Safety and Efficacy of Faropenem in Respiratory - and Urinary Tract Infections: A Prospective Observational Study in Pediatric Patients
Article Information
Apurba Ghosh1, Prashanth SN2, Vaibhav Jain3, Rajib Kumar Ray4, Shreyans Shah5, Monjori Mitra6*, Amitrajit Pal7*, Dattatray Pawar8, Akhilesh Sharma9
1Executive Director and Professor, Institute of Child Health, Kolkata, Dr Biresh Guha St, Park Circus, Ballygunge, Kolkata, West Bengal, India
2Professor and HOD, Department of Pediatrics, JSS Medical College and Hospital, Sri Shivarathreeshwara Nagara, Mysuru, Karnataka, India
3Senior Consultant, Vardhman Hospital, Sector D1, LDA Colony, Lucknow, Uttar Pradesh, India
4Senior Consultant, Sparsh Hospital, Saheed Nagar, Bhubaneswar, Odisha, India
5Consultant Pediatrician, Aatman Hospital, 5, Anveshan row house Bopal - Ghuma Road, BRTS, opp. Bopal, Bopal, Gujarat, India
6Professor of Pediatrics, Kolkata, Dr Biresh Guha St, Park Circus, Ballygunge, Kolkata, West Bengal, India
7Medical Advisor, Alkem Laboratories Private Limited, Alkem House, Devashish Building, Senapati Bapat Marg, Lower Parel, Mumbai, Maharashtra, India
8Head, Medical Affairs, Alkem Laboratories Private Limited, Alkem House, Devashish Building, Senapati Bapat Marg, Lower Parel, Mumbai, Maharashtra, India
9President & Chief Medical Officer, Alkem Laboratories Private Limited, Alkem House, Devashish Building, Senapati Bapat Marg, Lower Parel, Mumbai, Maharashtra, India
*Corresponding Authors: Monjori Mitra, Professor of Pediatrics, Kolkata, Dr Biresh Guha St, Park Circus, Ballygunge, Kolkata, West Bengal, India.
Amitrajit Pal, Medical Advisor, Alkem Laboratories Private Limited, Alkem House, Devashish Building, Senapati Bapat Marg, Lower Parel, Mumbai, Maharashtra, India.
Received: 18 January 2025; Accepted: 31 January 2025; Published: 21 February 2025
Citation:
Apurba Ghosh, Prashanth SN, Vaibhav Jain, Rajib Kumar Ray, Shreyans Shah, Monjori Mitra, Amitrajit Pal, Dattatray Pawar, Akhilesh Sharma. Safety and Efficacy of Faropenem in Respiratory- and Urinary Tract Infections: A Prospective Observational Study in Pediatric Patients. Journal of Pediatrics, Perinatology and Child Health. 9 (2025): 18-29.
View / Download Pdf Share at FacebookAbstract
Introduction: Faropenem, the only penem antibiotic for oral administration, is crucial for treating pediatric infections with limited supporting evidence.
Objectives: To assess the safety and efficacy of faropenem in pediatric infections, RTIs, and UTIs.
Methods: This prospective, multicentric, observational study (CTRI/2024/01/061255; 09/01/2024) enrolled male and female patients, aged ≥6 months to <18 years, diagnosed with RTIs or UTIs, treated with faropenem sodium dry syrup (100mg/5ml) at investigator's discretion. Follow-up assessments occurred on Days 7±2 and 14±2. Primary outcome was the incidence of TEAEs and SAEs. Secondary outcomes included proportions of patients with clinical improvement (≥50% symptom resolution without antibiotic change), clinical cure (complete symptom resolution), treatment failure (symptom worsening or therapy modification), and bacteriological cure (<1000 CFU/ml for UTI).
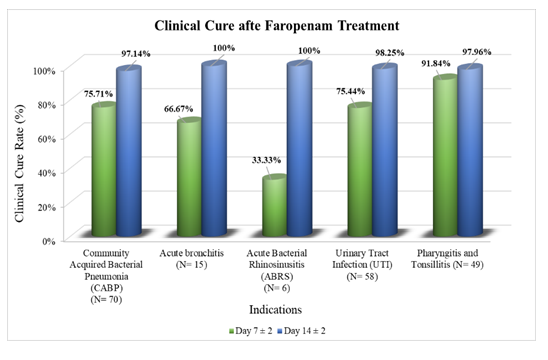
Results: Of 200 patients [54.50% females; mean age of 6.37±3.83 years], 197 completed the study without any reported TEAE/SAE. Clinical improvement at Day 7±2 was evident in all patients with pharyngitis, tonsillitis, and acute bronchitis; 98.57% with CABP, 89.47% with UTI, and 83.33% with ABRS. Early clinical cure rate at Day 7±2 was: pharyngitis and tonsillitis, 91.84%; CABP, 75.71%; UTI, 75.44%; acute bronchitis, 66.67%; ABRS, 33.37%. Clinical cure rate at Day 14±2 was: acute bronchitis and ABRS, 100.00%; UTI, 98.25%; pharyngitis and tonsillitis, 97.66%; CABP, 97.14%. Therapy modification was not required in any patient. Symptoms deteriorated in one patient with pharyngitis and tonsillitis. Bacteriological cure was observed in all evaluated patients with UTI.
Conclusions: Faropenem was found to be safe and effective for the treatment of RTIs and UTIs in children.
Keywords
Acute bacterial rhinosinusitis; Acute bronchitis; Communityacquired bacterial pneumonia; Faropenem; pharyngitis and tonsillitis; Respiratory tract infections; Urinary tract infections
Acute bacterial rhinosinusitis articles; Acute bronchitis articles; Community-acquired bacterial pneumonia articles; Faropenem; pharyngitis and tonsillitis articles; Respiratory tract infections articles; Urinary tract infections articles
Article Details
1. Introduction
Faropenem, a beta-lactam antibiotic within the penem class, has gained significant attention due to its broad-spectrum efficacy against a wide range of bacterial pathogens. Structurally, faropenem is a distinct entity within the beta-lactam family that differs from other penems in containing an oxygen atom instead of the characteristic sulfur atom present in the carbapenem class [1]. The salient attributes of this unique antibiotic include reduced intra-ring stress due to its structural features and enhanced activity and stability, notably against β-lactamases. It is readily absorbed, attains maximum plasma concentration within an hour, and has 72-84% bioavailability, 90-95% protein binding, and an 8-26% elimination rate (unaltered) [1,2]. Its pharmacokinetic property is particularly advantageous in pediatric populations, where oral administration facilitates compliance and ease of use. However, the administration of faropenem must be carefully guided by principles of antimicrobial stewardship to mitigate the escalating threat of antimicrobial resistance (AMR) [3].
Faropenem has been adopted in various regions, including Japan, India, and parts of Europe, as a second-line treatment for specific infections, especially when first-line antibiotics fail or when the causative pathogens exhibit resistance to standard therapies [4]. The broad-spectrum activity of the drug, effective against both gram-positive and gram-negative bacteria, positions faropenem as a promising therapeutic agent for respiratory tract infections (RTIs) and urinary tract infections (UTIs) [5]. Its role as a second-line treatment option is useful, particularly in today’s AMR landscape, where indiscriminate antibiotic usage could hasten the development of resistant strains [6].
Despite the known advantages of faropenem, the rise of AMR necessitates careful consideration of its use. Although resistance to faropenem remains relatively low, emerging reports of decreased susceptibility in strains of Escherichia coli and Klebsiella pneumoniae have been documented [5]. This emphasizes the need for judicious use, particularly in regions with a high prevalence of multi-drug resistant organisms.
Several studies have demonstrated the efficacy of faropenem in various clinical settings. Milatovic et al. [5] reported substantial in vitro activity of faropenem against 5,460 clinical bacterial isolates from Europe, highlighting its potential in treating infections caused by β-lactamase-producing bacteria. Another study by Bhalla [7] explored the application of faropenem in switch therapy protocols, where patients were transitioned from parenteral to oral administration during discharge, particularly in cases of RTIs and UTIs. The findings revealed that this approach not only enhanced patient compliance but also reduced the duration of hospital stay, contributing to cost-effective healthcare. Although the clinical activity of faropenem is proven in adult infections, it is imperative to investigate its applicability in children since respiratory pathogens affecting this age group are often less susceptible to antibacterial agents compared to those affecting adults [1,2].
Earlier studies have proven the safety, tolerability, clinical efficacy, and patient acceptance and compliance of faropenem in the pediatric population [2]. However, most of these findings were reported before the year 2000 (except the one by Yokota et al. [2] and are limited in number [4]. This prospective observational study sought to evaluate the treatment-related adverse events (TEAE) and clinical efficacy of faropenem sodium in each of the diseases RTIs (community-acquired bacterial pneumonia (CABP)/acute bronchitis, acute bacterial rhinosinusitis (ABRS), pharyngitis and tonsillitis) or UTIs for clinical cure or bacteriological cure or symptom resolution in pediatric patients aged ≥6 months and <18 years prescribed as a judicious treatment regimen by the physician.
2. Methods
2.1 Study Design and Setting
This prospective, observational, and multicentric study was conducted from January to June 2024 at five sites across India (Lucknow, Bhubaneswar, Kolkata, Mysuru, and Ahmedabad) (Supplementary Table S1). Site feasibility analysis confirmed the judicious prescription of faropenem by the investigators. Patients diagnosed with RTIs or UTIs received oral faropenem sodium dry syrup (100mg/5ml) at an initial dose of 5mg/kg, based on age, weight, severity of symptoms, and clinical response, with a maximum dose of 10mg/kg (maximum daily dose of 900 mg) three times daily. Patients were monitored from enrollment through Day 7±2 and Day 14±2.
The study received approval from independent ethics committees at all sites and followed the Declaration of Helsinki8 and New Drugs and Clinical Trials Rules, 2019 [9]. Written informed consent from parents and assent from participants aged ≥7 years were obtained before enrolment. Patient information was anonymized to ensure confidentiality. The trial was registered with the Clinical Trials Registry-India (CTRI/2024/01/061255; Registered on: 09/01/2024).
2.2 Inclusion and Exclusion Criteria
Male or female patients, aged ≥6 months and <18 years, were enrolled when clinically diagnosed with RTIs or UTIs based on the eligibility criteria. Diagnosis of CABP/acute bronchitis was confirmed based on the history of fever, acute onset or worsening of symptoms such as tachycardia, tachypnea, cough, dyspnea mostly, and hematological findings such as leukocytosis, immature neutrophils, or leukopenia if available. Diagnosis of ABRS was confirmed based on the presence of symptoms such as nasal inflammation, rhinorrhea, bad mood/productive cough, and nasal/postnasal discharge. UTI was confirmed based on the results of urine routine examination and culture and susceptibility test when available. Diagnosis of pharyngitis and tonsillitis (or tonsillopharyngitis) was diagnosed if the modified Centor (McIsaac) score was ≥ 3; since this scoring does not apply to patients younger than 3 years, this age group was enrolled based on the physician’s discretion. Details of inclusion and exclusion criteria are provided in Supplementary Methods 1.
2.3 Study Outcomes
The primary outcome was the proportion of patients with reported treatment-emergent adverse events (TEAEs; defined as AEs that occurred after faropenem administration) and serious AEs (SAEs) from baseline to Day 14. The secondary outcomes were the proportion of patients with clinical cure (complete resolution of signs and symptoms as per investigator’s judgment), clinical improvement (resolution of the majority of the symptoms in RTIs or UTI and not requiring change in antibiotic during the treatment period) at day 7±2 and day 14±2 from baseline, treatment failure (symptom deterioration or requirement of change in antibiotic during treatment phase), and bacteriological cure (defined as a colony count less than 1,000 CFU/ml as documented in clinical test reports of subjects with UTI) at Day 14±2.
2.4 Sample Size Estimation and Statistical Analysis
The sample size was determined to observe a clinical efficacy rate of 90% [2] with a 95% confidence interval (CI) and a 4.5% margin of error, considering 15% as the anticipated dropout rate.
All statistical methods were based on the International Council for Harmonization (ICH) E9 document ‘Statistical Principles for Clinical Trials’ and were carried out as per a comprehensive statistical analysis plan using SPSS version 28.0.1.1 (IBM Corp., Armonk, NY, USA). McNemar test was used to compare proportions and Wilcoxon signed-rank test was used to compare McIsaac scores. p<0.05 was considered statistically significant.
3. Results
3.1 Baseline characteristics
Among 200 enrolled subjects, 35% were diagnosed with CABP followed by 29% with UTI, and 24.50% with pharyngitis and tonsillitis. Acute bronchitis was diagnosed in 8.50% of enrolled subjects, and ABRS was diagnosed in 3.00% of the patients (Supplementary Table S2). Out of the enrolled participants, 197 completed the study and were included in the per-protocol (PP) analysis (Supplementary Table S3, Figure 1). Patients in the age group of 3-6 years comprised 46.50% of the total patients with an overall mean age of 6.37 (SD: 3.83) years. Diagnosis of CABP, UTI, pharyngitis, and tonsillitis was mostly evident in the age group of 3-6 years, while acute bronchitis was mostly diagnosed in subjects within 2 years of age. 54.50% of the subjects were female, among those diagnosed with various conditions: 66.67% for acute bacterial rhinosinusitis (ABRS), 58.82% for acute bronchitis, and 55.17% for urinary tract infections (UTI). In contrast, a male predominance was noted for community-acquired bacterial pneumonia (CABP) at 62.86% and for pharyngitis and tonsillitis at 61.22% (Table 1).
|
Total (N=200) |
CABP (n=70) |
UTI (n=58) |
Pharyngitis and Tonsillitis (n=49) |
Acute bronchitis (n=17) |
ABRS (n=6) |
|
|
Age (years) |
||||||
|
Mean ± SD |
6.37 ± 3.83 |
5.50 ± 2.69 |
7.15 ± 4.40 |
7.06 ± 3.9–4 |
4.47 ± 3.45 |
8.63 ± 5.89 |
|
Median (min, max) |
6.00 (1.00, 17.00) |
5.00 (1.00, 13.00) |
6.00 (1.40, 17.00) |
6.00 (1.00, 17.00) |
3.00 (1.00, 12.00) |
8.00 (2.00, 16.00) |
|
Age-group, n% |
||||||
|
1 to 2 years |
26 (13.00%) |
7 (10.00%) |
5 (8.62%) |
4 (8.16%) |
8 (47.06%) |
1 (16.67%) |
|
3 to 6 years |
93 (46.50%) |
43 (61.43%) |
26 (44.83%) |
22 (44.90%) |
2 (11.76%) |
1 (16.67%) |
|
7 to 10 years |
52 (26.00%) |
15 (21.43%) |
16 (27.59%) |
13 (26.53%) |
6 (35.29%) |
2 (33.33%) |
|
11 to 14 years |
20 (10.00%) |
5 (7.14%) |
6 (10.34%) |
8 (16.33%) |
1 (5.88%) |
0 (0%) |
|
15 to <18 years |
9 (4.50%) |
0 (0%) |
5 (8.62%) |
2 (4.08%) |
0 (0%) |
2 (33.33%) |
|
Weight (kg) |
||||||
|
Mean ± SD |
21.22 ± 10.96 |
19.40 ± 7.73 |
23.57 ± 12.27 |
21.68 ± 12.28 |
16.71 ± 9.37 |
28.63 ± 16.13 |
|
Median (min, max) |
18.00 (5.90, 60.00) |
18.00 (8.00, 42.00) |
20.00 (6.70, 52.00) |
18.00 (5.90, 60.00) |
12.00 (8.00, 44.20) |
28.50 (9.75, 48.00) |
|
Height (cm) |
||||||
|
Mean ± SD |
110.14 ± 23.74 |
108.41 ± 18.97 |
114.13 ± 22.83 |
112.14 ± 25.92 |
94.64 ± 29.52 |
119.50 ± 33.05 |
|
Median (min, max) |
108.00 (19.13, 170.00) |
109.00 (66.80, 152.00) |
109.50 (67.10, 163.00) |
108.00 (66.00, 169.00) |
86.00 (29.13, 158.00) |
125.00 (81.40, 170.00) |
|
Sex, n (%) |
||||||
|
Female |
109 (54.50%) |
26 (37.14%) |
32 (55.17%) |
19 (38.78%) |
10 (58.82%) |
4 (66.67%) |
|
Male |
91 (45.50%) |
44 (62.86%) |
26 (44.83%) |
30 (61.22%) |
7 (41.18%) |
2 (33.33%) |
Table 1: Demographic characteristics at baseline.
3.2 Clinical cure and improvement
No TEAE or SAE was reported in the study subjects with any indications. Clinical improvement or cure was assessed based on baseline symptoms, with a resolution of at least 50% considered as improvement. By Day 14±2, a complete clinical cure (100% resolution of symptoms) was achieved in all cases of pharyngitis, tonsillitis, and acute bronchitis, along with the majority of CABP (97.14%) and UTI (98.25%). Significant improvement was also seen in ABRS (100%). Clinical improvements (≥50% resolution) were observed by Day 7±2 in 98.57% of CABP, 89.47% of UTI, and 83.33% of ABRS cases, with all pharyngitis, tonsillitis, and bronchitis cases reaching 100% improvement (Supplementary Table S4, Figure 2, and Figure 3). By Day 7±2, significant symptom improvements were observed, such as tachypnea (CABP: 90.38%; acute Bronchitis: 100%), cough (CABP: 86.15%; acute Bronchitis: 66.67%), and painful micturition in UTI patients (82.93%). Resolution was complete for nearly all symptoms by Day 14±2, with CABP, UTI, and pharyngitis/tonsillitis showing 100% resolution for key symptoms like fever, tachypnea, and tender cervical nodes (Table 2).
|
Symptom |
Subjects with symptoms at baseline, n' |
Resolution at Day 7±2, r/n' (%) |
Resolution at Day 14±2, r/n' (%) |
p-value |
|
Community-Acquired Bacterial Pneumonia (CABP; n=70) |
||||
|
Fever |
70 |
67/70 (95.71%) |
70/70 (100.00%) |
0.25 |
|
Cough |
65 |
56/65 (86.15%) |
65/65 (100.00%) |
0.004** |
|
Tachypnea |
52 |
47/52 (90.38%) |
52/52 (100.00%) |
0.062 |
|
Tachycardia |
41 |
40/41 (97.56%) |
40/41 (97.56%) |
0.999 |
|
Increased work on breathing |
32 |
32/32 (100.00%) |
32/32 (100.00%) |
0.999 |
|
Sputum production |
21 |
21/21 (100.00%) |
21/21 (100.00%) |
0.999 |
|
Shortness of breath |
19 |
19/19 (100.00%) |
19/19 (100.00%) |
0.999 |
|
Dyspnea |
2 |
2/2 (100.00%) |
2/2 (100.00%) |
0.999 |
|
Immature neutrophils level |
2 |
0/2 (0%) |
1/2 (50.00%) |
0.999 |
|
Leukocytosis |
2 |
0/2 (0%) |
1/2 (50.00%) |
0.999 |
|
Headache |
1 |
1/1 (100.00%) |
1/1 (100.00%) |
0.999 |
|
Urinary Tract Infection (UTI; n=58) |
||||
|
Pain or burning while urinating |
41 |
34/41 (82.93%) |
41/41 (100.00%) |
0.015* |
|
Frequent urination |
35 |
29/35 (82.86%) |
35/35 (100.00%) |
0.031* |
|
Bloody urine |
10 |
7/10 (70.00%) |
10/10 (100.00%) |
0.25 |
|
Pressure in the groin |
9 |
9/9 (100.00%) |
9/9 (100.00%) |
0.999 |
|
Culture positive (majorly with pus cells) |
11 |
5/11 (45.45%) |
11/11 (100.00%) |
0.031* |
|
Pharyngitis and Tonsillitis (n=49) |
||||
|
Fever |
48 |
48/48 (100.00%) |
48/48 (100.00%) |
0.999 |
|
Tonsillar exudate or swelling |
47 |
43/47 (91.49%) |
47/47 (100.00%) |
0.125 |
|
Swollen, tender anterior cervical nodes§ |
43 |
43/43 (100.00%) |
42/43 (97.67%) |
0.999 |
|
Cough |
33 |
29/33 (87.88%) |
32/33 (96.97%) |
0.125 |
|
Others |
1 |
1/1 (100.00%) |
1/1 (100.00%) |
0.999 |
|
Acute bronchitis (n=17) |
||||
|
Cough |
15 |
10/15 (66.67%) |
15/15 (100.00%) |
0.062 |
|
Fever |
15 |
15/15 (100.00%) |
15/15 (100.00%) |
0.999 |
|
Tachypnea |
14 |
14/14 (100.00%) |
14/14 (100.00%) |
0.999 |
|
Tachycardia |
5 |
5/5 (100.00%) |
5/5 (100.00%) |
0.999 |
|
Shortness of breath |
13 |
13/13 (100.00%) |
13/13 (100.00%) |
0.999 |
|
Headache |
6 |
6/6 (100.00%) |
6/6 (100.00%) |
0.999 |
|
Sputum production |
5 |
5/5 (100.00%) |
5/5 (100.00%) |
0.999 |
|
Increased work on breathing |
2 |
2/2 (100.00%) |
2/2 (100.00%) |
0.999 |
|
Chest pain |
1 |
1/1 (100.00%) |
1/1 (100.00%) |
0.999 |
|
Dyspnea |
1 |
1/1 (100.00%) |
1/1 (100.00%) |
0.999 |
|
Acute Bacterial Rhinosinusitis (ABRS; n=6) |
||||
|
Nasal or postnasal discharge |
6 |
5/6 (83.33%) |
6/6 (100.00%) |
0.999 |
|
Productive cough |
5 |
2/5 (40.00%) |
5/5 (100.00%) |
0.25 |
|
Bad mood |
4 |
4/4 (100.00%) |
4/4 (100.00%) |
0.999 |
|
Rhinorrhea |
3 |
3/3 (100.00%) |
3/3 (100.00%) |
0.999 |
|
Inflammation of nasal mucosa |
3 |
2/3 (66.67%) |
3/3 (100.00%) |
0.999 |
- ‘Swollen, tender anterior cervical nodes’ worsened in one subject (FAK007) from Day 7±2 to Day 14±2.
NOTE: r is the number of subjects with symptom resolution; n' is the number of subjects with symptoms at baseline.
Table 2: Clinical symptom resolution at Day 7±2 and Day 14±2.
3.3 Treatment failure
None of the subjects required a change in antibiotics during the duration of the treatment. Thus, the proportion of subjects with treatment failure was 2.04% (1/49) in pharyngitis and tonsillitis and 0% in the other indications. Overall, the rate of treatment failure was 0.51% (1/197).
3.4 Efficacy of faropenem treatment in patients with UTI
In patients with UTIs, 71.93% had pus cells, and 19.30% had RBCs, at baseline. Urine culture and sensitivity tests were done on 20.69% of subjects at baseline and were positive with>105 CFU/ml colony counts. In the remaining patients, antibiotic therapy was started based on clinical diagnosis that primarily relied on symptoms like dysuria, frequent urination, hematuria, and pressure in the groin.
Following treatment with faropenem, there was a significant decrease in pus cells in 92.68% (38/41) of patients by Day 14 ± 2 and in 60.97% (25/41) of patients by Day 7 ± 2 (Supplementary Table S5). Of the 11 PP subjects with available baseline culture-positive data, 72.73% had Escherichia coli, 27.27% had Klebsiella pneumoniae, and all were resistant to the commonly used oral antibiotics. All subjects recovered by the end of treatment. Two subjects reported mild, unrelated weakness due to UTI, but both recovered by the end of the study.
3.5 Efficacy of faropenem treatment in patients with pharyngitis and tonsillitis: Analysis of McIsaac scores
Among 49 subjects with pharyngitis and tonsillitis, daily Centor (McIsaac) scores were recorded for 40. The median score showed significant changes from baseline to subsequent days (p<0.001 for all), except for Day 2 (p=0.157). (Supplementary Table S6 and Figure 4).
4. Discussion
Faropenem, a new antibiotic in the antimicrobial armamentarium should be judiciously used in children in the current era of antibiotic resistance where India has been earmarked as a hotspot of AMR. However, there is a dearth of recent evidence on faropenem usage in children, particularly in India. Accordingly, this prospective observational study investigated the safety and efficacy of faropenem in pediatric patients who underwent judicious treatment with this antibiotic for the treatment of RTIs or UTIs. Most subjects were diagnosed with CABP, followed by UTI, pharyngitis tonsillitis, acute bronchitis, and ABRS. Similar to observations from earlier studies, faropenem treatment was safe for pediatric subjects, with no reported TEAEs or SAEs in this study [2]. The clinical cure rates obtained in the present study (acute bronchitis: 100.00%; ABRS: 100.00%; UTI: 98.25%, pharyngitis and tonsillitis: 97.66%; CABP: 97.14%) align well with the clinical efficacy rates reported in a recent review on faropenem use in children for similar indications: 100% each in bronchitis, UTI, tonsillitis, and pneumonia, 90% in upper RTIs, and 50-100% in pharyngitis. These also align with the overall efficacy rates reported in earlier studies of faropenem in children: 91.0% by Yokota et al. 93.3% by Niinou et al., 96.0% by Furukawa and Okada, 96.8% by Nishimura et al., and 88.0-100.0% by Fujii et al., suggesting robust efficacy of faropenem in pediatric infections [2]. In this backdrop, the current study adds worthy information that might help physicians put this effective antibiotic to use in case of first-line antibiotic failure. Despite variations in treatment duration with faropenem, high clinical cure rates were maintained across all infections. This indicates that treatment duration may be effectively tailored based on the specific infection type and severity. It is noteworthy that by Day 14±2, faropenem treatment resulted in clinical improvement in all PP subjects. Early symptomatic improvement at Day 7±2 was evident in all subjects with pharyngitis and tonsillitis or acute bronchitis and in more than 83.00% of subjects with CABP, UTI, or ABRS. Change in antibiotic was not required for any subject. Symptom deterioration was observed in one subject at the last visit. Among subjects with pharyngitis and tonsillitis, a significant improvement in McIsaac score was observed as early as Day 3 post-treatment initiation and it was maintained till Day 14. Among subjects with UTI, a statistically significant reduction was observed in the number of pus cells, from baseline to Day 14±2; early symptomatic improvement was observed on Day 7±2. Based on the prescription pattern observed in this study, it was seen that patients infected with E. coli or K. pneumoniae, who displayed resistance to multiple commonly used antibiotics, were administered faropenem for UTI management. A recent study pointed to the efficacy of faropenem against cephalosporin-resistant strains of these bacteria [10], similar to other studies that have highlighted the role of faropenem in combatting causative pathogens of UTI [11,12]. Furthermore, faropenem is known to have a low minimum inhibitory concentration (MIC) [2,13,14]. Given rising resistance to cephalosporins and β-lactam antibiotics in pathogenic bacteria [15], the low MIC values of faropenem and its efficacy against resistant strains point towards its potential as a valuable therapeutic option. However, indiscriminate use must be avoided to mitigate further emergence of AMR.
Overall, the current study adds to the limited existing literature on the safety and efficacy of faropenem for the management of common infectious diseases, specifically in children. The study included pediatric patients of all age groups starting from 6 months to 18 years and covered multiple indications within the broader category of RTIs and UTIs, thus pointing towards a possible use of faropenem in each of these indications. Since the study enrolled patients from across India with diverse environments and bacterial prevalence, it can be hypothesized that faropenem might be effective against a broad range of bacterial species, as is also documented in the literature. However, confirmatory evidence necessitates an investigation of bacteriological eradication rates, which was beyond the scope of the current study. Another limitation of the current study is the lack of a comparator; randomized controlled trials comparing faropenem with other antibiotics would be fruitful in the future to obtain comparative data on the efficacy of faropenem relative to other commonly prescribed antibiotics used as the standard of care. Further studies on MIC values of clinical isolates would also add to the data pool on faropenem. It would be especially critical to obtain such data on resistant strains. The current study enrolled patients with a few common indications while faropenem is indicated in multiple other diseases. Future studies in larger cohorts involving polymicrobial infections will be beneficial to inform clinicians of the different indications in which faropenem may be prescribed.
Faropenem, an oral penam antibiotic, showed significant clinical cures at the completion of the treatment and significant early resolution of symptoms in all the enrolled subjects with RTIs and UTIs. The safety and effectiveness were well established in the study population, with minimal treatment failure rate. This antibiotic should be reserved for judicious use in the pediatric population in this era of antimicrobial resistance.
Acknowledgments
The authors also convey their gratitude to Medclin Research Pvt. Ltd. for the overall administration, coordination, data collection, and management of the study as a Contract Research Organization (CRO) and MedSign for EMR support.
Author Contributions
Conceptualization and Validation: AG, PSN, VJ, RKR, SS, MM, AP, DP, and AS. Methodology and Formal Analysis: AG, PSN, VJ, RKR, SS, MM, AP, DP, and AS. Project Administration: MM, AP, DP, and AS. Writing-Original draft: AP, and MM. Writing-Reviewing and Editing: AG, PSN, VJ, RKR, SS, MM, AP, DP, and AS, Supervision: AP and MM. All the authors have reviewed the manuscript and have agreed to publication.
Funding
Alkem Laboratories Private Limited., Mumbai, India.
Data availability
The data substantiating the results of this real-world study can be obtained from the corresponding author upon reasonable request.
Code availability
Not applicable
Declarations
Conflicts of interest
The authors AP, DP, and AS are full-time employees of Alkem Laboratories Private Limited., Mumbai, India, who sponsored the study. The other authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
The written and dated approval from the institutional ethics committees (IECs) was taken as per Medclin Research Pvt. Ltd.’s ethical committee submission standard operating procedure (SOP). The study was approved by the institutional ethics committees (IECs) of the participating sites.
References
- Schurek KN, Wiebe R, Karlowsky JA, et al. Faropenem: review of a new oral penem. Expert Rev Anti Infect Ther 5 (2007): 185-198.
- Nayak S, Pai U, Birla A. Role of Faropenem in Treatment of Pediatric Infections: The Current State of Knowledge. Cureus 14 (2022): e24453.
- World Health Organization (WHO). Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020. Geneva: WHO (2020).
- Gandra S, Takahashi S, Mitrani-Gold FS, et al. A systematic scoping review of faropenem and other oral penems: treatment of Enterobacterales infections, development of resistance and cross-resistance to carbapenems. JAC-Antimicrob Resist 4 (2022): dlac125.
- Milatovic D, Schmitz FJ, Verhoef J, et al. In vitro activity of faropenem against 5460 clinical bacterial isolates from Europe. J Antimicrob Chemother 50 (2002): 293-299.
- Poole K. Efflux-mediated antimicrobial resistance. J Antimicrob Chemother 56 (2005): 20-51.
- Bhalla A. Faropenem, a stable and orally bioavailable b-lactam, to counteract resistant pathogens and infectious diseases. A narrative review (2023).
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 310 (2013): 2191.
- Dixon JR. The International Conference on Harmonization Good Clinical Practice Guideline. Qual Assur 6 (1999): 65-74.
- Ishikawa K, Uehara Y, Mori N, et al. In Vitro Activity and Clinical Efficacy of Faropenem against Third-Generation Cephalosporin-Resistant Escherichia coli and Klebsiella pneumoniae. Antimicrob Agents Chemother 66 (2022): e0012522.
- Wagenlehner FME, Pilatz A, Naber KG, et al. Anti-infective treatment of bacterial urinary tract infections. Curr Med Chem 15 (2008): 1412-1427.
- Shah A, Sharma S, Unnikrishnan T. Experience of Faropenem for the management of urinary tract infection: Real-world experience from India. IP J Urol Nephrol Hepatol Sci 3 (2020): 77-81.
- Woodcock JM, Andrews JM, Brenwald NP, et al. The in-vitro activity of faropenem, a novel oral penem. J Antimicrob Chemother 39 (1997): 35-43.
- Marchese A, Debbia EA, Bryskier A, et al. Antimicrobial activity of faropenem, a new oral penem, against lower respiratory tract pathogens. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 5 (1999): 282-287.
- Singhal T. Antimicrobial Resistance: The “Other” Pandemic!: Based on 9th Dr. I. C. Verma Excellence Award for Young Pediatricians Delivered as Oration on 19th Sept. 2021. Indian J Pediatr 89 (2022): 600-606.
Supplementary File
Table S1: Number of subjects enrolled from each study site.
|
Site ID |
Site name |
Principal Investigator |
Number of subjects enrolled |
|
FAL |
Vardhman Hospital, Lucknow |
Dr. Vaibhav Jain |
63 |
|
FAB |
Sparsh Hospital, Bhubaneswar |
Dr. Rajib Kumar Ray |
60 |
|
FAK |
Institute of Child Health, Kolkata |
Dr. Apurba Ghosh |
40 |
|
FAM |
JSS Medical College, Mysuru |
Dr. Prashanth S N |
19 |
|
FAA |
Aatman Hospital, Ahmedabad |
Dr. Shreyans Shah |
18 |
|
Total number of subjects enrolled |
200 |
||
Supplementary Methods
Methods 1: Full Inclusion and Exclusion Criteria
Diagnosis of CABP/acute bronchitis was confirmed if the following criteria were present: (i) history of fever (oral temperature >38°C or axillary temperature >37.5°C) or hypothermia (oral temperature <35°C or axillary temperature <34.5°C), (ii) presence of at least one of the following hematology findings if available in clinical and medical records: leukocytosis (>15,000 WBC/mm3), >15% immature neutrophils (bands), leukopenia, and (iii) acute onset or worsening of at least two among the following within 5 days preceding enrolment: tachycardia (>6 months to <24 months: >160 beats/min; ≥24 months to <10 years: >140 beats/min; ≥10 years: >100 beats/min), tachypnea (>6 months to <12 months: >50 breaths/min; ≥12 months to <5 years: >40 breaths/min; ≥5 years: >20 breaths/min), and any of cough, dyspnea, grunting, sputum production, chest pain, cyanosis, or increased work of breathing. Diagnosis of ABRS was confirmed based on the following: (i) inflammation of nasal mucosa, purulent/mucopurulent nasal or postnasal discharge, or pathological shadow in the paranasal sinus on a radiogram, (ii) moderate or severe ABRS with a total score of the following symptoms ≥4: rhinorrhea (none: 0, mild/small amount: 1, moderate or severe: 2), bad mood/productive cough (none: 0, mild/small amount: 1, moderate or severe: 2), and nasal/postnasal discharge [none: 0 (serous), mild/small amount: 1 (mucopurulent, small amount), moderate or severe: 2 (moderate or larger amount)]. Diagnosis of UTI was confirmed based on results of urine culture test (colony count ≥ 105 CFU/ml) or urine routine test, if available. Diagnosis of pharyngitis and tonsillitis (or tonsillopharyngitis) was based on a modified Centor (McIsaac) score ≥ 3 calculated as a cumulative score, where each of the following was given a score of one: axillary body temperature ≥ 37.5ºC, absence of cough, swollen, tender anterior cervical nodes, and tonsillar exudate or swelling, in addition to a score based on age (3-14 years: score 1; 15-44 years: score 0; >45 years: score –1). Since this scoring system does not apply to patients younger than 3 years, this age group was enrolled based on the physician’s discretion.
The following were the exclusion criteria: severe cases of CABP including hypoxemia or sepsis as per investigator’s judgment, clinical evidence of infectious mononucleosis, leukopenia and/or thrombocytopenia, or diarrhea at screening (if available in clinical and medical records), receiving or received more than one dose of systemic antibiotic or medication (within 72 hours preceding enrollment) affecting bowel movement, known allergy or hypersensitivity to components of the study medication, participation in another clinical trial within past 30 days, any condition (epilepsy, cardiovascular disease, etc.) or concomitant medication, that in the opinion of the investigator, might affect study outcome, or any underlying condition in the patient, that in the opinion of the investigator, might worsen upon participation.
Table S2: Diagnosis of subjects at baseline.
|
Indication |
Number of subjects, N=200 |
|
Community-Acquired Bacterial Pneumonia (CABP) |
70 (35.00%) |
|
Urinary Tract Infection (UTI) |
58 (29.00%) |
|
Pharyngitis and Tonsillitis |
49 (24.50%) |
|
Acute bronchitis |
17 (8.50%) |
|
Acute Bacterial Rhinosinusitis (ABRS) |
6 (3.00%) |
Table S3: Subjects lost to follow-up.
|
Site ID |
Site name |
Subject ID |
Visit |
Reason for discontinuance |
|
FAK |
Institute of Child Health |
FAK008 |
Visit 2 |
The site tried to contact the subjects repeatedly. However, there was no response. Hence, the subjects were deemed to be lost to follow-up. |
Table S4: Clinical cure and clinical improvement rates upon treatment with faropenem.
|
Day 7±2, n (%) |
Day 14±2, n (%) |
%Difference [95% CI] |
p-value |
|
|
Clinical improvement rate |
||||
|
Community-Acquired Bacterial Pneumonia (CABP) (n=70) |
69 (98.57%) |
70 (100.00%) |
1.43%, [-1.35%, 4.21%] |
0.999 |
|
Urinary Tract Infection (UTI) (n=57) |
51 (89.47%) |
57 (100.00%) |
10.53% [2.56%, 18.49%] |
0.031* |
|
Pharyngitis and Tonsillitis (n=49) |
49 (100.00%) |
49 (100.00%) |
0% |
0.999 |
|
Acute bronchitis (n=15) |
15 (100.00%) |
15 (100.00%) |
0% |
0.999 |
|
Acute Bacterial Rhinosinusitis (ABRS) (n=6) |
5 (83.33%) |
6 (100.00%) |
16.67% [-13.15%, 46.49%] |
0.999 |
|
Clinical cure rate |
||||
|
Community-Acquired Bacterial Pneumonia (CABP) (n=70) |
53 (75.71%) |
68 (97.14%) |
21.43% [11.82%, 31.04%] |
<0.001*** |
|
Urinary Tract Infection (UTI) (n=57) |
43 (75.44%) |
56 (98.25%) |
22.81% [11.91%, 33.70%] |
<0.001*** |
|
Pharyngitis and Tonsillitis (n=49) |
45 (91.84%) |
48 (97.96%) |
6.12% [-0.59%, 12.84%] |
0.250 |
|
Acute bronchitis (n=15) |
10 (66.67%) |
15 (100.00%) |
33.33% [9.48%, 57.19%] |
0.062 |
|
Acute Bacterial Rhinosinusitis (ABRS) (n=6) |
2 (33.33%) |
6 (100.00%) |
66.67%, [28.95%, 100.00%] |
0.125 |
NOTE: The number of per-protocol subjects with each diagnosis at baseline is mentioned within parentheses.
Table S5: Change in urine routine and microscopy from baseline to Day 14±2.
|
Assessment days (N=57) |
n (%) |
Difference% [95% CI] from baseline |
p-value for change from baseline |
|
Presence of pus cells |
|||
|
Baseline |
41 (71.93%) |
NA |
NA |
|
Day 7±2 |
16 (28.07%) |
43.86% [30.98%, 56.74%] |
<0.001*** |
|
Day 14±2 |
3 (5.26%) |
66.67% [54.43%, 78.90%] |
<0.001*** |
|
Presence of epithelial cells |
|||
|
Baseline |
7 (12.28%) |
NA |
NA |
|
Day 7±2 |
3 (5.26%) |
7.02% [0.39%, 13.65%] |
0.125 |
|
Day 14±2 |
1 (1.75%) |
10.53% [2.56%, 18.49%] |
0.031* |
|
Presence of red blood cells |
|||
|
Baseline |
11 (19.30%) |
NA |
NA |
|
Day 7±2 |
4 (7.02%) |
12.28% [3.76%, 20.80%] |
0.015* |
|
Day 14±2 |
0 (0.00%) |
19.30% [9.05%, 29.54%] |
0.001** |
Abbreviations: CI = Confidence Interval; NA = Not applicable
Table S6: Change in McIsaac score in subjects with pharyngitis and tonsillitis.
|
Day after treatment initiation (n=40) |
McIsaac score |
||
|
Mean ± SD |
Median [IQ1, IQ3] |
p-value [Reference: Day 1] |
|
|
Day 1 |
4.20 ± 0.69 |
4.00 [4.00, 5.00] |
NA |
|
Day 2 |
4.15 ± 0.70 |
4.00 [4.00, 5.00] |
0.157 |
|
Day 3 |
3.73 ± 1.06 |
4.00 [3.00, 4.75] |
<0.001*** |
|
Day 4 |
3.20 ± 1.18 |
3.00 [2.00, 4.00] |
<0.001*** |
|
Day 5 |
2.65 ± 1.08 |
3.00 [2.00, 3.00] |
<0.001*** |
|
Day 6 |
2.23 ± 0.77 |
2.00 [2.00, 2.75] |
<0.001*** |
|
Day 7 |
2.25 ± 0.74 |
2.00 [2.00, 2.00] |
<0.001*** |
|
Day 8 |
1.95 ± 0.22 |
2.00 [2.00, 2.75] |
<0.001*** |
|
Day 9 |
1.95 ± 0.22 |
2.00 [2.00, 2.00] |
<0.001*** |
|
Day 10 |
1.95 ± 0.22 |
2.00 [2.00, 2.00] |
<0.001*** |
|
Day 11 |
1.95 ± 0.22 |
2.00 [2.00, 2.00] |
<0.001*** |
|
Day 12 |
1.95 ± 0.22 |
2.00 [2.00, 2.00] |
<0.001*** |
|
Day 13 |
1.95 ± 0.22 |
2.00 [2.00, 2.00] |
<0.001*** |
|
Day 14 |
1.95 ± 0.22 |
.00 [2.00, 2.00] |
<0.001*** |
Abbreviations: IQ1 = First/lower quartile; IQ3 = Third/upper quartile; NA = Not applicable; SD = Standard Deviation