Safety and Effectiveness of Lower Alteplase Dose Driven by Impending Clinical Deterioration Factors in very Elderly Submassive Pulmonary Embolism Patients. Case Series
Article Information
Juan Quintanilla1,2, Maria Fernanda Reyes-Chavez1, Carlos Jerjes-Sanchez1,2*, Melissa Galindo-Garza1,2, Aldo F Ponce-Barahona1,2, Vanessa Alegria-Saldivar1, Arturo Adrian Martínez Ibarra1,2, Armando Osorio-Salazar1, Jose Alfredo Salinas-Casanova1,2, Ricardo J Estrada-Mendizabal1, Renata Quevedo-Salazar1, Sofia Guardado-Vazquez1, Paola Gutiérrez-Gallegos1, Victor E Lozano-Corres1
1Tecnologico de Monterrey, Escuela de Medicina y Ciencias de la Salud, Monterrey, Nuevo Leon, Mexico
2Instituto de Cardiología y Medicina Vascular, TecSalud, Escuela de Medicina y Ciencias de la Salud, Tecnologico de Monterrey, San Pedro Garza Garcia, Nuevo Leon, Mexico
*Corresponding Author: Carlos Jerjes-Sanchez, Instituto de Cardiología y Medicina Vascular, TecSalud, Batallón San Patricio 112, Real de San Agustin, 66278 San Pedro Garza García, Nuevo Leon. México.
Received: 23 August 2022; Accepted: 07 September 2022; Published: 05 October 2022
Citation: Juan Quintanilla, Maria Fernanda Reyes-Chavez, Carlos Jerjes-Sanchez, Melissa Galindo-Garza, Aldo F Ponce-Barahona, Vanessa Alegria-Saldivar, Arturo Adrian Martínez Ibarra, Armando Osorio-Salazar, Jose Alfredo Salinas- Casanova, Ricardo J Estrada-Mendizabal, Renata Quevedo-Salazar, Sofia Guardado-Vazquez, Paola Gutiérrez-Gallegos, Victor E Lozano-Corres. Safety and Effectiveness of Lower Alteplase Dose Driven by Impending Clinical Deterioration Factors in very Elderly Submassive Pulmonary Embolism Patients. Case Series. Archives of Clinical and Medical Case Reports 6 (2022): 669-678.
View / Download Pdf Share at FacebookAbstract
Although thrombolysis improves the outcome and mortality in submassive (SM) and massive PE, its role is controversial because of the high rate of intracranial hemorrhage. In addition, safety and efficacy are unclear since randomized controlled studies have excluded very elderly patients because of frailty, multiple comorbidities, and a higher risk of bleeding. Therefore, the best thrombolytic regimen is unknown. Furthermore, it is unclear whether the decision-making for thrombolysis is performed according to the guidelines or based on clinical risk factors associated with poor outcomes. We report three very elderly SMPE associated with several impending clinical deterioration factors (ICDF) (in-transit thrombus, saddle thrombus, etc.). Therefore, we decided on 25 mg alteplase in one- or two-hour continuous infusion based on ICDF rather than clinical instability and systolic hypotension. In addition, we initiate DOACs around 48 hours after stopping unfractionated heparin (UFH). As a result, all patients improve right ventricular performance without bleeding complications. Our results suggest that the lower alteplase dose in one- or two-hour continuous infusion, followed by weight-adjusted UFH, was effective and safe, involving a complicated scenario as an intransit thrombus. Also, DOACs standard doses driven by the patient´s characteristics were unrelated to bleeding complications avoiding recurrence in very elderly SMPE.
Keywords
Alteplase; Massive Pulmonary Embolism; Pulmonary Embolism; Submassive Pulmonary Embolism; Thrombolysis
Alteplase articles; Massive Pulmonary Embolism articles; Pulmonary Embolism articles; Submassive Pulmonary Embolism articles; Thrombolysis articles
Alteplase articles Alteplase Research articles Alteplase review articles Alteplase PubMed articles Alteplase PubMed Central articles Alteplase 2023 articles Alteplase 2024 articles Alteplase Scopus articles Alteplase impact factor journals Alteplase Scopus journals Alteplase PubMed journals Alteplase medical journals Alteplase free journals Alteplase best journals Alteplase top journals Alteplase free medical journals Alteplase famous journals Alteplase Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Massive Pulmonary Embolism articles Massive Pulmonary Embolism Research articles Massive Pulmonary Embolism review articles Massive Pulmonary Embolism PubMed articles Massive Pulmonary Embolism PubMed Central articles Massive Pulmonary Embolism 2023 articles Massive Pulmonary Embolism 2024 articles Massive Pulmonary Embolism Scopus articles Massive Pulmonary Embolism impact factor journals Massive Pulmonary Embolism Scopus journals Massive Pulmonary Embolism PubMed journals Massive Pulmonary Embolism medical journals Massive Pulmonary Embolism free journals Massive Pulmonary Embolism best journals Massive Pulmonary Embolism top journals Massive Pulmonary Embolism free medical journals Massive Pulmonary Embolism famous journals Massive Pulmonary Embolism Google Scholar indexed journals Ultrasound articles Ultrasound Research articles Ultrasound review articles Ultrasound PubMed articles Ultrasound PubMed Central articles Ultrasound 2023 articles Ultrasound 2024 articles Ultrasound Scopus articles Ultrasound impact factor journals Ultrasound Scopus journals Ultrasound PubMed journals Ultrasound medical journals Ultrasound free journals Ultrasound best journals Ultrasound top journals Ultrasound free medical journals Ultrasound famous journals Ultrasound Google Scholar indexed journals Pulmonary Embolism articles Pulmonary Embolism Research articles Pulmonary Embolism review articles Pulmonary Embolism PubMed articles Pulmonary Embolism PubMed Central articles Pulmonary Embolism 2023 articles Pulmonary Embolism 2024 articles Pulmonary Embolism Scopus articles Pulmonary Embolism impact factor journals Pulmonary Embolism Scopus journals Pulmonary Embolism PubMed journals Pulmonary Embolism medical journals Pulmonary Embolism free journals Pulmonary Embolism best journals Pulmonary Embolism top journals Pulmonary Embolism free medical journals Pulmonary Embolism famous journals Pulmonary Embolism Google Scholar indexed journals Radiotherapy articles Radiotherapy Research articles Radiotherapy review articles Radiotherapy PubMed articles Radiotherapy PubMed Central articles Radiotherapy 2023 articles Radiotherapy 2024 articles Radiotherapy Scopus articles Radiotherapy impact factor journals Radiotherapy Scopus journals Radiotherapy PubMed journals Radiotherapy medical journals Radiotherapy free journals Radiotherapy best journals Radiotherapy top journals Radiotherapy free medical journals Radiotherapy famous journals Radiotherapy Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Submassive Pulmonary Embolism articles Submassive Pulmonary Embolism Research articles Submassive Pulmonary Embolism review articles Submassive Pulmonary Embolism PubMed articles Submassive Pulmonary Embolism PubMed Central articles Submassive Pulmonary Embolism 2023 articles Submassive Pulmonary Embolism 2024 articles Submassive Pulmonary Embolism Scopus articles Submassive Pulmonary Embolism impact factor journals Submassive Pulmonary Embolism Scopus journals Submassive Pulmonary Embolism PubMed journals Submassive Pulmonary Embolism medical journals Submassive Pulmonary Embolism free journals Submassive Pulmonary Embolism best journals Submassive Pulmonary Embolism top journals Submassive Pulmonary Embolism free medical journals Submassive Pulmonary Embolism famous journals Submassive Pulmonary Embolism Google Scholar indexed journals Thrombolysis articles Thrombolysis Research articles Thrombolysis review articles Thrombolysis PubMed articles Thrombolysis PubMed Central articles Thrombolysis 2023 articles Thrombolysis 2024 articles Thrombolysis Scopus articles Thrombolysis impact factor journals Thrombolysis Scopus journals Thrombolysis PubMed journals Thrombolysis medical journals Thrombolysis free journals Thrombolysis best journals Thrombolysis top journals Thrombolysis free medical journals Thrombolysis famous journals Thrombolysis Google Scholar indexed journals Spondyloarthritis articles Spondyloarthritis Research articles Spondyloarthritis review articles Spondyloarthritis PubMed articles Spondyloarthritis PubMed Central articles Spondyloarthritis 2023 articles Spondyloarthritis 2024 articles Spondyloarthritis Scopus articles Spondyloarthritis impact factor journals Spondyloarthritis Scopus journals Spondyloarthritis PubMed journals Spondyloarthritis medical journals Spondyloarthritis free journals Spondyloarthritis best journals Spondyloarthritis top journals Spondyloarthritis free medical journals Spondyloarthritis famous journals Spondyloarthritis Google Scholar indexed journals
Article Details
Pulmonary embolism (PE) is the third leading cause of mortality after ST-elevation myocardial infarction and stroke [1,2]. Also, PE remains a significant cause of death in special groups, including pregnant women [2], cancer, traumatic injuries, and very elderly patients [1–3]. Although thrombolysis improves outcomes and mortality in submassive (SM) [4,5] and massive PE [6], respectively, its role is controversial because of the high intracranial hemorrhage rate, especially in >60 years [5]. Furthermore, although 100 mg of alteplase in a two-hour infusion improves mortality compared to heparin alone, minor bleeding complications in octogenarians have increased [7]. Additionally, second and third-generation thrombolytic efficacy and safety are unclear since randomized controlled studies have excluded >75 years [5] because of frailty, multiple comorbidities, and higher bleeding complication risks. Therefore, we are unaware of the best thrombolytic regimen for this population [8]. On the other hand, it is unclear whether advanced treatment decisions are made according to the guidelines [8] or depend on the clinician's perception of clinical severity indicators associated with poor outcomes in SMPE patients [1]. In a post-hoc analysis of the PEITHO trial [9], the authors state the need to recognize more appropriate candidates for thrombolysis through clinical indicators of severity (systolic blood pressure <110 mmHg, respiratory rate >20 breaths/minute, or chronic heart failure) [9]. Recent exploratory analysis suggests thrombolysis decision-making is based on impending clinical deterioration factors other than systolic blood pressure <90 mmHg in very elderly SMPE patients [1]. Both observations indicate the necessity of identifying a subgroup with a higher risk profile that could benefit from advanced treatment in SMPE patients. Therefore, we report that 25 mg alteplase in a one- or two-hour continuous infusion was safe and effective in three very elderly SMPE patients. Additionally, we decided on advanced treatment based on impending clinical deterioration factors (1) rather than clinical instability and systolic hypotension [8].
1. Case 1
ER Clinical Presentation
A 79-year-old woman with a history of right knee arthroplasty, frailty, and GI bleeding six months early. She started with syncope and dyspnea three days earlier. Table 1 shows pre-thrombolysis vital signs. Physical examination showed venous distention, a loud second heart sound, tenderness, warmth edema, and erythema in the left leg. Chest X-ray was not diagnostic. Table 1 shows pre-thrombolysis, ECG, laboratory, and biomarker findings. Table 1 also displays bedside transthoracic echocardiography (TTE) (Video 1) (Figure 1A), computed tomography pulmonary angiography (CTPA) (Figure 1B), and lower limbs ultrasound findings. We confirmed an SMPE and started enoxaparin at 1mg/kg twice daily. On the second day, the dyspnea and D-dimer and cardiac biomarkers improved significantly (Table 2). However, forty-eight hours later, we observed progressive biomarkers increase (Table 2) and new T wave inversion in V1-V3 on ECG despite clinical stability. Therefore, we decided on a treatment escalation using the lower alteplase dose (Table 1) based on three impending clinical deterioration factors [1] increasing biomarkers measurement (Table 2), remaining right ventricular dysfunction (TAPSE 11 mm), and ECG new dynamic ST changes, suggesting enoxaparin therapeutic failure and the possibility of impending clinical instability. We also considered severe pulmonary hypertension and chronic renal disease for clinical decision-making. Additionally, age-associated frailty was determinant for using a lower alteplase regimen and weight-adjusted unfractionated heparin (UFH) as an adjunctive treatment [4] without major or minor bleeding complications (Table 1). Table 1 shows post-thrombolysis TTE, CTPA (Figures 1A and 1B), and ultrasound findings. We also identified biomarkers that improved after thrombolysis and during the follow-up (Tables 1 and 2). She was discharged seven days after with apixaban 5 mg BID. During the six-month follow-up, she was asymptomatic and without recurrences. She had macroscopic hematuria with apixaban temporary discontinuation in a two years-follow-up. Currently, she is in functional class I on apixaban 2.5 mg BID for extension treatment.
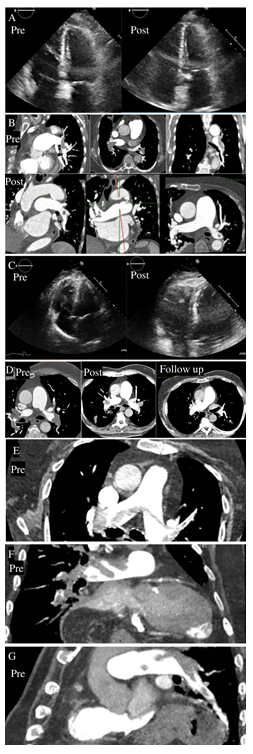
Figure 1: Pre and post-thrombolysis image studies. A) Case 1 pre and post-thrombolysis echo. B) Case 1 pre and post-thrombolysis CTPA. C) Case 2 pre and post-thrombolysis echo. D) Case 2 pre, and post-thrombolysis and follow-up CTPA. E) Case 3 pre and post-thrombolysis CTPA.
|
Before alteplase |
After alteplase |
Before alteplase |
After alteplase |
Before alteplase |
After alteplase |
||
|
Variable |
Case 1 |
Case 1 |
Case 2 |
Case 2 |
Case 3 |
Case 3 |
|
|
Systolic BP (mmHg) |
136 |
125 |
140 |
161 |
122 |
140 |
|
|
Heart rate (bpm) |
72 |
68 |
84 |
82 |
116 |
66 |
|
|
Respiratory Rate (rpm) |
22 |
18 |
24 |
22 |
26 |
16 |
|
|
O2 Saturation (%) |
89 |
98 |
97 |
96 |
86 |
100 |
|
|
sPESI |
1 |
- |
1 |
- |
1 |
- |
|
|
ECG |
Non-specific findings |
T wave inversion V1-V4 |
RBBB, RV overload, and ST dynamic changes |
No RBBB |
S1Q3T3, ST dynamic changes, negative T waves |
Improved dynamic ST changes, atrial fibrillation |
|
|
TTE |
PSAP 76 mmHg, TAPSE 11 mm, RV/LV >2:1, and McConnell sign |
PSAP 59 mmHg, improved RV systolic function, TAPSE 18 mm |
Systolic septal flattening, McConnell’s sign, in-transit thrombus type A |
No in-transit thrombus, RV performance improvement |
Severe RVD, McConnell sign, PASP 46 mmHg, TAPSE of 14.7 mm. |
- |
|
|
CTPA |
Main pulmonary arteries thrombi |
Substantial thrombus burden reduction |
Main pulmonary arteries thrombi |
Significant thrombus burden decrease |
Saddle thrombus |
- |
|
|
LLUS |
DVT left femoral and popliteal veins |
Decrease in thrombi burden |
DVT right tibioperoneal vein |
DVT right popliteal vein and right posterior tibial vein. |
- |
||
|
Hemoglobin (gr/dL) |
14.5 |
- |
17 |
- |
17.5 |
- |
|
|
Leucocytes (cells/mm²) |
15,400 |
- |
9,400 |
- |
13,670 |
- |
|
|
Platelets (cells/mm²) |
263,000 |
- |
256,000 |
- |
235,000 |
- |
|
|
Creatinine (mg/dL) |
2.8 |
- |
1.2 |
- |
0.8 |
- |
|
|
eGFR (mL/min) |
40 |
- |
91 |
- |
119 |
- |
|
|
D-dimer ng/mL |
8,149 |
6,700 |
7,000 |
- |
6, 452 |
14,191 |
|
|
BNP pg/dL |
2,349.50 |
640 |
648.7 |
423 |
226.4 |
167.9 |
|
|
hscTnI ng/L |
178 |
19.1 |
304 |
315.5 |
125.1 |
81.2 |
|
|
Alteplase |
25 mg in 2 hours |
- |
25 mg in 1 hour |
- |
25 mg in 1 hour |
- |
|
|
Adjunctive treatment |
* UFH 24 hours |
* UFH 24 hours |
* UFH 24 hours |
||||
|
Anticoagulation |
Apixaban 5 mg BID |
Rivaroxaban 15 mg BID |
Apixaban 5 mg BID |
Table 1: Pre and post-thrombolysis vital signs, ECG, laboratories, risk stratification and biomarker findings.
RBBB: Right Bundle Branch Block; TTE: Transthoracic Echocardiogram; CTPA: Computed Tomography Pulmonary Angiography; LLUS: Lower Limbs Ultrasound; sGFR: Glomerular Filtration Rate; BNP: B-Type Natriuretic Peptide; hs-cTnI: High-Sensitive Cardiac Troponin I; * Weight-Adjusted Unfractionated Heparin: a constant infusion (12 U/Kg per hour, maximum 1,000 U/h) adjusted to maintain an activated partial thromboplastin time of 50–70 s for 24–48 h.
|
Variable |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
Day 8 |
Day 9 |
Day 10 |
Day 11 |
Day 12 |
|
Case 1 |
||||||||||||
|
DD ng/mL |
8,149 |
7,022 |
3,453 |
3,596 |
- |
11,200 |
19,600 |
6,700 |
6,900 |
2,495 |
2,139 |
2,932 |
|
BNP pg/dL |
2,349 |
1,767 |
1,741 |
500 |
- |
1,030 |
1,016 |
640 |
721 |
529 |
399 |
150 |
|
hscTnI ng/L |
178.7 |
127.2 |
80.4 |
10.5 |
- |
24.4 |
26.1 |
19.1 |
16.9 |
12.2 |
13.3 |
10.4 |
|
Case 2 |
||||||||||||
|
DD ng/mL |
7,000 |
- |
3,773 |
- |
829 |
- |
- |
- |
- |
- |
- |
- |
|
BNP pg/dL |
648.7 |
423 |
125 |
- |
88.9 |
- |
- |
- |
- |
- |
- |
- |
|
hscTnI ng/ |
304 |
315.5 |
140.1 |
- |
43.6 |
- |
- |
- |
- |
- |
- |
- |
|
Case 3 |
||||||||||||
|
DD ng/mL |
6, 452 |
14,191 |
8,636 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
BNP pg/dL |
226.4 |
167.9 |
61.3 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
hscTnI ng/L |
125.1 |
125.1 |
24.4 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Table 2: Biomarker outcome in very elderly pulmonary embolism patients.
DD: D- Dimer; BNP: B-Type Natriuretic Peptide; hscTnI: High Sensitivity Cardiac Troponin I
2. Case 2
2.1 Community Hospital Clinical Presentation
An 81-year-old male patient presents shortness of breath, eight weeks of edema in the lower extremities, and one week of worsening dyspnea. His medical history includes hypertension, diabetes, ischemic heart disease, and coronary bypass (sixteen years ago). He had clinical stability (130/80 mmHg) and oxygen desaturation (88%). ECG had the right bundle branch block, right ventricular pressure overload, and ST dynamic changes. The patient was diagnosed with non-ST elevation myocardial infarction and received UFH and dual antiplatelet therapy.
2.2 ER Clinical Presentation
The patient was transferred to our hospital. Physical examination revealed obesity and warm extremities. Hoffman’s sign was negative. Pre-thrombolysis vital signs are shown in Table 1. The chest X-ray revealed a bilateral Westermark sign, lung infarction, and right diaphragm elevation. Table 1 shows pre-thrombolysis ECG, laboratory, and biomarker findings. Table 1 also displays bedside TTE (Video 2) (Figure 1C), computed tomography pulmonary angiography (CTPA) (Figure 1D), and lower limbs ultrasound findings. After a fast-track meeting with the PREVENTION team [10], we decided lower the alteplase dose (Table 1) based on seven impending clinical deterioration factors [1]: right bundle branch block, ST dynamic changes, higher BNP and hs-cTnI measurements, right ventricular hypokinesia, a significant thrombus burden, and an in-transit thrombus type A. Additionally, age was determinant for using a lower alteplase regimen and weight-adjusted unfractionated heparin (UFH) as an adjunctive treatment [4] without major or minor bleeding complications (Table 1). Table 1 shows post-thrombolysis TTE (Video 2) (Figure 1C), CTPA (Figure 1D), and ultrasound findings. Table 2 shows biomarkers findings. After 24 hours on UFH, we started rivaroxaban with biomarkers improvement (Table 1) (Table 2). At discharge, the patient was asymptomatic with rivaroxaban 20 mg OD. CTPA showed no thrombus in the pulmonary arteries (Figure 1D) in a one-month follow-visit. The patient is alive, without recurrence, in a 19-month follow-up.
3. Case 3
3.1 ER Clinical Presentation
An 83-year-old male arrives with sudden dyspnea and O2 saturation of 86%. He has a past medical history of hypertension, hypothyroidism, ectopic atrial beats, and deep venous thrombosis. Table 1 shows pre and post-thrombolysis vital signs. The physical exam showed a loud second heart sound and bilateral edema. The chest X-ray had a bilateral Westermark sign. Table 1 shows pre and post-thrombolysis baseline ECG, laboratory, and biomarker findings. Table 1 also displays bedside TTE, CTPA (Figure 1E, 1F, 1G), and lower limbs ultrasound findings. Based on clinical presentation and results, we confirmed an SMPE. After a fast-track meeting with the PREVENTION team [10], we decided on advanced treatment based on four impending clinical deterioration factors [1]: hypoxemia <90%, saddle thrombus, right ventricular hypokinesis, and TAPSE of 14.7 mm. Therefore, we started lower the alteplase dose and UFH (Table 1). After thrombolysis, we observed clinical, ECG (Table 1) and biomarkers improvement (Table 2). The patient was discharged on the fifth-day post-thrombolysis without major or minor bleeding complications. Currently, the patient is alive, receiving apixaban without recurrence or bleeding complications in a 3-month follow-up.
We performed a systematic review from 1990 to December 2021, including SM and massive PE patients treated with a systemic lower alteplase dose (<50 mg) through PubMed, ScienceDirect, and Wiley. We used the following terms: (“pulmonary embolism/drug therapy” [MESH]) and “thrombolytic therapy” [MESH]) and (low dose)) or (quarter dose)) or (safe dose) and (1990:2021[pdat])) not (catheter directed)) not (children)) and (alteplase [MESH terms])) not (stroke)) not (catheter)) not (myocardial infarction)) not (pleural infection)) not (hemodialysis)) not (prosthetic valve thrombosis)) not (endovascular)) not (tenecteplase). We identified thirteen papers, including 61 massive and 87 SMPE patients (Table 2) (references in supplementary material).
4. Discussion
Our cases had three relevant findings. First, alteplase 25 mg in short-term continuous infusion and adjunctive treatment weight-adjusted UFH [4] was effective and safe in very elderly SMPE patients, including an in-transit thrombus. Second, our results extended the safety and effectiveness of direct-acting oral anticoagulants (DOAC), improving quality of life and simplifying PE treatment after thrombolysis [11–13]. Finally, early recognition of clinical severity indicators to identify those that could potentially benefit from thrombolysis use through control trials in SMPE is mandatory [1,9,14,15]. There is a worldwide trend in reducing thrombolysis regimens in younger [16] and very elderly ST-elevation myocardial infarction [17], left atrial thrombus [18], obstructive mechanical valve thrombosis [19], and PE with [20] or without COVID-19 [21–24]. However, reduced-dose regimens evidence is insufficient to support their efficacy and safety in PE [15]. The rationality for reducing the alteplase dose to 50 mg [21] is based on the lungs receiving a total cardiac output and convergence point for the whole molecules improving efficacy. Furthermore, after the first passage, repetitious recycling of those molecules occurs by continuously re-entering the lungs, causing “multiple hits” [11]. Therefore, the effectiveness and safety of a “safe dose” is supported by 304 SM and massive PE patients [11,12,21]. However, these studies did not include very elderly patients, so their effectiveness and safety are unknown in this population. Furthermore, the optimal thrombolysis regimen remains uncertain since randomized clinical trials excluded this population [5]. For our patients, the decision-making of the antithrombotic approach was challenging because they are at high risk of thromboembolic and bleeding complications because of age-related changes [25]. Because of this, we have decided to use the lower alteplase dose (25 mg) in a one- or two-hour infusion for those with higher thrombus burden and in-transit thrombus instead of a prolonged lower-dose infusion (Tables 1 and 3). As a result, our patients' in-hospital outcome was free of major, clinically relevant, minor bleeding complications and recurrence. The systematic review identified the safety and effectiveness of prolonged lower-dose (<25 mg) alteplase infusion in recent surgery, pregnancy, cancer, in-transit thrombus, and very elderly SMPE and massive patients (Table 3). Our results also reproduce the effectiveness and safety of the “safer” thrombolysis dose (10 mg alteplase bolus in one minute, followed by 15 mg in 2 hours), UFH 10 units /Kg/ hour for 24 hours, followed by DOAC maintenance dose, 15 minutes after heparin discontinuation [13]. This regimen reduces PSAP, improves right ventricular performance, eliminates ICU stay, and promotes early discharge in 42 SMPE patients [13]. Unfortunately, the results lack the patient's age and thrombolysis decision-making and have not been published extensively. Nevertheless, the rationality for reducing the alteplase dose to 25 mg is similar to 50 mg reduction [21]. Independent of the infusion time, the “safe,” [21] “safer,” (13), ultra-low- [26], and lower alteplase doses in short- or long-term infusion seem to improve the outcome with no increase in significant bleeding complications (Table 1). After the “safe” dose [21], DOAC were safe and effective, modifying the quality of life associated with parenteral anticoagulation and reducing in-hospital stays and costs for patients sixty-seven years of age or younger [11,12]. We reproduced the safety and effectiveness of DOAC when initiated 24 hours after discontinuing UFH [11,12]. We also decided to avoid the loading dose to reduce bleeding risk. Additionally, we are selecting apixaban or rivaroxaban depending on the patient´s characteristics (frailty, bleeding risk, and renal function). Finally, PEITHO-3 will allow any class of DOAC after 48 hours of low-molecular-weight heparin or UHF adjunctive treatment. The results will provide current and robust evidence for the safety and effectiveness of DOAC following a lower alteplase dose [15]. Currently, despite guideline recommendations [8], clinical decision-making for thrombolysis use does not seem to depend on systolic hypotension [1,4,9,11–13,21,26,27], including eighty-seven SMPE patients identified in our systematic review (Table 3). Therefore, proper recognition through clinical severity indicators is mandatory [1,9,14]. In our cases, the number of impending clinical deterioration factors [1] historically related to poor outcomes and pulmonary thrombus burden drove the clinical decision-making for one- or two-hour continuous infusion. PEITHO-3 [15] is a randomized, placebo-controlled, double-blind, multicenter, and multinational trial with long-term follow-up to compare the efficacy and safety of a reduced-dose alteplase regimen with standard heparin anticoagulation. The study will enroll 659 SMPE patients and fulfill at least one clinical criterion of severity: systolic blood pressure <110mm Hg, respiratory rate >20 breaths/min, or history of heart failure [9,15]. The primary efficacy outcome is the composite of all-cause death, hemodynamics, or recurrence within 30 days of randomization. Key secondary outcomes to be included are fatal or GUSTO severe or life-threatening bleeding, net clinical benefit (primary efficacy outcome plus severe or life-threatening bleeding), and all-cause death within 30 days [15]. If the hypothesis of PEITHO-3 is confirmed, international clinical practice guidelines [8] will most likely revisit their recommendations by including reperfusion and reduced-dose systemic thrombolysis as first-line treatments in a new phenotype of SMPE [15]. Conversely, if the hypothesis is rejected, catheter-directed thrombolysis may become the only option for improving the prognosis of SMPE patients [14,15].
Table 2: Systematic review shows demographic characteristics, thrombolysis regimen, indication, success, bleeding complications, and mortality.
Pat: Patients; MB: Major Bleeding; mB: Minor Bleeding; MPE: Massive Pulmonary Embolism; UHF: Unfractionated Heparin; OAC: Oral Anticoagulation; *Two doses. § Firstdose of 25 mg with hemodynamic improvement, a second dose of 25 mg with complete resolution.
¶ 6 mg dose per day for two days. •First dose of 10 mg, second dose of 15 mg. ?Continuous 1mg/h for 24 hours. ?Author postulated that a safer dose could be used. NR: Not reported. PASP: Pulmonary artery systolic pressure.
5. Limitations
We acknowledge that the data in this case series is insufficient to establish the safety and effectiveness of one- or two-hour continuous infusion followed by DOAC in elderly SMPE patients. We cannot identify definitive clinical criteria to recognize “more appropriate” candidates for thrombolysis among submassive PE patients [9]. Nevertheless, our findings are another piece of evidence that supports a worldwide trend, the requirement for clinical severity indicators at presentation [1,4,9,11–13,21,26,27] rather than hemodynamic monitoring and systolic hypotension [8] to start advanced treatment in well-selected SMPE patients [15]. Additionally, our results could help to generate hypotheses that should validate in randomized controlled trials to come [9,15].
6. Conclusion
Our results suggest that the lower alteplase dose in one- or two-hour continuous infusion, followed by weight-adjusted UFH, was effective and safe, involving a complicated scenario as an in-transit thrombus. Also, after stopping UHF, standard doses of DOAC driven by the patient´s characteristics were unrelated to bleeding complications avoiding recurrence in very elderly SMPE patients.
Authors Contributions
Juan Quintanilla; Was the physician in charge of patient care, creating the alteplase dose, and then reading, reviewing, and approving the manuscript. Maria Fernanda Reyes-Chavez; Project coordination, data extraction from the articles for the systematic review, creation of tables, images, and videos, writing, and case revision. Then read, reviewed, and approved the manuscript. Carlos Jerjes-Sanchez; Coordination and conduction of the project with the cardiology residents and students. Supervised the tables, images, and videos. Revise cases and write the manuscript. Melissa Galindo-Garza; Coordination and planification of the project with the cardiology residents. She performed journal article searching and data extraction for the systematic review, created table No.2, and revised the cases. Then, read, reviewed, and approved the manuscript. Aldo F Ponce-Barahona; Contributed to searching journal articles for the systematic review, creating tables, revising the cases, write the first case. Read, reviewed, and approved the manuscript. Vanessa Alegria-Saldivar; Contributed to the data extraction from the articles for the systematic review, creating tables and images, and writing and revising the cases. Read, reviewed, and approved the manuscript. Arturo Adrian Martínez Ibarra; Searching for journal articles and data extraction for the systematic review, creating table No.2, revising the cases, and making the final manuscript. Read, reviewed, and approved the manuscript. Armando Osorio-Salazar; Contributed to creating tables, writing, and revising the cases and manuscript. Read, reviewed, and approved the manuscript. José Alfredo Salinas-Casanova- Contributed to the data extraction from the articles for the systematic review, creation of table, revision of the cases, and final manuscript. Read, reviewed, and approved the manuscript. Ricardo J. Estrada-Mendizabal; Contributed to table creation, writing, and revising the cases and manuscript. Read, reviewed, and approved the manuscript. Renata Quevedo-Salazar; Contributed to table creation, writing, and revision of the cases. Read, reviewed, and approved the manuscript. Sofía Guardado Vázquez; Contributed tables creation, writing, and revision of the cases. Read, reviewed, and approved the manuscript. Paola Gutierrez-Gallegos; Contributed to table creation, writing, and revising the cases and manuscript. Read, reviewed, and approved the manuscript. Victor E. Lozano-Corres; Contributed table creation and writing of the cases. Read, reviewed, and approved the manuscript.
References
- Castillo-Perez M, Jerjes-Sánchez C, Rodríguez D, et al. Clinical outcomes of very elderly patients treated with ultrasound-assisted catheter-directed thrombolysis for pulmonary embolism: a systematic review. J Thromb Thrombolysis [Internet] (2021).
- Jerjes-Sánchez C, Rodriguez D, Farjat AE, et al. Pregnancy-Associated Venous Thromboembolism: Insights from GARFIELD-VTE. TH Open 05 (2021): e24-34.
- Rodriguez D, Jerjes-Sanchez C, Fonseca S, et al. Thrombolysis in massive and submassive pulmonary embolism during pregnancy and the puerperium: a systematic review. J Thromb Thrombolysis 50 (2020): 929-941.
- Jerjes-Sánchez C, Villarreal-Umaña S, Ramírez-Rivera A, et al. Improving adjunctive treatment in pulmonary embolism and fibrinolytic therapy. The role of enoxaparin and weight-adjusted unfractionated heparin. J Thromb Thrombolysis 27 (2009): 154-162.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for Patients with Intermediate-Risk Pulmonary Embolism. New England Journal of Medicine 370 (2014): 1402-1411.
- Jerjes-Sanchez C, Ramírez-Rivera A, García M de L, et al. Streptokinase and Heparin versus Heparin Alone in Massive Pulmonary Embolism: A Randomized Controlled Trial. J Thromb Thrombolysis 2 (1995): 227-229.
- Zengin A, Karatas MB, Çanga Y, et al. Terapia Trombolítica em Octogenários com Embolia Pulmonar Aguda. Arquivos Brasileiros de Cardiologia [Internet] (2021).
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). European Heart Journal (2019): ehz405.
- Barco S, Vicaut E, Klok FA, et al. Improved identification of thrombolysis candidates amongst intermediate-risk pulmonary embolism patients: implications for future trials. Eur Respir J 51 (2018): 1701775.
- del Toro-Mijares R, Jerjes-Sánchez C, Rodríguez D, et al. Equipo de respuesta rápida para tromboembolismo venoso del Hospital Zambrano Hellion (PREVENTION -Team): Mejorando el manejo del tromboembolismo pulmonar y trombosis venosa profunda. ACM 90 (2020): 2799.
- Sharifi M, Bay C, Schwartz F, et al. Safe-Dose Thrombolysis Plus Rivaroxaban for Moderate and Severe Pulmonary Embolism: Drip, Drug, and Discharge: Thrombolysis plus rivaroxaban in PE. Clinical Cardiology 37 (2014): 78-82.
- Sharifi M, Vajo Z, Freeman W, et al. Transforming and Simplifying the Treatment of Pulmonary Embolism: “Safe Dose” Thrombolysis Plus New Oral Anticoagulants. Lung 193 (2015): 369-374.
- Sharifi M, Karandish K, Schroeder B, et al. Quarter or Safer Dose Thrombolysis in Submassive Pulmonary Embolism. Circulation (2019): 140.
- Obi M, Packer CD. Submassive Pulmonary Embolism: A Re-evaluation of Hemodynamic Instability. Cureus [Internet] (2019).
- Sanchez O, Charles-Nelson A, Ageno W, et al. Reduced-Dose Intravenous Thrombolysis for Acute Intermediate–High-risk Pulmonary Embolism: Rationale and Design of the Pulmonary Embolism International THrOmbolysis (PEITHO)-3 trial. Thromb Haemost (2021): a-1653-4699.
- Gurewich V. Therapeutic Fibrinolysis. Journal of the American College of Cardiology 68 (2016): 2099-2106.
- Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or Primary PCI in ST-Segment Elevation Myocardial Infarction. N Engl J Med 368 (2013): 1379-1387.
- Wolff G, Kelm M, Westenfeld R, et al. Low-Dose Thrombolysis for the Management of Left Atrial Thrombus Formation During Percutaneous Mitral Valve Repair. JACC: Cardiovascular Interventions 12 (2019): e9-10.
- Özkan M, Gündüz S, Güner A, et al. Thrombolysis or Surgery in Patients With Obstructive Mechanical Valve Thrombosis. Journal of the American College of Cardiology 79 (2022): 977-989.
- Betancourt-del Campo H, Jerjes-Sanchez C, Castillo-Perez M, et al. Systemic thrombolysis and anticoagulation improved biomarker measurements in massive-like pulmonary embolism and severe COVID-19 pneumonia: a case report. Brown RA, Bouzas-Mosquera A, Cankovic MZ, Rampat R, Sayers M, Thomson R, editors. European Heart Journal - Case Reports (2020): ytaa448.
- Sharifi M, Bay C, Skrocki L, et al. Moderate Pulmonary Embolism Treated With Thrombolysis (from the “MOPETT” Trial). The American Journal of Cardiology 111 (2013): 273-277.
- Weng C, Wang X, Huang L, et al. Low-dose urokinase thrombolytic therapy for patients with acute intermediate-high-risk pulmonary embolism: A retrospective cohort study. Er F, editor. PLoS ONE 16 (2021): e0248603.
- Hoang BH, Do PG, Le LD, et al. Safety, Efficacy of an Accelerated Regimen of Low-Dose Recombinant Tissue-Type Plasminogen Activator for Reperfusion Therapy of Acute Pulmonary Embolism. Clin Appl Thromb Hemost (2021): 107602962110379.
- Alcántara Carmona S, Pérez Redondo M, Nombela Franco L, et al. Local low-dose urokinase thrombolysis for the management of haemodynamically stable pulmonary embolism with right ventricular dysfunction. EuroIntervention 14 (2018): 238-246.
- Bauersachs RM. Managing venous thromboembolism with novel oral anticoagulants in the elderly and other high-risk patient groups. European Journal of Internal Medicine 25 (2014): 600-606.
- Guru PK, Giri AR, Sanghavi DK, et al. Ultra-Low-Dose Systemic Tissue Plasminogen Activator in High-Risk Submassive Pulmonary Embolism. Mayo Clinic Proceedings 97 (2022): 1158-1163.
- Jerjes-Sanchez C, Ramirez-Rivera A, Arriaga-Nava R, et al. High dose and short-term streptokinase infusion in patients with pulmonary embolism: prospective with seven-year follow-up trial. J Thromb Thrombolysis 12 (2001): 237-247.
Supplementary Material
References Table 3
- Biteker M, Duran NE, Gündüz S, et al. Treatment of Pulmonary Embolism with Low-Dose Prolonged Infusion of Tissue-Type Plasminogen Activator in an 85-Year-Old Woman: Letters to the Editor. Journal of the American Geriatrics Society 57 (2009): 745-746.
- Biteker M, Duran NE, Ozkan M. Successful treatment of massive pulmonary embolism in a pregnant woman, with low-dose, slow infusion of tissue plasminogen activator. Turk Kardiyol Dern Ars 38 (2010): 32-34.
- Yildiz M, Karakoyun S, Acar RD, et al. Effectiveness of low-dose prolonged infusion of tissue plasminogen activator in a nonagenarian patient with acute pulmonary embolism and main pulmonary artery thrombus. Blood Coagulation & Fibrinolysis 24 (2013): 95-96.
- Sen F, Karavelioglu Y, Arisoy A. Low-dose tissue plasminogen activator in the treatment of a massive pulmonary thromboembolism in a colon cancer patient treated with bevacizumab: A case report. Oncology Letters 8 (2014): 2779-2781.
- Aykan AC, Boyaci F, Hatem E. Successful treatment of a pulmonary embolism with low dose prolonged infusion of tissue typed plasminogen activator in a 37 year old female in early postoperative period. Anadolu Kardiyol Derg 14 (2014): 400-402.
- Shen L, Li Y, Hernandez-Arenas LA, et al. Successful treatment of a pulmonary embolism with low dose of tissue plasminogen activator after thoracic surgery. The American Journal of Emergency Medicine 34 (2016): 2259.e5-2259.e6.
- Aykan AC, Gokdeniz T, Aykan DA, et al. Low dose prolonged infusion of tissue type plasminogen activator therapy in massive pulmonary embolism. European Heart Journal 37 (2016).
- Zencirkiran Agus H, Uygur B, Guler A, et al. Successful treatment of massive pulmonary embolism with low-dose tissue plasminogen activator after meniscus surgery. Blood Coagulation & Fibrinolysis 29 (2018): 559-561.
- Kalkan ME, Yildiz M, Ak HY, et al. Safety of low-dose prolonged infusion of tissue plasminogen activator therapy in patients with thromboembolic events in the intensive care unit. Kardiologiia 60 (2020): 86-90.
- Lozier JN, Elinoff JM, Suffredini AF, et al. Low-dose, short course alteplase treatment of submassive pulmonary embolism: a case series from the National Institutes of Health Clinical Center. Blood Coagulation & Fibrinolysis 29 (2018): 701-707.
- Zhang LY, Gao BA, Jin Z, et al. Clinical efficacy of low dose recombinant tissue-type plasminogen activator for the treatment of acute intermediate-risk pulmonary embolism. SMJ 39 (2018): 1090-1095.
