mingorance Crepaldi, Rudá Alessi, Marco Martins. SARS-CoV-2 and Exposure to Pollution of the Population near an Industrial Area in the Metropolitan Region in São Paulo State, Brazil. Fortune Journal of Health Sciences 5 (2022): 58-87
View / Download Pdf Share at FacebookAbstract
Background Industrial installations close to residential areas could cause health risks. Since coronavirus disease 2019 (COVID-19) emerged in December 2019, the epidemic caused by severe acute respiratory syndrome Corona virus 2 (SARS-CoV-2), which causes clusters of severe respiratory illness, has rapidly developed into a pandemic. The purpose of this study was to evaluate the association between pre-existing diseases, such as obstructive pulmonary disease (OPDs), rhinitis, sinusitis, pharyngitis, conjunctivitis, dermatitis and primary hypothyroidism (PH), in residents close to an industrial area exposed to long-term air pollutants and an increased risk of complications when infected with SARS-CoV-2. Methods With a focus on the area affected by the Capuava Petrochemical Complex (CPC) (Region 1) and combining the AERMOD dispersion model with the Weather Research Forecast (WRF) (2016), we evaluated the Greater ABC region, Brazil. The concentrations of the nitrogen dioxide (NO2), carbon monoxide (CO), particulate matter (PM10), sulfur dioxide (SO2) and volatile organic compounds (VOCs) were analyzed in 2017 and these data were correlated with data obtained in a survey of 2004 residents 8-72 years of age of both sexes; 1002 (Region 1), and 1002 of them reside within the areas surrounding various industrial areas (Region 2). SARS-CoV-2 cases were collected from the Greater ABC region. Result Region 1 showed higher average concentrations of all pollutants analyzed. Among the 2004 total residents, there were significant differences between Region 1 and Region 2 in the incidence of cases of rhinitis, sinusitis, pharyngitis, OPDs, conjunctivitis, dermatitis and PH demonstrating that there is a higher incidence of the evaluated diseases in residents who live closer to the CPC. Compared with residents with these diseases, the residents of Region 1 had a higher relative risk of complications when infected with SARS-CoV-2 than did the residents of Region 2. Conclusion To pro
Keywords
SARS-CoV-2, atmospheric pollution, inflammation, autoimmune, Metropolitan Region, Brazil
SARS-CoV-2 articles, atmospheric pollution articles, inflammation articles, autoimmune articles, Metropolitan Region articles, Brazil articles
Article Details
1. Introduction
Industrial installations close to residential areas, together with unfavorable topographic conditions for the dispersion of pollutants, could cause health risks [1]. The health risks associated with residing near petrochemical industrial complexes represent a critical concern due to potential air pollution emissions. Metropolitan regions are important for public health because of the large number of people living in them, and the air pollution levels in metropolitan regions are usually higher than those in other areas. Industrial plants can increase the concentrations of hazardous substances in surrounding areas and cause respiratory symptoms among residents [2]. Exposure to air pollution has been associated with compromised pulmonary immune defense mechanisms in both animals and humans [3, 4]. In the last decade, associations between exposure to air pollution and acute health outcomes have been demonstrated in a number of studies [4, 5, 6, 7, 8, 9] and air pollution also increases morbidity, especially in individuals with asthma and/or chronic obstructive pulmonary disease (COPD) [5, 6]. A study conducted in Spain used the International Study on Asthma and Allergies in Children's questionnaire to survey the population. They observed a higher prevalence of nocturnal cough among children (aged 6–7 years)
and adolescents (aged 13–14 years) who lived within a petrochemical industrial complex in Tarragona than among children and adolescents who lived in areas of the country in which no petrochemical complexes were located [10]. In the 1960s, the prevalence of asthma and allergic diseases began to increase worldwide. Currently, more than 300 million people are affected by these diseases [11]. Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality in high- and low-income countries and represents a significant public health burden worldwide [12]. In the United States, COPD affects over 6.5% of adults, is the third leading cause of death, and incurs 700,000 inpatient stays, 1.5 million emergency room visits, and USD 32 billion in cost annually [13]. In Wuhan, Hubei Province, China, several cases of severe pneumonia have emerged in humans [14, 15]. The novel coronavirus (2019-nCoV), in the world, was initially reported in China at the end of 2019 [16) and the disease rapidly spread internationally, raising global public health concerns [17, 18]. The World Health Organization (WHO) has defined this new syndrome using the acronym COVID-19 for Coronavirus Disease 2019 [19] and declared COVID-19 as a global pandemic on March 11, 2020 [19].
The International Committee on Taxonomy of Viruses (ICTV) [20] named the disease as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the causative pathogen was identified as a novel corona virus [21, 22]. Since coronavirus disease 2019 (COVID-19) emerged in Wuhan, China in December 2019 [17,18,19,23], the epidemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes clusters of severe respiratory illness [24], has rapidly developed into a pandemic, a rapid and robust response by the global scientific community has described many essential aspects of SARS-CoV-2 transmission and its natural history [17,25,26,27,28], but critical questions remain [26]. Several factors are involved in the transmission of SARS-CoV-2 between individuals, including the environment in buildings and human behavior [19,29,30,31,32,33]. SARS-CoV-2 is mainly transmitted human-to-human through close contact, respiratory droplets, fomites, and contaminated surfaces [34, 35, 36, 37]. Studies in the literature indicate that in the last two decades, there was the transmission of three new corona viruses to humans, causing serious acute pathologies, such as acute respiratory syndrome (SARS). The first outbreak of the disease, SARS-CoV, was reported in November 2002 in Guangdong, China [38] and in 2012 the second outbreak occurred in Saudi Arabia due to Middle East respiratory syndrome coronavirus (MERS-CoV) [39]. Coronaviruses (CoVs) are grouped into four genera, including alpha, beta, gamma, and delta, and belong to the corona viridae family [40, 41]; birds are infected by those belonging to the gamma and delta genera while viruses belonging to the alpha and beta genera, infect mammals, including humans [42, 43]. Like other viruses, Coronavirus has its genetic material wrapped in a protein coat known as a capsid, and is approximately 60-100 nm in diameter.
At the beginning of April 2020, marked differences in the rate of transmission and in the mortality associated with outbreaks of COVID-19 in different countries were noted. Early studies concluded that the risk factors associated with the development of the disease are older age [14] history of smoking [44] and heart disease [45]. Subsequently, the number of infected people increased rapidly, and a month later, the outbreak had developed into a national crisis, with infected individuals diagnosed all over the country [27]. COVID-19 has been associated with high rates of infection and lethality, especially in patients with comorbidities [46]. It is characterized by sore throat, headaches [29], fever, cough, fatigue, shortness of breath, pneumonia, and other respiratory tract symptoms [17, 47].
Blood tests performed between September 2019 and March 2020 in Italy revealed anti-SARS-CoV-2, antiviral immunoglobulin G (IgG) and/or immunoglobulin M (IgM) antibodies [48]. Anti-SARS-CoV-2 antibody response analyses in patients with COVID-19 showed that within 13 days after symptom onset, seroconversion of IgG or IgM was present in almost 100% of patients [49]. Some patients with COVID 19 present on radiographic images ground-glass lung changes and in many cases, progresses to death of acute respiratory distress syndrome (ARDS) [16, 24,]. As of June 16, 2020, there were 7,941,791 confirmed cases of COVID-19 and 434,796 deaths worldwide and 867,624 confirmed cases of COVID-19 and 43,332 deaths in Brazil (Johns Hopkins University 2020; World Health Organization Coronavirus Disease (COVID-19) 2020) [50]. The association between air pollution and respiratory infections has been a matter of public health concern in recent years [51]; however, both public and scientific interest has heightened since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic [52]. Petroleum processing can emit various organic compounds that can threaten human health [53]. Petrochemical industrial complexes (PICs) generate various air pollutants such as volatile organic compounds (VOCs), sulfur dioxide (SO2), particulate matter (PM), and nitrogen oxides (NOx) [54, 55,56,57,58]; VOCs are organic compoundscontaining one or more carbon atoms; they have very high vapor pressure and readily evaporate into the atmosphere at room temperature. Some examples include acetone, benzene, ethylene glycol, formaldehyde, methylene chloride, perchloroethylene, toluene, xylene, and 1,3-butadiene [59]. High NO2 concentrations are significantly associatedwith respiratory mortality [60, 61] and also generate harmful secondary pollutants such as nitric acid (HNO3)and136 ozone (O3) [62].
The question of whether COVID-19 can be spread by airborne transmission has been asked both in the scientific community and by the general public since the arrival of the virus. Similar to fine particulate matter (PM2.5), viruses are among the inhalable biological particles that may be airborne. Recent studies have shown that viruses become airborne through sneezing and coughing and that they may be dispersed further as they commingle with PM2.5 [63]. Coarse particulate matter (PM10-2.5) consists of aerodynamically inhalable particles between 10μm and 2.5 μm in size. Such particles are generally found near roadways and dust-generating industries. They are considered to be of regulatory interest because they may penetrate up to the level of the lower respiratory tract and the portion of the lung in which gas exchange occurs [63].
There are few epidemiologic studies that relate air pollution and chemical industries to rhinitis, sinusitis, pharyngitis and obstructive pulmonary diseases (OPDs) such as asthma and COPD. Studies in South America have identified associations between residential proximity to a petrochemical plant and asthma, rhinitis, cough and wheezing [55, 64, 65]. Previous studies have found that air pollution is a risk factor for respiratory infection because it carries microorganisms and affects the body's immunity [65]. Furthermore, recent studies suggest that the cause of death of many COVID-19 patients is related to cytokine storm syndrome [66, 67]. In this syndrome, which is also known as hypercytokinemia, uncontrolled release of proinflammatory cytokines occurs [68]. It is a severe reaction of the immune system that leads to a chain of destructive processes in the body that can end in death. São Paulo State (SP), the most populous and industrialized state in Brazil, has approximately 45 million inhabitants and 7,012 industries [69]. Our previous studies [ 70,71,72] involved subjects living in a densely populated area of SP surrounding the Capuava Petrochemical Complex (CPC). We reported primary hypothyroidism (PH) [71, 72] and an increase in the incidence of chronic autoimmune thyroiditis (CAT) over the years [70] and showed evidence that iodine should not be considered the agent responsible for autoimmune thyropathies in Santo André [73]. Some of the results of the current study have been previously reported in the form of an abstract and poster presented at the ABC University Medical Congress (Supplementary material (SM)) (Zaccarelli-Marino et al. 2016a, b, 2017a, b, 2018 a,b). The purpose of this study was to evaluate the association between pre-existing diseases, such as obstructive pulmonary disease (OPDs), rhinitis, sinusitis, pharyngitis, conjunctivitis, dermatitis and primary hypothyroidism (PH), in residents close to an industrial area exposed to long-term air pollutants and an increased risk of complications when infected with SARS-CoV-2.
2. Methods
2.1 Study Population and Data Collection
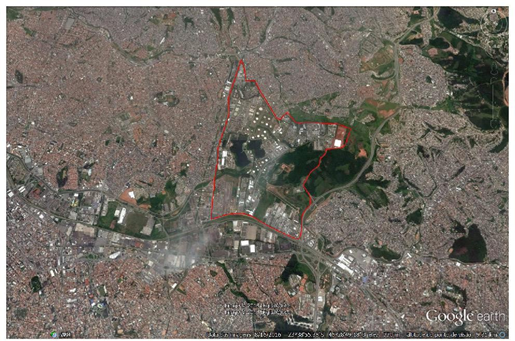
This is a cohort study. We evaluated residents living in the area close to the Capuava Petrochemical Complex (CPC) (Fig. 1) in the Greater ABC region from July 2003 through June 2005.
The residents were divided into two groups (A and B) based on their proximity to the industrial areas; those who were considered non exposed (not including a consideration of ambient air pollution) lived in the surroundings of a different industrial area, mainly steel industries in which no petroleum byproducts are manufactured. The research population was defined after the purpose of this study had been explained to the participants. Prior to data collection, written informed consent was obtained (where appropriate, from the father or mother of the participant or from another responsible person), and authorization for this realization was obtained from the residents. The data collection consisted of obtaining information on the residents, and a home-based questionnaire was administered to participating residents 8-72 years of age of both sexes who had lived in the area since 2003. The survey was developed and applied by the authors of this study. Group A comprised 1002 residents, males and females, living in the surroundings of the CPC. This industrial area was named Region 1 and is occupied by 14 industries that produce polyethylene and polypropylene from naphtha distillation as well as various intermediary substances that are used as raw materials in the manufacture of other products. The area is located on the boundaries of Santo André, Mauá and São Paulo (located 0.5 km, 1.0 km, and 2.0 km from the CPC, respectively) in the State of São Paulo (SP), Brazil. Group B comprised 1002 residents, males and females, living in the surroundings of various industrial areas; these industrial areas harbor mainly steel industries that generate no petroleum byproducts. The area occupied by Group B is located on the boundaries of Santo André, São Bernardo do Campo, and São Caetano do Sul (7.5 km, 8.0 km, and 8.5 km from Region 1, respectively) in SP, Brazil. This industrial area was named Region 2, and the residents of this region were treated as the control group. Information on the city's population density was obtained from the Brazilian Institute of Geography and Statistics (IBGE) [74].
Regions 1 and 2 were each divided into five cities; 334 residents were evaluated according to their distance from the CPC. Each resident had lived in the same home in either Region 1 or Region 2 for more than 10 years, and the controls were matched to the participants with respect to their social and economic situations. The socioeconomic conditions of the participants were evaluated through the questionnaire. The residents were selected based on their personal reporting of similar salaries and social habits (these residents do not have the economic means to move far from the polluted areas). The residents were considered adults if they were at least 18 years of age, and children and adolescents were under the age of 18. When the residents were children or adolescents, the questions were presented to their parents or to the person(s) responsible for the children. The visits occurred once in each house; to guarantee maximal participation, we included weekends as a time for visits.
During the study period (2003-2005), there was no preselection of residents from Regions 1 or 2, and only spontaneous answers were considered. The inclusion criteria were age between 8 and 72 years and having lived in the same house in either Region 1 or Region 2 for more than 10 years. Residents who worked at the CPC were excluded. Written questionnaires (WQs) have been widely used in epidemiologic studies. The International Study of Asthma and Allergies in Childhood (ISAAC) WQ has been previously validated by a comprehensive study [75]. The ISAAC was an important milestone among epidemiological studies on the prevalence of asthma and allergic illnesses in children and adolescents. The ISAAC was designed to evaluate the prevalence of asthma and allergic problems in children in different parts of the world using the standard method (self-administered written questionnaire and/or video questionnaire [76,77]. The written questionnaire (WQ) self-application of ISAAC was the more utilized instrument due to its ease of understanding, low cost, and independence of a trained interviewer [76, 77]. The questionnaire "The International Study of Asthma and Allergies in Childhood (ISAAC)" is an important epidemiological survey instrument established in 1991 to investigate asthma, rhinitis, and eczema in adolescents. ISAAC for nasal symptoms was chosen for the epidemiological diagnosis of rhinitis in children and adolescents; it included questions about sneezing, coryza, watery eyes, and itchy eyes [75].
The European Community Respiratory Health Survey II (ECRHS II) was chosen for the epidemiological diagnosis of asthma; it includes questions about the prevalence of wheezing, coughing, panting, previous diagnoses, and use of medication for the treatment of asthma. It is also used to determine the incidence of allergic diseases, asthma, and reduced pulmonary function and how ambient factors may be associated with allergic diseases and low pulmonary function [78]. The survey respondents were asked their names (initials), age (years), sex (male, female), address and time at local residency, profession and education (adults), education (children and adolescents), and questions about their social and economic situation (adults) and medical history. Rhinitis was investigated through questions based on the same questionnaire: symptoms of sneezing, coryza, watery eyes, itchy eyes, nasal itching, rhinorrhea, blocked nose, cough, sputum production, shortness of breath and rhinitis. All of the symptoms were evaluated in children, adolescents, and adults. Sinusitis was investigated through questions about frontal headache or pain in the jaw region, posterior secretion, watery eyes, itchy eyes and sinusitis; pharyngitis was investigated through questions regarding oropharyngeal pain, scratchiness in the throat, and difficulty swallowing, and OPDs such as asthma and COPD were investigated through questions based on the questionnaire. OPD was based on personal reporting of symptoms such as cough, sputum production and shortness of breath, chest wheezing, and asthma or COPD. Responses regarding rhinitis, sinusitis, pharyngitis, and OPDs such as asthma and COPD were only considered positive when the patient had been diagnosed and treated by a doctor and had been prescribed medication to treat these pathologies.
We selected only residents who presented with OPD with asthma and COPD and took medications for these pulmonary pathologies. Individuals who were taking medication that could interfere with this study, including those undergoing treatment for other otorhinolaryngological and pulmonary pathologies, were excluded. Individuals with a history of smoking or who suffered from other otorhinolaryngological pathologies, emphysema, bronchiectasis, lung surgery, or other lung diseases were also excluded. Conjunctivitis was based on personal reports of signs and symptoms such as pink or red color in the white part of the eye, watery eyes, itchiness, a gritty feeling in the eye, irritation and/or burning sensation in the eye, pain in the eye, and photophobia. The response for conjunctivitis was only considered positive when its diagnosis and treatment had been performed by a physician. We selected only residents presenting with conjunctivitis who were taking medications for this pathology. Individuals who were taking medication to treat other eye diseases or who had a history of smoking, eye surgery, viral conjunctivitis, or bacterial infection of the eye were excluded. Dermatitis was based on personal reports of symptoms such as redness, swelling, intense itching, and skin lesions such as red bumps, blisters, and pustules. The response to dermatitis was only considered positive when the residents' information about their diagnosis and treatment was based on a diagnosis made by a physician. We selected only residents presenting with dermatitis who were taking medications for this skin pathology. PH was evaluated through a questionnaire; we selected only residents presenting with PH and using thyroid hormone.
2.2 SARS-CoV-2 Database
The number of SARS-CoV-2 cases was collected from each city in the Greater ABC region, a traditionally industrial region of São Paulo State, Brazil, that is part of the São Paulo Metropolitan Region. Five cities were chosen: Santo André, Mauá, the Eastern Region of São Paulo City, São Bernardo do Campo and São Caetano do Sul. All information about SARS-CoV-2 cases was obtained from the five ABC Paulista prefectures, the São Paulo State Health Secretariat, and the Brazilian Ministry of Health [50, 79].
2.3 Atmospheric Pollutants
The concentrations of atmospheric pollutants were analyzed in 2017 by numerical simulation with the AERMOD model using meteorological data for the period 2005 to 2009, data on the physical characteristics of the environment (topography and type of land use), and data on pollutant sources (information on the physical characteristics of the sources and their emissions), and these data were correlated with the results of research for the period 2003 to 2005. AERMOD is a dispersion model developed by the American Meteorological Society (AMS) and the U.S. Environmental Protection Agency [80] and made available for public use. The area influenced by atmospheric emissions from the CPC was evaluated using a combination of the AERMOD dispersion model with the Weather Research Forecast (WRF) meteorological model [81].
According to recommendations made by the U.S. Environmental Protection Agency [80], the dispersion model should only be applied when 5 years of meteorological data are available. This was accomplished by the fields provided by the WRF model; based on a combination of the meteorological fields with the pollutant emissions data, the concentration isopletes for the regulated pollutants were calculated for the receptor grid [81]. The map of the concentrations of each pollutant was used to identify the hot spots in which the population was more exposed. The only documentation on the building downwash algorithm in AERMOD (American Meteorological Society/U.S. Environmental Protection Agency Regulatory Model), referred to as PRIME (Plume Rise Model Enhancements), refers to the fact that recent field and wind tunnel studies have shown that AERMOD can overpredict concentrations by factors of 2–8 for certain building configurations.
While a wind tunnel equivalent building dimension study (EBD) can be conducted to approximately correct the overprediction bias, previous field and wind tunnel studies indicate that there are notable flaws in the PRIME building downwash theory. Although AERMOD/PRIME may provide accurate and unbiased estimates (within a factor of 2) for some building configurations, a major review and update are needed so that accurate estimates can be obtained for other building configurations for which significant overpredictions or underpredictions are common due to downwash effects [82]. Two categories of pollutants were analyzed: 1) volatile organic compounds (VOCs), the main source of which is evaporation and leakage from storage tanks; and 2) NO2, CO, PM10, and SO2, which are emitted from chimneys after processing and controlled by São Paulo State legislation (Fig. 1). Although Brazilian legislation does not regulate VOC concentrations, the dispersion curves of VOCs were also analyzed due to the impact of these compounds on health.
3. Ethical Statement
This research was approved by the Committee of Ethics in Research of the ABC School of Medicine, SP, Brazil and registered under number 087/2002. Statistical Analysis The likelihood of an individual’s developing rhinitis, sinusitis, pharyngitis, obstructive pulmonary diseases such as asthma and chronic obstructive pulmonary disease, conjunctivitis, dermatitis, and primary hypothyroidism in each city were computed for each combination and compared through Wald tests with Bonferroni correction for multiple comparisons. The association between both regions (Region 1 and Region 2) and diseases (rhinitis, sinusitis, pharyngitis, obstructive pulmonary diseases such as asthma and chronic obstructive pulmonary disease, conjunctivitis, dermatitis, and primary hypothyroidism) was compared by the chi-square test. The different incidences and relative complication risks of SARS-CoV-2 in association with diseases such as rhinitis, sinusitis, pharyngitis, obstructive pulmonary diseases, conjunctivitis, dermatitis and primary hypothyroidism were compared using the chi-square test. A polynomial regression that employed the logarithm of the odds of diseases as response and pollutant concentration up to the third degree, along with their interactions, was fitted to the pollutant, which exhibited a consistent trend of increase in concentration with increasing distance from the Capuava Petrochemical Complex (CPC).
4. Results
Demographic Characteristics and Determination of Risk Factors for SARS-CoV-2
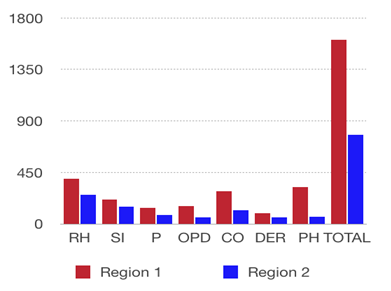
Among the total residents (2004), there were significant differences in the distributions of individuals with rhinitis, sinusitis, pharyngitis, obstructive pulmonary diseases, conjunctivitis, dermatitis and primary hypothyroidism in Region 1 and Region 2, demonstrating that there is a higher incidence of the evaluated diseases in areas closer to the CPC (Tables 1 and 2).
Table 1. Incidence of rhinitis (RH), sinusitis (SI), pharyngitis (P), obstructive pulmonary diseases (OPDs),
conjunctivitis (CO), dermatitis (DER) and primary hypothyroidism (PH) among individuals in Regions 1 and 2
(number of residents in each group = 1,002)
|
Disease/Relation |
RH |
SI |
P |
OPD |
CO |
DER |
PH |
TOTAL |
|
Region 1 |
395 |
214 |
143 |
156 |
284 |
95 |
324 |
1611 |
|
Region 2 |
253 |
150 |
76 |
55 |
121 |
58 |
65 |
778 |
|
Ratio Region 1/ Region 2 |
1,56 |
1,43 |
1,88 |
2,84 |
2,35 |
1,64 |
4,98 |
2,07 |
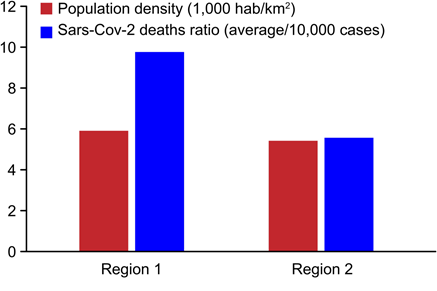
Table 2. Population densities, distances of regions from the Capuava Petrochemical Complex (CPC), and SARS- CoV-2 deaths per 10,000 cases.
|
Regions and industrial area distance |
Region 1 |
Region 2 |
|
Population density (1,000 hab/km2) |
5.90 |
5.40 |
|
Industrial area distance (km) |
1.20 |
8.00 |
|
SARS-CoV-2 deaths ratio (average/10,000 cases) |
9.7 |
5.5 |
Brazilian Institute of Geography and Statistics (IBGE). Coronavirus 2 (SARS-CoV-02) data for five prefectures of Paulista,the São Paulo State Health Secretariat and the Brazilian Ministry of Health Compared with other residents who reported having had one or more of the evaluated diseases, the residents in Region 1 had a higher relative risk of complications from SARS-CoV-2 than the residents in Region 2 associated with each disease between the regions (Fig. 2).
Table 2 indicates the population density, the distances of the regions from the CPC, and the number of SARS-CoV-2 deaths per 10,000 cases. Fig. 3 shows the absolute relation between the number of SARS-CoV-2 deaths in each region and the proximity of the region to the CPC. The tables and graphs demonstrate that the closer to Region 1 an individual lives, the higher is the risk of death from SARS-CoV-2.
Table 3 displays the number of confirmed cases and deaths due to coronavirus 2 (SARS-CoV-02) in Region 1 and Region 2 according to ABC Paulista prefectures, the São Paulo State Health Secretariat, and the Brazilian Ministry of Health.
Table 3: Number of confirmed cases and deaths relative to coronavirus 2 (SARS-CoV-02) in the cities of Santo André (North), Mauá and eastern São Paulo (Region 1) and Santo André (South), São Bernardo do Campo and São Caetano do Sul (Region 2).
|
Region |
Region 1 |
Region 2 |
||||
|
Cities |
Santo André (north) |
Mauá |
São Paulo (east)* |
Santo André (south) |
São Bernardo |
São Caetano |
|
Confirmed |
2,348 |
560 |
2,181 |
2,348 |
3,482 |
1,592 |
|
Deaths |
101.5 |
106 |
121 |
105.5 |
274 |
69 |
|
Deaths ratio/10,000 |
4.5 |
18.9 |
5.6 |
4.5 |
7.8 |
4.3 |
*São Rafael (São Mateus). Consulted on June 16, 2020, from Coronavirus 2 (SARS-CoV-02) data. Five prefectures of ABC Paulista, the State Health Secretariat of São Paulo and the Brazilian Ministry of Health
Based on the dispersion model, it was possible to estimate the average concentrations of NO2, CO, PM10, and VOCs for Region 1 and Region 2; these are presented in Table 4.
Table 4: Average concentrations of NO2, CO, PM10, SO2 and VOCs obtained via the simulated plume along with the distance from the CPC.
|
Location |
Distance (km) |
NO2 |
CO |
PM10 |
SO2 |
VOCs |
|
(µg/m3) |
(µg/m3) |
(µg/m3) |
(µg/m3) |
(µg/m3) |
||
|
Region 1 |
0.5 |
13.16 |
3.95 |
0.93 |
1.36 |
477.4 |
|
1 |
10.21 |
2.84 |
1.49 |
3.54 |
313.0 |
|
|
2 |
4.32 |
1.21 |
0.83 |
2.03 |
69.3 |
|
|
Region 2 |
7.5 |
2.15 |
0.69 |
0.19 |
0.34 |
10.5 |
|
8 |
1.82 |
0.59 |
0.16 |
0.33 |
9.2 |
|
|
8.5 |
1.84 |
0.59 |
0.16 |
0.31 |
10.3 |
Region 1, which is closer to the CPC than Region 2, showed higher average concentrations of all pollutants, and we also found differences in the incidence of rhinitis, sinusitis, pharyngitis, obstructive pulmonary diseases, conjunctivitis, dermatitis, and primary hypothyroidism in these two regions (Table 1).
5. Discussion
The global population, in late December 2019, has suffered from the COVID-19 created by the novel SARS-CoV-2 and the disease has spread rapidly internationally, raising global public health concerns [17,18]. Some individuals who contract SARS-CoV-2 and have a positive diagnosis are asymptomatic, meaning they have no symptoms and their health is not affected at all. However, other patients have a wide range of severe symptoms and may even die.In Italy, Pollán et al. [83], found that between 21.9% and 35.8% of patients were asymptomatic, with this cohort representing between 367,000 and 1,042,000 cases. It is interesting to note that five independent reports from different nations; the US [84], Netherlands [85], Germany [86], Singapore [87] and the United Kingdom [88], found that individuals who did not suffer from the infection yet had T cells specific for SARS-CoV-2-S, which could be derived from memory T cells created by historical infections with the global coronavirus that causes the common cold.
Different groups of people suffering from SARS-CoV-2: 80% have no or only mild symptoms, 15% have severe symptoms and require supplemental oxygen, and 5% have critical symptoms that require ventilation and other forms of invasive treatment [89,90] reviewed 11 patients infected with COVID-19 who responded in various ways to the virus and exhibited a number of symptoms. Some of the patients had a mild case of the virus without pneumonia; others were suffering from COVID-19 pneumonia but had no virus in their samples. Certain patients had standard cold symptoms for a short period of time, testing negative for SARS-CoV-2, but later (within the next 14 days) returned positive tests; these patients are considered long-term virus carriers and have come to experience mild cases of COVID-19 pneumonia [91].
The number of secondary infections of COVID-19 varies widely, and most outbreaks of many secondary infections occur under environmental factors [92, 93] and it is important to take steps to minimize the environmental factors that promote the infection. In this study, environmental factors are important for SARS-CoV-2 and our epidemiological surveys were conducted through questionnaires completed from July 2003 to June 2005 by residents living close to the Capuava Petrochemical Complex (CPC), and our findings showed different incidences of rhinitis, sinusitis, pharyngitis, OPD, conjunctivitis, dermatitis, and PH among a total of 2,004 residents from two different regions (Region 1, which is close to the CPC, and Region 2, which is further away; the respondents included 1,002 residents from each region) (Table 1) and differences in the risk of developing complications from SARS-CoV-2 for the residents of the two regions (Fig. 2). The transmission of other respiratory viruses, such as influenza or human coronaviruses [94,95,96] and the possible exposures for COVID-19 infection by SARS-CoV-2 are contact with facial membranes and inhalation of virus carried in airborne particles (inhalable or breathable particles), and environmental factors can have an influence significant in the infection. According to our results, the average concentrations of NO2, CO, PM10, SO2, and VOCs obtained from the simulated plumes in the cities studied are more significant in areas closer to the CPC (Table 4); this suggests that people living in those areas are more exposed to atmospheric pollutants than people who live far from the petrochemical industries and is consistent with many other studies [72, 97, 98].
The concentrations of atmospheric pollutants were analyzed in 2017 using numerical simulations with the AERMOD model using meteorological data for the period from 2005 to 2009, correlating these data with the research done in 2003 to 2005. Proper control of these environmental factors plays a significant role in preventing the spread of COVID-19 [99]. The residents of Region 1 are close to the CPC, it is essential to understand the potential dynamics of vulnerability to SARS-CoV-2 transmission and COVID-19 spread. Researchers have shown that short-term (hours, days) and long-term (months, years) exposure to PM2.5 is associated with aggravation of asthma and respiratory symptoms and with increased hospital admissions. The adverse health effects of acute (hours to days) and chronic (months to years) exposure to air pollution range from minor irritation of the upper respiratory system to an impact on morbidity in the form of chronic respiratory diseases (asthma, chronic obstructive pulmonary disease (COPD), heart disease, diabetes, hypertension, lung cancer, and death [ 5, 6, 9, 100, 101, 102]. More specifically, both acute and chronic exposure to fine particulate matter (PM2.5) has been shown to induce a systemic inflammatory response that can increase the risk of cardiorespiratory morbidity [7, 103, 104].
PM2.5 consists of particles with median aerodynamic diameter of 2.5 μm or less; particles of this size are small enough to invade even the smallest airways, and PM2.5 is associated with adverse health impacts. It generally results from activities that burn fossil fuels, such as operating fuel-powered automotive engines, smelting, and metal processing. The World Health Organization estimated that PM2.5 accounted for 3.1 million deaths and approximately 3.1% of the global disability-adjusted life years (DALYs) in 2010 [105]. Because the petroleum refining and petrochemical industries produce emissions at many stages of their operations, releasing pollutants such as volatile organic compounds, greenhouse gases, and particulate matter [98]. We believe that our findings are related to CPC emissions and that these emissions can significantly decrease the air quality and cause short-term and long-term health impacts for people living near the sites and in the same regions as these industries. It is important to know the atmospheric lifetime of VOCs to gain an understanding of the distances they might travel in air. A higher atmospheric lifetime indicates that VOCs can travel a greater distance in the atmosphere, possibly leading to impacts much farther from the emission source [98].
A wide variety of hazardous chemicals may originate from industrial facilities and other activities to the environment, and these chemicals are harmful to human health and to the environment [98]. Exposure to air pollution is a known risk factor for asthma and COPD. Individuals with asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome (ACOS) have a more rapid decline in lung function, more frequent exacerbations, and poorer quality of life than those with asthma or COPD alone [8]. Air pollution has indirect systemic effects linked to pro-inflammation and oxidation mechanisms of the lungs and alterations in immunological processes increase the population's vulnerability to COVID-19 [106]. This is consistent with studies that linked prolonged exposure to air pollution to acute respiratory inflammation, asthma attacks, and death from cardiorespiratory disease [107, 108, 109, 110], and the possibility of a detrimental effect of air pollution on the prognosis of patients affected by COVID-19 is plausible. In this study, the relative SARS-CoV-02 risk was higher for residents with rhinitis, sinusitis, pharyngitis, OPD, conjunctivitis, dermatitis, and PH who live closer to the CPC than the risk for residents who do not have these diseases, and these residents displayed increased vulnerability to COVID-19. Chemical composition influences ecotoxicity, cytotoxicity, and genotoxicity in different ways so that different biological outcomes are expected even in cases of similar number and mass concentrations [111]. According to the World Health Organization, 41 million people died from noncommunicable diseases (NCDs) worldwide in 2016; this represents 71% of global deaths [112]. An important cause of these NCDs is industrial air pollution. However, its quantity and composition vary considerably with location. As an example, living in proximity to industrial facilities appears to be an adverse condition. Environmental air pollution contributes to this huge burden through the initiation and promotion of respiratory diseases that are leading causes of death [113]. The respiratory tract is one of the parts of the body that is most involved in susceptibility to air pollution. In particular, asthma and COPD had a major impact on the overall worldwide mortality of 4.2 million caused by respiratory diseases in 2008 [113].
In addition to mortality, the chronic nature of asthma and COPD causes considerable morbidity. In 2012, 6.62 million disability-adjusted life years (DALYs) for COPD alone worldwide [114] indicated an immense socioeconomic impact of non-communicable respiratory diseases. It is well established that individuals with both asthma and COPD and those with asthma - COPD overlap syndrome (ACOS) have more frequent exacerbations, a more rapid decline in lung function, and worse quality of life than individuals with asthma or COPD alone [ 115, 116, 117, 118]. It is recognized that these conditions or their expression may be influenced by both host and environmental factors [118] including exposure to air pollution. Research has shown that short-term exposure to O3 is associated with decreased lung function [119] and increased hospitalizations for COPD [6, 120, 121], whereas long-term exposure to O3 is associated with increased respiratory mortality [122]. Similarly, ground-level ozone (O3) or ambient O3 pollution is another major source of air pollution that is also associated with adverse health impacts. The World Health Organization has estimated that 0.1% of the global DALYs in 2010 can be attributed to O3 exposure [123].
Concentrations of NO2, CO, PM10, SO2, and VOCs that are above the levels specified in the São Paulo air quality standards are present inside the CPC area (Region 1); this can be related to the main signs and symptoms associated with exposure to VOCs, including conjunctival irritation, nose and throat discomfort, allergic skin reactions and dyspnea [124]. The health effects of VOCs may include eye, nose, and throat irritation, which are also the initial symptoms of COVID-19. It is essential to know the atmospheric lifetime of VOCs to understand the distances they might travel in the air. A higher atmospheric lifetime indicates that VOCs can travel a greater distance in the atmosphere, possibly impacting areas much farther away from the emission source [98]. This supports our findings, which showed that the highest levels of VOCs were localized in proximity to the CPC (Table 4); the levels in the limited area of the CPC were higher than those allowed in the standard for air quality, and increased incidences of rhinitis, sinusitis, pharyngitis, OPD, conjunctivitis, dermatitis, and PH were found in this area. According to our results, 143 residents of the region closer to the CPC had pharyngitis (Table 1), affected by air pollution. In the case of COVID-19, one of the initial symptoms is sore throat. Hence, while sore throat may be the symptom described by the patient, the examination might also reveal nasopharyngitis [125], and residents near the CPC should be alert to the risk of COVID-19. Brazil is currently among the group of countries with the highest prevalence of allergic rhinitis in the world [126]. Rhinosinusitis (RS) is characterized by inflammation of the mucosae of the nose and paranasal sinuses, and it is one of the most prevalent disorders of the upper airways. According to our results, 395 of the residents in the region closer to the CPC had rhinitis, and 214 of them had sinusitis (Table 1). Due to their high degree of contact with the environment and their limited defense mechanisms, the nasal cavity is one of the organic systems that are most vulnerable to environmental pollutants [127]; that ould tend to increase the vulnerability of this population to COVID-19. Riediker et al. [128] found that rhinoconjunctival tissue is sensitive to irritant stimuli during ongoing allergic inflammation; thus, symptoms of allergic rhinoconjunctivitis might be exacerbated in areas with increased levels of air pollutants. Conjunctivitis is most associated with O3 and NO2 exposure, although PM10 and SO2 exposure are also correlated [129]. Our findings showed that levels of NO2, SO2, and MP10 that exceeded the levels indicated in the São Paulo air quality standard were localized in proximity to the CPC, where we found more cases of conjunctivitis. According to our results, 284 of the residents in Region 1 had conjunctivitis (Table 1). The dense innervation of the ocular surface is extremely sensitive to chemical substances present in the environment. Additionally, human eyes are protected only by a thin layer of tear film, causing them to be very susceptible to the harmful effects of air pollution [129, 130, 131]; if these residents are affected by COVID-19, they could be at higher risk of dangerous eye complications such as retinopathies. Air pollution is associated with COPD risk [132].It may also be a factor in transforming asthma into COPD [8].
In this study, 156 residents of the region closer to the CPC had OPD (Table 1), a condition that is also affected by air pollution. It causes breathlessness in most patients with severe chronic respiratory disease, and in cases of COVID-19, one of the initial symptoms is shortness of breath. Elevated exposure to NO2 has been associated with hypertension [133], heart and cardiovascular diseases [134, 135, 136], increased rates of hospitalization [134, 137], significant deficits in the development of lung function in children [138, 139], reduced lung function or lung injury in adults [140, 141] and diabetes [133]. The strategy employed by SARS-CoV-2 to enter the cell encompasses the binding of the virus peak glycoprotein to host cell receptors using ACE2 and the transmembrane cell protease serine 2 (TMPRSS2) [142]. ACE2 is a transmembrane protein expressed on the surface of several cells in the body, such as the epithelium of the respiratory system. Certainly, several studies have already demonstrated the relationship between the ACE2 protein and the entry mechanisms of some coronaviruses, such as HCoV-NL63, SARS-CoV and the new SARS-CoV-2 (causer of COVID-19). Above all, as SARS-CoV-2 enters the cell mainly through the binding of the spike protein of the virus with the ACE2 receptor, the increased expression of this molecule on the surface of these patients' cells may increase the chance of infection and even influence the severity of the disease [143].
Interdisciplinary research in air pollution and biomedical science can explain how exposure to air pollutants may affect respiratory viral infections, especially in populations already at risk of increased morbidity and mortality rates after infection with respiratory viruses such as SARS-CoV-2. Aging has been associated with a decline in immune defenses and respiratory function that can result in a higher predisposition to respiratory infections. Moreover, among the chronic diseases that affect the elderly population, COPD and asthma can accelerate pulmonary function decline and increase mortality risk [144]. Our findings showed that higher values of NO2 were localized in regions that are in proximity to the CPC (Table 4). NO2 is a common tracer of air pollution/industrial activity associated with morbidity and mortality [145,146]. This study shows that the closer participants lived to Region 1, the higher was their risk of death from SARS-CoV-2. This finding should direct the attention of authorities to these populations. Exposure to ambient air pollution has recently been implicated in the occurrence and development of autoimmune diseases [147]. Autoimmune diseases represent a broad spectrum of disorders characterized by direction of the body's immune responses against its own tissues, resulting in prolonged inflammation and subsequent tissue damage [147]. According to our results, 324 residents of Region 1 had PH (Table 1). In iodine-sufficient regions, the primary cause of PH is CAT [148] and sufficiency of iodine [73, 149], and an increase in CAT incidence over the years [70] was demonstrated in a previous study in Region 1. This finding could draw attention to a risk factor for complications of SARS-CoV-2. Previous studies have highlighted that pathogenic viruses mutate to adapt to their host, bypassing the immune system and increasing their infectivity. During the early stages of the pandemic, one study [150] reported the presence of 14 protein S-associated mutations. Of these 14 mutations, only one mutation (the D614G) proved essential for increasing virus incidence and global spread [150]. Region 1 is unique because its residents live near petrochemical industries, and environmental factors could affect the cases of rhinitis, sinusitis, pharyngitis, OPD, conjunctivitis, and PH. This chronic exposure could be an important contributor to disease and to the higher risk of complications of SARS-CoV-2 in individuals with long term exposure to air pollutants.
China, where the COVID-19 epidemic started, is severely affected by air pollution [145,146]. An association between short-term exposure to air pollution and COVID-19 infection has also been described for the recent outbreak in China [65]. In recent decades, environmental pollution has increased and air quality has deteriorated in China, mainly due to the country's rapid industrialization [151]. The spatial analysis performed in our study was conducted on a regional scale and combined with the number of deaths that occurred in 66 administrative regions in Italy, Spain, France, and Germany [152]. The results showed that of the 4,443 fatality cases, 3,487 (78%) occurred in five regions located in northern Italy and central Spain. Additionally, the same five regions show the highest NO2 concentrations and downwards airflow, a factor that prevents efficient dispersion of air pollution. These results indicate that long-term exposure to NO2 may be one of the most critical contributors to fatalities caused by the SARS-CoV-2 virus in these regions and perhaps across the whole world [152].
Since the presence of comorbidities appeared to be a determinant of the etiology and severity of COVID-19 symptoms [14, 17, 153], a role of atmospheric pollution in contributing to the high levels of SARS-CoV-2 lethality in northern Italy has been hypothesized [106]. In this study, we also show that higher values of NO2 localized in regions in proximity to CPC could increase the risk of complications in cases of SARS-CoV-2. Air pollution has been termed the "silent killer" by the World Health Organization [154] because its effects often go unnoticed or are not easily measured. In the present case of SARS-CoV-2, air pollution may facilitate the upsurge of more severe symptoms of the disease of the eye [129, 130, 131], nose [127], and throat [125], since the virus may travel through the cranial nerves, producing anosmia, ageusia (lack of taste) and possibly retinopathy. To date, only a limited number of epidemiological studies have actually identified and/or quantified the risk of rhinitis, sinusitis, pharyngitis, OPD, conjunctivitis, dermatitis, and PH. Our study adds evidence that chronic exposure to air pollution may contribute to significant morbidity and that it may contribute to SARS-CoV-2 risk in individuals with preexisting diseases. Better knowledge of the risks posed by exposure to environmental pollutants and SARS-CoV-2 may help us understand and develop preventive strategies to modify the progressive deterioration of lung function that leads to death.
The results of this survey may help to identify susceptible populations that are vulnerable to air pollution and to target preventive strategies. In addition to the increasing frequency of health service use, we can put forth the hypothesis that individuals living near the CPC, a petrochemical industrial complex, have more symptoms that affect their daily lives and have lower health-related quality of life. This is a pioneering study related to inflammatory diseases, such as diseases of the upper and lower airways, conjunctivitis, thyroid diseases, and air pollution, and of the impact of these diseases on SARS-CoV-2 pandemic infection in a population living in the area surrounding a petrochemical complex in Brazil. We recommend that patients affected by SARS-CoV-2 be followed up for a long period of time after medical discharge as they may experience consequences of COVID-19 such as vascular complications and pulmonary fibrosis. Given the magnitude of the problem of air pollution and the major global health problems generated by SARS-CoV-2 and COVID-19, it is essential that any link between air pollution and respiratory, immunological and hormonal diseases be firmly established.
This study has several strengths, including its use of large-scale population-based data covering a 15-year period and its use of well-defined geographic regions in residents living in the CPC influence area in the Greater ABC region. The data used in this study were also linked to data on provincial air pollution and to population survey data gathered over a period of multiple years to permit a broader evaluation of the impact of risk factors for SARS-CoV-2 and the development of COVID-19 in the population. The study is also strengthened by its adjustment for important health risk factors, including rhinitis, sinusitis, pharyngitis, OPD, conjunctivitis, dermatitis, and PH, in the analysis of the relationship between air pollution and SARS-CoV-2. Future research in this area should be conducted.
6. Conclusions
This study found that residents with rhinitis, sinusitis, pharyngitis, obstructive pulmonary diseases, conjun-ctivitis, dermatitis, and primary hypothyroidism who were exposed to higher levels of NO2, CO, PM10, SO2, and VOCs had an increased risk of compli-cations from SARS-CoV-2 infection.
Given the asymptomatic characteristic of coronavirus disease in its initial development and the risks posed by its complications, we suggest that SARS-CoV-2 be continuously evaluated in chemical plant employees and in residents living near industrial areas. To protect public health from the transmission of SARS-CoV-2, further research into the dynamics of COVID-19 infection in polluted regions is urgently needed. Furthermore, it is necessary to develop control measures, guidelines and strategies to maintain atmospheric air quality at an adequate level. To reduce the possibility of the virus mutating and causing more waves of infection, an early diagnosis quickly and accurately is essential, as people living in polluted regions are more vulnerable to disease and complications with sequelae or even not having a recovery evolving towards death.
List of abbreviations
SARS-CoV-2 - severe acute respiratory syndrome coronavirus 2
OPD - obstructive pulmonary diseases
PH - primary hypothyroidism
CPC - Capuava Petrochemical Complex
WRF - Weather Research Forecast
NO2 - nitrogen dioxide
CO - carbon monoxide
PM10 - particulate matter
SO2 - sulfur dioxide
VOCs - Volatile organic compounds
WHO - World Health Organization
COVID-19 - Corona Virus Disease 2019
COPD - chronic obstructive pulmonary diseases
PIC - petrochemical industrial complex
NOx - nitrogen oxides
HNO3 - nitric acid
O3 - ozone
PM2.5 - particulate matter
SP - São Paulo State
IBGE - Brazilian Institute of Geography and Statistics
CAT - chronic autoimmune thyroiditis
AMS - American Meteorological Society
U.S. EPA - United States Environmental Protection Agency
PRIME - Plume Rise Model Enhancements
EBD - building dimension study
WQ - Written questionnaires
ISAAC - International Study of Asthma and Allergies in Childhood
ECRHS II - European Community Respiratory Health Survey II
RH - rhinitis
SI- sinusitis
oP- pharyngitis
CO - conjunctivitis
DER – dermatitis
IgG - antiviral immunoglobulin G
IgM - immunoglobulin M
ARDS - acute respiratory distress syndrome
MERS-CoV - Middle East respiratory syndrome coronavirus
CoVs - Coronaviruses
SM - Supplementary material
Declarations
Ethics approval and consent to participate This research was approved by the Committee of Ethics in Research of the ABC School of Medicine, SP, Brazil, and registered under number 087/2002.
Consent for publication
Not applicable
Availability of data and materials
The data generated and used in the analysis of the present study are included in published articles.
Competing interests
The authors declare that they have no competing interests.
Funding
This research received no external funding.
Authors' Contributions
Maria Angela Zaccarelli-Marino participated in the design of the study, the acquisition of the data, the interpretation of the results, and the drafting of the manuscript and critically revised the manuscript. Thalles Zaccarelli Balderi participated in the design of the study, the acquisition of the data, the interpretation of the results, the statistical analysis and the critical revision of the manuscript. Felipe Mingorance Crepaldi participated in the design of the study, the acquisition of the data, the interpretation of the results and the literature revision. Rudá Alessi participated in the design of the study and the acquisition of the data and critically revised the manuscript. Marco Antonio Garcia Martins participated in the design of the study and the interpretation of the results. He also critically revised the manuscript and supervised the study. All authors have read and approved the final manuscript.
Acknowledgments
The authors are grateful to the residents of Santo André, Mauá, São Paulo, São Bernardo do Campo and São Caetano do Sul, São Paulo State, Brazil, for their participation and collaboration. We would also like to thank the parents of the younger participants and those responsible for providing the information for their understanding. The authors are grateful to Professor Maria de Fatima Andrade, PhD, of the University of São Paulo, Department of Atmospheric Sciences of the Institute of Astronomy, Geophysics and Atmospheric Sciences, São Paulo, SP, Brazil, for her support and collaboration regarding atmospheric pollutants. The authors are grateful to the Public Ministry of São Paulo state, Brazil, for their collaboration, support and trust
References
- Ribeiro H, Assunção J. Historical overview of air pollution in Sao Paulo metropolitan area, Brazil: influence of mobile sources and related health effects. 2001. Department of Environmental Health-University of Sao Paulo, Brazil (2001).
- Zhang J, Mauzerall DL, Zhu T, Liang S, Ezzati M, Remais JV. Environmental health in China: progress towards clean air and safe water. Lancet 375 (2010): 1110-1119.
- Billionnet C, Sherrill D, Annesi-Maesano I. Estimating the health effects of exposure to multi-pollutant Ann Epidemiol 22 (2012): 126-141.
- Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 117 (2012): 100-111.
- Burnett RT, Cakmak S, Brook JR. The effect of the urban ambient air pollution mix on daily mortality rates in 11 Canadian cities. Can J Public Health 89 (1998): 152-156.
- Ko FWS, Hui DSC. Air pollution and chronic obstructive pulmonary disease. Respirology 17 (2012): 395-401.
- Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc 52 (2002): 470-484.
- To T, Zhu J, Larsen K et al. Progression from asthma to chronic obstructive pulmonary disease. Is air pollution a risk factor? Am J Respir Crit Care Med 194 (2016): 429-438.
- Lavigne E, Villeneuve PJ, Cakmak S. Air pollution and emergency department visits for asthma in Windsor, Canada. Can J Public Health 103 (2012): 4-8.
- Rovira E, Cuadras A, Aguilar X, Esteban L, Borràs-Santos A, Zock JP, Sunyer J. Asthma, respiratory symptoms and lung function in children living near a petrochemical site. Environ Res 133 (2014): 156-163.
- Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 120 (2007): 1031-1035.
- Liu Y, Yan S, Poh K, Liu S, Iyioriobhe E, Sterling DA. Impact of air quality guidelines on COPD sufferers. Int J Chron Obstruct Pulmon Dis 11 (2016): 839-872.
- Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest 147 (2015): 31-45.
- Wu F, Zhao S, Yu B et al. A new coronavirus associated with human respiratory disease in China. Nature 579 (2020): 265-269.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382 (2020): 727–33.
- Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 272 (2020): ciaa272.
- Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395 (2020): 507-513.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8 (2020): 420–2.
- WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. Geneva: World Health Organization (2020).
- Gorbalenya A, Baker S, Baric R, de Groot R, Drosten C, Gulyaeva A et al. The species severe acutev respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5 (2020): 536–544.
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature 581 (2020): 221–4.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579 (2020): 270–3.
- Li Q, Guan X, Wu P et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382 (2020): 1199-1207.
- Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (2020): 497-506.
- Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill 25 (2020): 2000062.
- Bi Q, Wu Y, Mei S et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 20 (2020): 911-919.
- Chan JFW, Yuan S, Kok KH et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395 (2020): 514-523.
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 172 (2020): 577-582.
- Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, Cui Y, Fu RB, Dong YZ, Chi XY, Zhang MY, Liu K, Cao C, Liu B, Zhang K, Gao YW, Lu B, Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis 26 (2020): 1583–91.
- Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, Sun L, Duan Y, Cai J, Westerdahl D, Liu X, Xu K, Ho KF, Kan H, Fu Q, Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 582 (2020): 557–60.
- Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K. Air, surface environmental, and personal protective equipment contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 323 (2020): 1610–2.
- West R, Michie S, Rubin GJ, Amlôt R. Applying principles of behaviour change to reduce SARS-CoV-2 transmission. Nat Hum Behav 4 (2020): 451–9.
- Ye G, Lin H, Chen S, Wang S, Zeng Z, Wang W, Zhang S, Rebmann T, Li Y, Pan Z, Yang Z, Wang Y, Wang F, Qian Z, Wang X. Environmental contamination of SARS-CoV-2 in healthcare premises. J Infect (2020): S0163-4453(20)30260-7.
- Cheng VCC, Wong SC, Chen JHK, Yip CCY, Chuang VWM, Tsang OTY, Sridhar S, Chan JFW, Ho PL, Yuen KY. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol 41 (2020): 493–8.
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV- 2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 55 (2020): 105924.
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26 (2020): 681–7.
- Transmission of SARS-CoV-2: implications for infection prevention precautions. Scientific brief on 9 July 2020. Geneva: World Health Organization (2020).
- Zhong NS, Zheng BJ, Li YM, Poon Xie ZH, Chan K H, Li P, Tan SY, Chang Q, Xie JP, Liu X Q, Xu J, Li D. X, Yuen KY, Peiris & Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet 362 (2003): 1353–1358.
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, & Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. The N Engl J Med 367 (2012): 1814–1820.
- van Regenmortel MH, Fauquet C M, Bishop DH, Carstens E, Estes M, Lemon S. Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press San Diego (2000) USA.
- Adams MJ, Lefkowitz EJ, King AM, Harrach B, Harrison RL, Knowles NJ, et al. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses . Archives of virology 161 (2016): 2921–2949.
- Yin Y, & Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. (Carlton, Vic.) 23 (2018): 130–137.
- Tang Q, Song Y, Shi M, Cheng Y, Zhang W, & Xia X Q. Inferring the hosts of coronavirus using dual statistical models based on nucleotide composition. Scientific reports 5 (2015): 17155.
- Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, Wei S, Deng Y, Liu J, Liu HG, Yang M, Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 133 (2020): 1032-1038.
- Shi S, Qin M, Shen B et al. Association of cardiac injury with mortality in hospitalized patients With COVID-19 in Wuhan, China. JAMA Cardiol 5 (2020): 802-810.
- Kakodkar P, Kaka N, Baig MN. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus 12 (2020): e7560.
- Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus- infected pneumonia in Wuhan, China. JAMA 323 (2020): 1061-1069.
- Apolone G, Montomoli E, Manenti A, Boeri M, Sabia F, Hyseni I, et al. Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy. Tumori (2020); 300891620974755.
- Long, Q-X, Liu, B-Z, Deng, H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26 (2020): 845–848.
- Johns Hopkins University (2020) Coronavirus. http://www.conre3.org.br>portal>coronavirus. Accessed 17 June (2020)
- Su W, Wu X, Geng X, Zhao X, Liu Q, Liu T. The short-term effects of air pollutants on influenza-like illness in Jinan, China. BMC Public Health 19 (2019): 1319.
- Lewis D. Is the coronavirus airborne? Experts can't agree. Nature 580 (2020): 175.
- Godoi RH, Godoi AF, Junior SJG et al. Healthy environment--indoor air quality of Brazilian elementary schools nearby petrochemical industry. Sci Total Environ 463-464 (2013): 639-646.
- Rodríguez S, Querol X, Alastuey A, Viana MM, Alarcón M, Mantilla E, Ruiz CR. Comparative PM10-PM2.5 source contribution study at rural, urban and industrial sites during PM episodes in Eastern Spain. Sci Total Environ 328 (2004): 95-113.
- Wichmann FA, Müller A, Busi LE, Cianni N, Massolo L, Schlink U, Porta A, Sly PD. Increased asthma and respiratory symptoms in children exposed to petrochemical pollution. J Allergy Clin Immunol 123 (2009): 632-638.
- Tanyanont W, Vichit-Vadakan N. Exposure to volatile organic compounds and health risks among residents in an area affected by a petrochemical complex in Rayong, Thailand. Southeast Asian J Trop Med Public Health 43 (2012): 201-211
- Hsu CY, Chiang HC, Shie RH, Ku CH, Lin TY, Chen MJ, Chen NT, Chen YC . Ambient VOCs in residential areas near a large-scale petrochemical complex: spatiotemporal variation, source apportionment and health risk. Environ Pollut 240 (2018): 95-104.
- Omidvarborna H, Baawain M, Al-Mamun A. Ambient air quality and exposure assessment study of the Gulf cooperation council countries: a critical review. Sci Total Environ 636 (2018): 437-448.
- Minnesota Department of Health. Volatile organic compounds (VOCs) in your home (2010).
- Chen TM, Gokhale J, Shofer S, Kuschner WG. Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci 333 (2007): 249-256.
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health 12 (2013): 43.
- Khoder MI. Atmospheric conversion of sulfur dioxide to particulate sulfate and nitrogen dioxide to particulate nitrate and gaseous nitric acid in an urban area. Chemosphere 49 (2002): 675-684.
- Frontera A, Cianfanelli L, Vlachos K, Landoni G, Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: the "double-hit" hypothesis. J Infect 81 (2020): 255-259.
- Moraes et al. Moraes AC, Ignotti E, Netto PA, Jacobson Lda S, Castro H, Hacon Sde S (2010) Wheezing in children and adolescents living next to a petrochemical plant in Rio Grande do Norte, Brazil. J Pediatr 86 (2010): 337-344.
- Zhu Y, Xie J, Huang F, Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci Total Environ 727 (2020): 138704.
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res 7 (2020): 11.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395 (2020): 1033-1034.
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 76 (2012): 16-32.
- IBGE (2016) Brazilian institute of geography and statistics (2016).
- Zaccarelli-Marino MA. Chronic autoimmune thyroiditis in industrial areas in Brazil: a 15-year survey. J Clin Immunol 32 (2012): 1012-1018.
- Zaccarelli-Marino MA, André CDS, Singer JM. Overt primary hypothyroidism in an industrial area in São Paulo, Brazil: the impact of public disclosure. Int J Environ Res Public Health 13 (2016): 1161.
- Zaccarelli-Marino MA, Alessi R, Balderi TZ, Martins MAG. Association between the occurrence of primary hypothyroidism and the exposure of the population near to industrial pollutants in São Paulo State, Brazil. Int J Environ Res Public Health 16 (2019): 3464.
- Marino MA, Martins LC, Esteves RZ, Kasamatsu TS, Maciel RM. Urinary iodine in patients with auto-immune thyroid disorders in Santo André, SP, is comparable to normal controls and has been steady for the last 10 years. Arq Bras Endocrinol Metabol 53 (2009): 55-63.
- IBGE (2020) Brazilian institute of geography and statistics (2020).
- Vanna AT, Yamada E, Arruda LK, Naspitz CK, Solé D. International study of asthma and allergies in childhood: validation of the rhinitis symptom questionnaire and prevalence of rhinitis in schoolchildren in São Paulo, Brazil. Pediatr Allergy Immunol 12 (2001): 95-101.
- Asher MI, Keil U, Anderson HR et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 8 (1995): 483-491.
- Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 351 (1998): 1225-1232.
- Jarvis D, Burney P. The European community respiratory health survey II. Eur Respir J 20 (2002): 1071-1079.
- Brazil Ministry of Health. Painel coronavirus (2020).
- Air emissions factors and quantification (2008). Accessed 9 December 2009
- Kumar A, Patil RS, Dikshit AK, Islam S, Kumar R. Evaluation of control strategies for industrial air pollution sources using American Meteorological Society/Environmental Protection Agency Regulatory Model with simulated meteorology by Weather Research and Forecasting Model. J Clean Prod 116 (2016): 110-117.
- Petersen RL, Guerra SA, Bova AS. Critical review of the building downwash algorithms in AERMOD. J Air Waste Manag Assoc 67 (2017): 826-835.
- Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez OM, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet (London, England) 396 (2020): 535–544.
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 181 (2020): 1489–1501.e15.
- Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba N, Endeman H, et al. Phenotype and kinetics of SARS CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Science immunology 5 (2020): eabd2071.
- Braun J, Loyal, Frentsch M, Wendisch D, Georg P, Kurth F, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 587 (2020): 270–274.
- Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584 (2020): 457–462.
- Meckiff BJ, Ramírez-Suástegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4+ T Cells in COVID-19. Cell 183 (2020): 1340–1353.e16.
- Rokni M, Ghasemi V & Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: Comparison with SARS and MERS. Reviews in medical virology 30 (2020): e2107.
- Dong X, CaoYY, Lu XX, Zhang JJ, Du H, Yan YQ, Akdis CA & Gao YD. Eleven faces of coronavirus disease 2019. Allergy 75 (2020): 1699–1709.
- Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L & Wang M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 323 (2020): 1406–1407.
- Furuse Y, Sando E, Tsuchiya N, Miyahara R, Yasuda I, Ko YK, et al. Clusters of coronavirus disease in communities, Japan, January–April 2020. Emerg Infect Dis (2020).
- Nishiura H, Oshitani H, Kobayashi T, Saito T, Sunagawa T, Matsui T, Wakita T, MHLW COVID-19 Response Team, Motoi Suzuki. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19), medRxiv preprint (2020): 0029272.
- Nicas M, Sun G. An integrated model of infection risk in a health care environment. Risk Anal 26 (2006): 1097–108.
- Nicas M, Jones RM. Relative contributions of four exposure pathways to influenza infection risk. Risk Anal 29 (2009): 1292–303.
- Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 19 (2019): 101.
- Kalabokas PD, Hatzianestis J, Bartzis JG, Papagiannakopoulos P. Atmospheric concentrations of saturated and aromatic hydrocarbons around a Greek oil refinery. Atmos Environ 35 (2001): 2545-2555.
- Ragothaman A, Anderson W. Air quality impacts of petroleum refining and petrochemical industries. Environments 4 (2017): 66.
- Azuma K, Kagi N, Kim H, Hayashi M. Impact of climate and ambient air pollution on the epidemic growth during COVID-19 outbreak in Japan. Environ Res 190 (2020): 110042.
- Andersen ZJ, Raaschou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, Tjønneland A, Overvad K, Sørensen M. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care 35 (2012): 92-98.
- Hoffmann B, Luttmann-Gibson H, Cohen A et al. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ Health Perspect 120 (2012): 241-246.
- Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, Schlaug G, Gold DR, Mittleman MA. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med 172 (2012): 229-234.
- Fink R, Erzen I, Medved S. Environmentally induced health impacts among elderly with cardio-vascular disease. Health Med 6 (2012): 3841-3849.
- Nachman KE, Parker JD. Exposures to fine particulate air pollution and respiratory outcomes in adults using two national datasets: a cross-sectional study. Environ Health 11 (2012): 25.
- Health effects of particulate matter. WHO (2013).
- Contini D, Costabile F. Does air pollution influence COVID-19 outbreaks? Atmosphere 11 (2020): 5.
- Bates DV, Baker-Anderson M, Sizto R. Asthma attack periodicity: a study of hospital emergency visits in Vancouver. Environ Res 51 (1990): 51-70.
- Schwartz J, Dockery DW. Particulate air pollution and daily mortality in Steubenville, Ohio. Am J Epidemiol 135 (1992): 12-19.
- Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 147 (1993): 826-831.
- Dockery DW, Pope CA. Acute respiratory effects of particulate air pollution. Annu Rev Public Health 15 (1994): 107-132.
- Lionetto MG, Guascito MR, Caricato R, Giordano ME, De Bartolomeo AR, Romano MP, Conte M, Dinoi A, Contini D. Correlation of oxidative potential with ecotoxicological and cytotoxicological potential of PM10 at an urban background site in Italy. Atmosphere 10 (2019): 733.
- Noncommunicable diseases (2018).
- Global status report on noncommunicable diseases (2010).
- Ambient air pollution: a global assessment of exposure and burden of disease. (2016)
- Kauppi P, Kupiainen H, Lindqvist A, Tammilehto L, Kilpeläinen M, Kinnula VL, Haahtela T, Laitinen T. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma 48 (2011): 279-285.
- Miravitlles M, Soriano JB, Ancochea J, Muñoz L, Duran-Tauleria E, Sánchez G, Sobradillo V, García-Río F.Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med 107 (2013): 1053-1060.
- Menezes AMB, Oca MMD, Pérez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, Muiño A, Jardim JRB, Valdivia G, Tálamo C. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 145 (2014): 297-304.
- Papaiwannou A, Zarogoulidis P, Porpodis K et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 6 (2014): S146-S151.
- Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, Washko GR, O'Connor GT, Mittleman MA. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med 188 (2013): 1351-1357.
- Medina-Ramón M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol 163 (2006): 579-588.
- Halonen JI, Lanki T, Tiittanen P, Niemi JV, Loh M, Pekkanen J. Ozone and cause-specific cardiorespiratory morbidity and mortality. J Epidemiol Community Health 64 (2010): 814-820.
- Jerrett M, Burnett RT, Pope CA, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med 360 (2009): 1085-1095.
- Global burden of diseases. World Health Organization (2011).
- United States Environmental Protection Agency. Indoor Air Quality (IAQ). Technical Overnew of Volatile Organic Compounds (2017).
- Renner B, Mueller CA, Shephard A. Environmental and non-infectious factors in the aetiology of pharyngitis (sore throat). Inflamm Res 61 (2012): 1041-1052.
- Solé D, Wandalsen GF, Camelo-Nunes IC, Naspitz CK. Prevalence of symptoms of asthma, rhinitis, and atopic eczema among Brazilian children and adolescents identified by the International Study of Asthma and Allergies in Childhood (ISAAC) - Phase 3. J Pediatr (Rio J) 82 (2006): 341-346.
- Shusterman D. Upper respiratory tract disorders. In: LaDou J (ed) Occupational and environmental medicine. Appleton and Lange, Stanford, CT (1997): 291-304
- Riediker M, Monn C, Koller T, Stahel WA, Wüthrich B. Air pollutants enhance rhinoconjunctivitis symptoms in pollen-allergic individuals. Ann Allergy Asthma Immunol 87 (2001): 311-318.
- Chang CJ, Yang HH, Chang CA, Tsai HY. Relationship between air pollution and outpatient visits for nonspecific conjunctivitis. Invest Ophthalmol Vis Sci 53 (2012): 429-433.
- Hong J, Zhong T, Li H et al. Ambient air pollution, weather changes, and outpatient visits for allergic conjunctivitis: a retrospective registry study. Sci Rep 6 (2016): 23858.
- Fu Q, Mo Z, Lyu D, Zhang L, Qin Z, Tang Q, Yin H, Xu P, Wu L, Lou X, Chen Z, Yao K. Air pollution and outpatient visits for conjunctivitis: a case-crossover study in Hangzhou, China. Environ Pollut 231 (2017): 1344-1350.
- Schikowski T, Adam M, Marcon A et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur Respir J 44 (2014): 614-626.
- Shin S, Bai L, Oiamo TH, Burnett RT, Weichenthal S, Jerrett M, Kwong JC, Goldberg MS, Copes R, Kopp A, Chen H. Association between road traffic noise and incidence of diabetes mellitus and hypertension in Toronto, Canada: a population-based cohort study. J Am Heart Assoc 9 (2020): e013021.
- Mann JK, Tager IB, Lurmann F, Segal M, Quesenberry CP, Jr., Lugg MM, Shan J, Van Den Eeden SK. Air pollution and hospital admissions for ischemic heart disease in persons with congestive heart failure or arrhythmia. Environ Health Perspect 110 (2002): 1247-1252.
- Pope CA, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 109 (2004): 71-77.
- Gan WQ, Davies HW, Koehoorn M, Brauer M. Association of long-term exposure to community noise and traffic-related air pollution with coronary heart disease mortality. Am J Epidemiol 175 (2012): 898-906.
- Abbey DE, Colome SD, Mills PK, Burchette R, Beeson WL, Tian Y. Chronic disease associated with long- term concentrations of nitrogen dioxide. J Expo Anal Environ Epidemiol 3 (1993): 181-202.
- Gauderman WJ, McConnell R, Gilliland F, London S, Thomas D, Avol E, Vora H, Berhane K, Rappaport EB, Lurmann F, Margolis HG, Peters J. Association between air pollution and lung function growth in southern California children. Am J Respir Crit Care Med 162 (2000): 1383-1390.
- Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med 164 (2001): 2067-2072.
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353 (2005): 1685-1693.
- Bowatte G, Erbas B, Lodge CJ et al. Traffic-related air pollution exposure over a 5-year period is associated with increased risk of asthma and poor lung function in middle age. Eur Respir J 50 (2017): 1602357.
- Djomkam A, Olwal CO, Sala TB & Paemka L. Commentary: SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Frontiers in oncology 10 (2020): 1448.
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, and Acton S. “A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9”. Circulation Research 87 (2000): e1–e9.
- Bentayeb M, Simoni M, Baiz N, Norback D, Baldacci S, Maio S, Viegi G, Annesi-Maesano I. Adverse respiratory effects of outdoor air pollution in the elderly. Int J Tuberc Lung Dis 16 (2012): 1149-1161.
- He L, Zhang S, Hu J, Li Z, Zheng X, Cao Y, Xu G, Yan M, Wu Y. On-road emission measurements of reactive nitrogen compounds from heavy-duty diesel trucks in China. Environ Pollut 262 (2020): 114280.
- He MZ, Kinney PL, Li T, Chen C, Sun Q, Ban J, Wang J, Liu S, Goldsmith J, Kioumourtzoglou MA. Short- and intermediate-term exposure to NO(2) and mortality: a multi-county analysis in China. Environ Pollut 261 (2020): 114165.
- Zhao CN, Xu Z, Wu GC et al. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev 18 (2019): 607-614.
- Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med 335 (1996): 99-107.
- de Freitas CU, Campos RAG, Silva MAR, Panachão MR, de Moraes JC, Waissmann W, Roberto Chacra A, Maeda MY, Rodrigues RSM, Belchor JG, Barbosa SO, Santos RT. Can living in the surroundings of a petrochemical complex be a risk factor for autoimmune thyroid disease? Environ Res 110 (2010): 112-117.
- Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking Changes in SARS- CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 182 (2020): 812– 827.e19.
- Zhang XY, Wang YQ, Niu T, Zhang XC, Gong SL, Zhang YM, Sun JY. Atmospheric aerosol compositions in China: spatial/temporal variability, chemical signature, regional haze distribution and comparisons with global aerosols " published in Atmos. Atmos Chem Phys 12 (2012): 779-799.
- Ogen Y. Assessing nitrogen dioxide (NO(2)) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci Total Environ 726 (2020): 138605.
- Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 395 (2020): e52.
- WHO. Air pollution, the invisible killer (2018).
Supplementary material ( SM)
Zacarelli-Marino M, Benedito M, Leitão M, Sato L,Crepaldi F, Martins P, Pinto M, Vincentin R, Balderi T (2018a) Association between lower and upper airway pathologies and environmental pollutants in residents close to industrial areas in the state of São Paulo, Brazil. In: ABC University Medical Congress. Annals ABCS Health Sci, Santo André, p 14
Zacarelli-Marino M, Biselli R, Casulli B, Cebrian G, Moreira C, Kodama P, Sayeg A, Cunha L (2017a) Obstructive pulmonary diseases in industrial areas in Brazil: an epidemiological study. In: ABC University Medical Congress. Annals ABCS Health Sci, Santo André, p 44
Zacarelli-Marino M, Contreiro P, Loreto A, Aguiar A, Almeida R, Martins L (2016a) Allergic conjunctivitis in industrial areas in Brazil: an epidemiological study. In: ABC University Medical Congress. Annals ABCS Health Sci, Santo André, p 115
Zacarelli-Marino M, Giacovone C, Martins H, Takara M, Falcone B, Balderi T, Martins L (2016b) Dermatitis in industrial areas in Brazil: an epidemiological study. In: ABC University Medical Congress. Annals ABCS Health Sci, Santo André, p 116
Zacarelli-Marino M, Gutierrez M, Gabriel N, Ribeiro P, Giro L, Novais T, Bosco R, Araújo K, Teodoro V, Balderi T (2018b) Association between the occurrence of primary hypothyroidism and the exposure of the population to industrial pollutants in São Paulo State, Brazil. In: ABC University Medical Congress. Annals ABCS Health Sci, Santo André, p 13
Zacarelli-Marino M, Moreira C, Sayeg A, Kodama P, Cunha L, Kaufman F, Biselli R, Casulli B, Cebrian G, Balderi T (2017b) Rhinitis, Sinusitis and Pharyngitis in Industrial Areas in Brazil: an epidemiological study. In: ABC University Medical Congress. Annals ABCS Health Sci, Santo André, p 43