Rutin: A Novel A2AR Antagonist with Promising Therapeutic Potential for Cancer Immunotherapy
Article Information
Sarah Kandoussia, Soumaya Rafiia, Yassine El Ghallabb, Souha Sahraouia,c, Abdallah Badoua*
aLaboratory of Immuno-Genetics and Human Pathologies, Faculty of Medicine and Pharmacy of Casablanca, Hassan II University, Casablanca, Morocco
bLaboratory of Drugs Sciences, Biomedical Research and Biotechnology, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
cMohammed VI Center of Oncology, CHU Ibn Rochd, Faculty of Medicine and Pharmacy of Casablanca, Hassan II University, Casablanca, Morocco
*Corresponding Author: Abdallah Badou, Laboratory of Immuno-Genetics and Human Pathologies, Faculty of Medicine and Pharmacy of Casablanca, Hassan II University, Casablanca, Morocco.
Received: 21 February 2025; Accepted: 03 March 2025; Published: 21 August 2025.
Citation: Sarah Kandoussi, Soumaya Rafii, Yassine El Ghallab, Souha Sahraoui, Abdallah Badoua. Rutin: A Novel A2AR Antagonist with Promising Therapeutic Potential for Cancer Immunotherapy. Journal of Bioinformatics and Systems Biology. 8 (2025): 51-57.
View / Download Pdf Share at FacebookAbstract
Tumor cells evade immune surveillance through various mechanisms, including the upregulation of inhibitory immune checkpoints like the A2AR receptor. Targeting these checkpoints to reactivate anti-tumor immunity has become a major focus of cancer immunotherapy research. Recent efforts have explored the potential of natural molecules to modulate these checkpoints, aiming to minimize the side effects and high costs associated with antibody-based immunotherapies. In this study, we investigated rutin and eugenol, two naturally occurring compounds with reported therapeutic benefits, as potential A2AR antagonists. Our in silico docking analysis reveals that rutin binds to A2AR with high affinity and strong binding force, engaging three key amino acid residues implicated in tumor progression. This suggests that rutin may function as a potent A2AR antagonist, potentially reversing immune suppression and hindering tumor development. Furthermore, in vitro experiments using human peripheral blood mononuclear cells (PBMCs) demonstrated that rutin is non-toxic up to 900μM and, notably, exhibits a dose-dependent effect on succinate dehydrogenase (SDH) activity, a marker of mitochondrial function and metabolic activity. These findings suggest that rutin not only binds strongly to A2AR but also influences PBMC activity, warranting further investigation of its therapeutic potential in cancer immunotherapy.
Keywords
Rutin, Cell Proliferation, SDH, A2AR, Immune Checkpoints, Natural molecule, Flavonoids.
Rutin articles; Cell Proliferation articles; SDH articles; A2AR articles; Immune Checkpoints articles; Natural molecule articles; Flavonoids articles.
Article Details
1. Introduction
The immune system maintains homeostasis by defending against exogenous threats while preventing autoimmunity. This delicate balance is achieved through complex regulatory mechanisms, including immune checkpoints (ICPs). These are crucial modulators of immune cell activation, preventing overactivation and autoimmunity, but also influencing anti-tumor immunity[1]. However, ICPs can be co-opted by the tumour, allowing it to evade the immune system's surveillance. As a result, the immune cells primed to target the cancer cells are instead constrained by diverse molecular pathways, which inhibit their activation and, consequently, their effector functions[1]. Among several checkpoint molecules, we can highlight the Adenosine Receptor (A2a). Adenosine signaling through its A2A receptor impairs an effective anti-tumor immune response by inhibiting the infiltration of cytotoxic cells such as CD8+ T cells and Natural killer (NK) cells. Additionally, this signaling suppresses T cell proliferation and cytokine production[2]. A recent study showed that the pharmacological blockade of A2A-R by the antagonist SCH58261 in mouse models of squamous cell carcinoma of the head and neck suppressed tumor growth, induced a reduction in CD4+ Foxp3+ regulatory T cells, and improved the antitumor immune response of CD8+ T cells. This suggests that the A2A receptor may be a promising therapeutic target for treating this type of cancer[3]. Furthermore, other antagonists, including CPI-444, are currently being tested in combination with anti-PD-1 and anti-CTLA-4 therapies. This combination has demonstrated very potent results in preclinical models of colon adenocarcinoma metastasis, stimulating CD8+ T cells and eliminating tumors[4].
Recently, to inhibited the immune checkpoints molecules, several studies are directed towards the use of natural products to avoid the negative side effects that can occur during immunotherapy based on antibodies and also to make this therapy more accessible to the majority of patients[5, 6].
In our study, we evaluated Rutin and Eugenol as A2A-R antagonists, based on their beneficial effects demonstrated across multiple studies. We then assessed the optimal dosage range for the most promising candidate, ensuring it was not toxic to human peripheral blood mononuclear cells using the MTT assay. Rutin, a widely distributed flavonoid, has been extensively studied for its anti-cancer properties in colorectal cancer, melanoma, and hepatic carcinoma[7]. Chen et al. reported the anti-neuroblastoma effect of rutin, which involved inhibiting the growth and chemotactic ability of LAN-5 cells. Additionally, rutin is known to inhibit cancer cell growth by modulating the cell cycle and/or inducing apoptosis, as well as by inhibiting proliferation, angiogenesis, or metastasis in colorectal cell lines[8]. Eugenol, the active molecule of Syzigium aromaticum, is found in various aromatic plants like nutmeg, basil, cinnamon, and bay leaves. Recent studies have shown that eugenol possesses antioxidant, antimutagenic, antigenotoxic, anti-inflammatory, and anticancer properties[9]. The molecular mechanisms of eugenol-induced apoptosis in melanoma, leukemia, and osteosarcoma have been well documented [10, 11]. Furthermore, eugenol is a potent inhibitor of melanoma cell proliferation[12].
2. Material and methods
2.1 Ligands and receptor preparation
ChemOfficeProfessional version 18.0.0.231(http://www.cambridgesoft.com/) was used to generate a better ligand conformation for docking in Protein Data Bank(PDB) file format, the drawed3D geometry structures of Rutin, Eugenol and the experimental antagonists CPI-444 and SCH58621 used as controls were optimized to a minimum energy (Allinger 1977). All rotatable bonds present in the ligands were treated as non-rotatable. The Gasteiger charges were added to the ligand atoms prior to docking (Gasteiger and Marsili 1980). The crystal structure of A2AR (PDB ID: 6GDG) was retrieved from RCSB Protein Data Bank (https://www.rcsb.org/). All water molecules and heteroatoms were removed from the crystal structures, and polar hydrogen atoms were added using Discovery Studio Visualizerv19.1.0.18287 (https://www.3ds.com/) Grid box preparation and docking.
Docking experiments were conducted with Rutin, Eugenol, Caffeine and both antagonists CPI-444 and SCH58621 on the A2A receptor. Grid box parameters were set by using the graphic user interface AutoDock Tools (ADT), version 1.5.6, of Molecular Graphics Laboratory(MGL) software packages ofThe Scripps Research Institute (Michel et al. 2011).The center grid box was set at x = 78.122, y = 89.064 and z = 85.083 points, the number of points in x, y and z dimensions was adjusted to84, 120 and 76. The spacing value between grid points was 1 Å.The molecular docking program AutoDock Vina (version 1.1.2) (Trott and Olson 2010) was used to perform the docking experiment. The Lamarkian Genetic Algorithm was used during the docking process to explore the best conformation space for the ligand with a population size of 150 individuals. The maximum number of generations was set at 27000 and the evaluations one was set at 2500000. Other parameters were set as default:
1. Receptor preparation
The receptor, initially downloaded in PDB format, was prepared for docking using AutoDock Tools. All extraneous molecules, including water, were removed. This allowed for explicit water molecule modeling and the optimization of hydrogen atom placement for receptor stabilization. The prepared receptor was subsequently converted to the PDBQT docking format.
2. Ligands preparation
The preparation of the receptor was done by AutoDock Tools software. The purpose was to make the molecules more rigid by stabilizing the rotary regions, then we converted it into the docking format which is the PDBQT.
2.2 Docking
Molecular docking was performed using AutoDock Vina to evaluate the binding affinity between the A2AR receptor and the ligands, utilizing their respective PDBQT formats. A Vina configuration file, defining the grid box dimensions encompassing the receptor and ligands, was generated. AutoDock Vina was then employed to calculate the binding free energy for each ligand.
2.3 Analysis of docking results
Docking results were visualized using Discovery Studio, beginning with the receptor and ligands in PDBQT format. Interactions between the A2AR receptor and each ligand were analyzed, including bond length calculations. Interacting residues were identified and labeled to pinpoint the amino acid region involved in ligand binding.
2.4 PBMC preparation and cell culture
Fresh venous blood was collected from two healthy adult volunteers (18-45 years old) after obtaining written informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Histopaque (d = 1.077 g/ml; Sigma, USA) at 900g for 25 minutes at 18-20°C. The isolated cells were washed three times with RPMI-1640 (400g, 15 minutes) and resuspended in RPMI-1640 supplemented with L-glutamine, 10% heat-inactivated newborn calf serum (Sigma, USA), 26.3 g/L penicillin, and 4.2 g/L streptomycin. Cell counts were determined using a hemocytometer and Trypan blue exclusion, with microscopic verification. PBMCs were cultured in triplicate at 37°C with 5% CO2 (Heracell 150i CO2 incubator, Thermo Fisher Scientific, France) in culture plates. Cells were incubated with varying doses of Rutin (78095-25MG-F, Fluka) with and without 5 μg/mL phytohemagglutinin (PHA; Sigma, USA).
2.5 MTT assay
Cell viability was assessed using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. PBMCs (5 x 105 cells/mL) were cultured in 96-well plates (Hiwaka, Japan) at 37°C with 5% CO2 for 96 hours, with varying concentrations of Rutin, in the presence or absence of PHA. Following incubation, 20 μL of MTT solution (5 mg/mL in RPMI, filter-sterilized at 0.2 μm and stored at -20°C) was added to each well, and plates were incubated for 4 hours at 37°C in a humidified 5% CO2 atmosphere. After centrifugation (800g, 20 minutes), 100 μL of DMSO was added to each well to dissolve the resulting formazan crystals. Optical density, reflecting SDH enzymatic activity, was measured. The percentage of SDH enzymatic activity was calculated as:
% SDH enzymatic activity = (OD test / OD control) * 100.
Higher OD values indicate greater cell viability due to the reduction of MTT by metabolically active cells.
2.6 Statistical analysis
Statistical analysis was performed using unpaired Student’s test. The value of P < 0.05 was considered statistically significant.
3. Results:
In order to study the interaction between A2AR and the two substances studied (Eugenol and Rutin), we first prepared the A2AR receptor, Eugenol and Rutin by the software Autodock tools, then we evaluated the affinity between A2AR and each substance using AutoDock Vina software. The affinity results in an energy score of the bond between the two molecules. The lower the energy score, the stronger the affinity. A comparison was made with two control antagonists, CPI-444 and SCH58261, and a chemical caffeine which have shown a potent effects on the inhibition of A2AR in the literature.
Table 1: The energy score of the interaction of therapeutic molecules with A2AR in (Kcal/mol).
|
Substance name |
Energy score (Kcal/mol) |
|
Rutin |
-12,8 |
|
Eugenol |
-6,6 |
|
CPI-444 |
-9,4 |
|
SCH58261 |
-9,5 |
|
Caffein |
-6,7 |
This table shows the energy scores (in Kcal/mol) for the interaction of five different therapeutic molecules with the A2AR receptor. The lower (more negative) the energy score, the stronger the binding affinity between the molecule and the receptor.
These results showed that eugenol had an energy score of -6.6 Kcal/mol with A2AR, which was lower than the control compounds studied, demonstrating its weak affinity with A2AR. In contrast, rutin had an energy score of -12.8 Kcal/mol, which was lower than the energy scores of -6.7 Kcal/mol, -9.4 Kcal/mol, and -9.5 Kcal/mol for the A2AR antagonist controls, Caffeine, CPI-444, and SCH58261, respectively. (Table1)
This strong affinity of rutin with A2AR prompted us to investigate the strength of this binding in more detail, using the discovery studio visualiser software to determine the different types of bonds and the binding distance between our receptor and rutin. We also analyzed these parameters in the molecules CPI-444, SCH58261, and Caffeine, which served as controls.
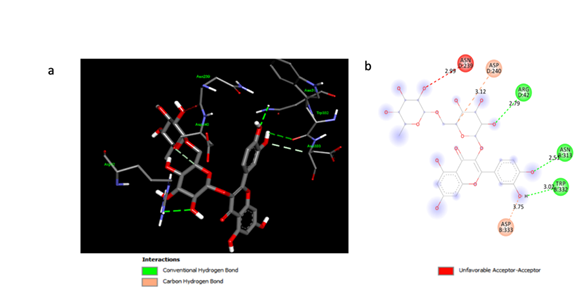
(a) The 3D structure highlights rutin binding to key residues ASN8:313, TRP8:332, and ASP8:333 (and ASP0:240), with conventional hydrogen bonds (green), a carbon-hydrogen bond (pink), and an unfavorable acceptor-acceptor interaction (red). (b) The 2D diagram simplifies this interaction, showing bond distances (Å) for the non-covalent (red), carbon-hydrogen (pink), and hydrogen (green) bonds.
The docking study identified the Rutin binding site on the A2AR receptor and revealed the formation of three hydrogen bonds between the two molecules. These interactions are illustrated in 2D (Figure 1b) and 3D (Figure 1a) representations. The observed hydrogen bond distances were 2.51, 2.79, and 3.02 Å.
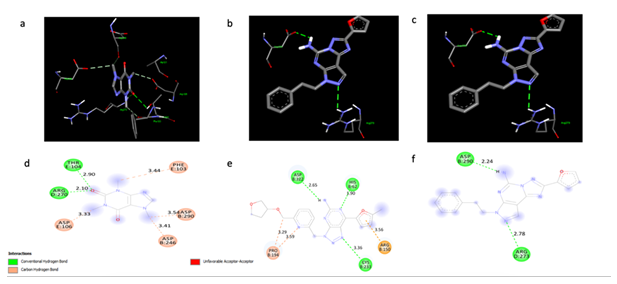
The interactions between A2AR and control molecules: caffeine, CPI-444, and SCH58261. 3D (a, b, c) and 2D (d, e, f). The 3D structures illustrate how each molecule fits into the A2AR binding pocket, while the 2D diagrams highlight specific interacting residues and the types of non-covalent interactions formed. In the 2D schematics, red indicates non-covalent bonds, pink represents carbon-hydrogen bonds, and green denotes hydrogen bonds. Distances between interacting residues are shown in Ångströms. These visualizations allow for a comparative analysis of the binding modes and potential affinities of these control molecules for the A2AR receptor.
The three control molecules, caffeine, SCH58261, and CPI-444, formed two, two, and three hydrogen bonds with the A2AR receptor, respectively. The bond distances observed were comparable to those of Rutin (Figure 2a, b). Rutin's binding site on A2AR involves three amino acid residues: Arginine, Asparagine, and Tryptophan (Figure 1a, b). In contrast, caffeine interacts with Arginine and Threonine; SCH58261 with Arginine and Aspartate; and CPI-444 with Aspartate, Cysteine, and Histamine (Figure 2a, b).
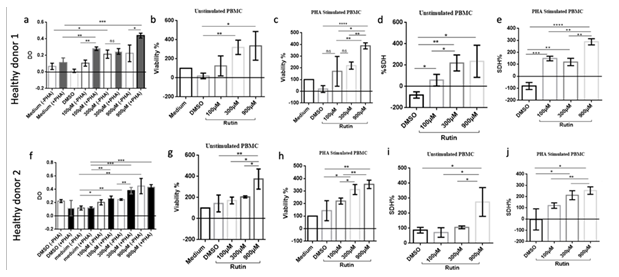
This figure shows Rutin's effects on PBMC viability and SDH activity in two healthy donors, with/without PHA stimulation. (a, f) OD of stimulated/unstimulated PBMCs at 0, 100, 300, and 900 μM Rutin. (b, g) Unstimulated PBMC viability (%). (c, h) PHA-stimulated PBMC viability (%). (d, i) Unstimulated PBMC SDH activity (%). (e, j) PHA-stimulated PBMC SDH activity (%). For all panels, data are presented as mean ± SEM from triplicate experiments. Statistical significance is indicated by (*p<0.05, **p<0.01, *p<0.001, ****p<0.0001).
In order to assess Rutin's impact on PBMC activity, cells from two healthy donors were cultured with varying concentrations of Rutin (100µM, 300µM, and 900µM) in the presence and absence of phytohemagglutinin (PHA). Cell viability, optical density (OD), and succinate dehydrogenase (SDH) activity were measured. Rutin did not induce cell toxicity, even at high doses (900 µM), as demonstrated by the observed increase or maintenance of cell viability across all concentrations tested (Fig.3 a, b, f, g). Interestingly, Rutin significantly increased succinate dehydrogenase (SDH) activity, both in the presence and absence of PHA (Fig.3 c, d, h, i). This enhancement of mitochondrial SDH enzymatic activity suggests a potential increase in PBMC proliferation and/or metabolic activity. These findings indicate that Rutin, rather than being toxic, may stimulate PBMC metabolic function and potentially promote cell proliferation.
Numerous studies have sought to develop novel cancer immunotherapies by targeting inhibitory immune checkpoints, such as the A2AR receptor, to reinvigorate anti-tumor immune responses. The use of natural products has garnered significant interest as a means to mitigate the side effects and high costs associated with antibody-based immunotherapies, aiming to improve patient accessibility. This study investigates the potential antagonistic effects on A2AR of two naturally derived compounds with reported therapeutic properties: rutin and eugenol. Furthermore, we determined the optimal, non-toxic dosage range for the most promising candidate using the MTT assay on human peripheral blood mononuclear cells.
We started by determining the affinity level of our molecules of interest, the rutin and eugenol, with the A2AR receptor, using the Autodock vina software. The affinity was measured via the energy score in kcal / mol. The lower the score, the better is the affinity. Our results showed that eugenol had an energy score of -6.6 Kcal / mol with A2AR, which represents a low affinity. However, the affinity with the rutin was strong as the energy calculated by the software was -12.8 Kcal / mol. Then we analyzed therapeutic molecules known in the literature, which regulate the immunosuppressive effect of A2AR such as CPI-444 and SCH58261 and Caffeine so we could compare them to the Rutin. . Eini et al. have shown, in an in vivo study using murine models in which tumor was induced with the carcinogen 3-methylcholanthrene, that treatment with caffeine promotes an effective anti-tumor immune response during tumor initiation by blocking A2AR. As a result, the production of IFNγ increased, and the response of tumor-specific memory T lymphocytes was greatly improved [6]. Similarly, CPI-444, an active and selective A2AR antagonist, has demonstrated effectiveness in vitro and in vivo[4] and is currently being evaluated in clinical trials for patients with advanced cancers (NCT03454451) . Pharmacological blockade of A2AR by the selective antagonist SCH58261 delayed tumor growth in a head and neck squamous cell carcinoma mouse model and significantly reduced the population of CD4+ Foxp3+ regulatory T cells. Furthermore, this blockade enhanced the anti-tumor response of CD8+ T cells[3]. Additionally, A2AR blockade with SCH58261 showed great potential in managing retinal neurodegenerative diseases characterized by microglial reactivity, such as glaucoma and ischemic diseases[13].
In this sense, we opted for a comparative study in silico to assess the affinity of this molecule and the sites where it binds with A2AR compared to caffeine. The results of the binding energy score of these molecules with the A2AR receptor are -6.7 Kcal / mol, -9.4 Kcal / mol and -9.5 Kcal / mol for CPI-444 and SCH58261 and Caffeine respectively. These scores are higher than that of rutin, which has the strongest affinity for A2AR. In contrast, eugenol displayed a lower affinity compared to the controls.
Furthermore, this energy score between rutin and A2AR is lower than the energy scores of the caffeine antagonist controls, CPI-444 and SCH58261 (-6.7 Kcal / mol, -9.4 Kcal / mol and -9.5 Kcal / mol respectively) with A2AR. This low binding energy score for rutin indicates a strong affinity between rutin and the A2AR receptor.
Then, using the discovery studio visualization software, we tried to see if the binding between A2AR and rutin was strong enough, by analyzing the two parameters which are the number of covalent bonds of hydrogen and the bond distance between our receptor and rutin. 2D and 3D configurations of the interactions between A2AR and rutin showed that the latter had three hydrogen bonds with distances were 2.51, 2.79 and 3.02 Ångström. They were similar to those of the controls. However, the distance is short between the two molecules which shows a strong and important binding force. This suggests that rutin may be strongly linked to A2AR.
After having these interesting results of the interaction between rutin and A2AR, it was important to identify the binding site, to have an idea about the signaling that results by the contact between our A2AR receptor and rutin, via the amino acids: Arginine, Asparagine and Tryptophan. These three amino acids have positive scientific evidence in the treatment of cancer. Lots of studies showed that the Arginine is linked to inflammation[14];they claim that in colorectal cancer, high levels of Arginine promote and inhibit tumor growth, depending on the tumor development stage[15]. There is also the influence of arginine on metastases through polyamine and nitrate oxide in some cancers[16]. Regarding the other amino acid which is asparagine, a study has shown that arginine depletion has a lower toxicity profile and can effectively reduce the level of pro-cancer biochemicals in patients[17]. And maintaining intracellular asparagine levels is essential for the growth of cancer cells[18]. Likewise,for the third amino acid, a high consumption of the Tryptophan, helps tumors to overcome immune barriers and cancer progression[19]. These results suggest that the binding of rutin to A2AR via these amino acids could activate a cascade of signaling pathways that will lead to upregulation or downregulation of the anti-tumor immune response.
Since we found that Rutin is a promising candidate as an A2AR antagonist, we sought to determine its potential toxicity and effects on cell proliferation. To this end, we employed the MTT assay on human PBMCs. Our results demonstrated that Rutin did not induce significant cellular toxicity, even at relatively high doses (up to 900 μM). We also observed an increase in SDH enzymatic activity, a marker of mitochondrial function and metabolic activity, in the presence of Rutin, both with and without PHA stimulation. This increase suggests that Rutin may have a positive effect on PBMC proliferation and/or metabolic activity. Chen et al. suggest that while rutin can exhibit cytotoxic effects on cancer cells, its impact on healthy cells is less pronounced. However, the specific mechanisms and the extent of toxicity are likely dependent on the cell type and experimental conditions. Therefore, it's crucial to consider the specific context when evaluating rutin's toxicity[8]. Sahiner et al. further supports rutin's anticancer potential, showing its inhibitory effect on cancer cells while exhibiting good biocompatibility with blood and fibroblast cells[20]. Asfour & Mohsen et al. also highlights rutin's enhanced cytotoxic activity against colon carcinoma cells when encapsulated in pH-sensitive nanospheres[21].
These findings are encouraging and suggest that Rutin could be a promising candidate for the development of novel therapies targeting the A2AR receptor, particularly in combination with agents stimulating immune activation. However, further studies will be needed to determine the exact mechanism by which Rutin exerts these effects and to evaluate its therapeutic potential in vivo.
4. Conclusion
In silico screening proved valuable in analyzing the interaction between rutin and the A2AR receptor, a key inhibitor of the immune response implicated in cancer progression. Our docking results demonstrate that rutin binds to A2AR with high affinity and strong binding force, engaging three amino acid residues known to be involved in tumor progression. This suggests that rutin may act as a potent A2AR antagonist, potentially enhancing the immune system's ability to control tumor growth. Furthermore, our in vitro studies using human peripheral blood mononuclear cells (PBMCs) and the MTT assay revealed that rutin is not toxic to these cells, even at high concentrations. Importantly, we observed a dose-dependent effect of rutin on SDH activity, a marker of mitochondrial function and metabolic activity, suggesting a potential influence on PBMC proliferation and/or metabolic activity. This dual observation of potent A2AR binding and non-toxic, potentially proliferative effects on PBMCs supports the further investigation of rutin as a promising candidate for cancer immunotherapy. Future studies will focus on elucidating the precise molecular mechanisms underlying rutin's interaction with A2AR and its impact on immune cell function, as well as evaluating its efficacy in in vivo tumor models.
Competing Interest
The authors declare no conflicts of interest.
This work was supported with grants from The Moroccan Ministry of Higher Education and Research (grant PPR1, type B for AB): PPR1/B; The Moroccan Ministry of Higher Education, Research and Innovation, the Moroccan Ministry of Industry, Commerce, Green and Digital Economy, and the Digital Development Agency (Alkhawarizmi grant for AB): Alkhawarizmi/2020/08; and the Intra-Africa Academic Mobility Grant provided by the EUROPEAN COMMISSION, ref number 624289-PANAF-1-2020-1-KEPANAFMOBAF.
References
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27 (2015):450–461.
- Young A, Mittal D, Stagg J, et al. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discovery 4 (2004), 879–888.
- Ma S-R, Deng W-W, Liu J-F, et al. Blockade of adenosine A2A receptor enhances CD8+ T cells response and decreases regulatory T cells in head and neck squamous cell carcinoma. Mol Cancer 16 (2017).
- Willingham SB, Ho PY, Hotson A, et al. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti-PD-(L)1 and Anti-CTLA-4 in Preclinical Models. Cancer Immunol Res 6 (2018): 1136–1149.
- Rawangkan A, Wongsirisin P, Namiki K, et al. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor that Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules (Basel, Switzerland) 23 (2018).
- Eini H, Frishman V, Yulzari R, et al. Caffeine promotes anti-tumor immune response during tumor initiation: Involvement of the adenosine A2A receptor. Biochem Pharmacol 98 (2015): 110–118.
- Ganeshpurkar A, Saluja AK. The Pharmacological Potential of Rutin. Saudi Pharmaceut J 25 (2017): 149–164.
- Chen, H, Miao Q, Geng M, et al. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci World J 2013 (2013): 269165.
- Morshedi A, Dashti RMH, Rafati A, et al. P19.4 The effect of syzygium aromaticum (clove) and vitamin C on learning and memory in rats. Clin Neurophysiol 117 (2006): 211–212.
- Ghosh R, Nadiminty N, Fitzpatrick JE, et al. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chemist 280 (2005): 5812–5819.
- Jaganathan SK & Supriyant E. Antiproliferative and Molecular Mechanism of Eugenol-Induced Apoptosis in Cancer Cells. Mole 17 (2013): 6290–6304.
- Pisano M, Pagnan G, Loi M, et al. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol Cancer 6 (2007): 8.
- Madeira MH, Boia R, Elvas F, et al. Selective A2A receptor antagonist prevents microglia-mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure-induced transient ischemic injury. Translational Research: J Laboratory Clin Med 169 (2016): 112–128.
- Wu T, Wang C, Ding L, et al. Arginine Relieves the Inflammatory Response and Enhances the Casein Expression in Bovine Mammary Epithelial Cells Induced by Lipopolysaccharide. Mediators of Inflammation, 2016 (2016): 9618795.
- Ma Q, Williamson K, O’rourke D, et al. The effects of l-arginine on crypt cell hyperproliferation in colorectal cancer. J Surg Res 8 (1999).
- Al-Koussa H, El Mais N, Maalouf H, et al. Arginine deprivation: a potential therapeutic for cancer cell metastasis? A review. Cancer Cell Int 20 (2021): 150.
- Fung MKL & Chan G. Drug-induced amino acid deprivation as strategy for cancer therapy. J Hematol Oncol 10 (2017): 144.
- Krall AS, Xu S, Graeber TG, et al. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun 7 (2016).
- Prendergast GC. Why tumours eat tryptophan. Nat 478 (2011): 192–194.
- Sahiner N, Sagbas S, Sahiner M, et al. Degradable Natural Phenolic Based Particles with Micro- and Nano-size Range. Recent Patents on Materials Science 11 (2018).
- Asfour MH & Mohsen AM. Formulation and evaluation of pH-sensitive rutin nanospheres against colon carcinoma using HCT-116 cell line. J Adv Res 9 (2017): 17–26.