Role of Cardiac IKs Current in Repolarization Reserve Process During Late Sodium Current (INaL) Activation
Article Information
Richard Printemps1, Céline Salvetat1, Jean-François Faivre2, Marie Le Grand1, Patrick Bois2*, Hamid Moha ou Maati1,3*
1CRO PHYSIOSTIM, ZI de Brenas, F-81440 Lautrec, France
2CNRS, ERL 7003, Laboratoire STIM, Université de Poitiers, F-86073 Poitiers, France
3Institut de Génomique Fonctionnel IGF CNRS INSERM UMR5203, 141 rue de la Cardonille, F-34090 Montpellier, France
*Corresponding author(s): Hamid Moha ou Maati, CRO PHYSIOSTIM, ZI de Brenas, F-81440 Lautrec, France; IGF, CNRS, INSERM, Université de Montpellier, Labex ICST, F-34094 Montpellier, France
Patrick Bois, CNRS, ERL 7003, Laboratoire STIM, Université de Poitiers, F-86073 Poitiers, France
Received: 26 June 2019; Accepted: 16 July 2019; Published: 19 July 2019
Citation: Richard Printemps, Céline Salvetat, Jean-françois Faivre, Marie Le Grand, Patrick Bois, Hamid Moha ou Maati. Role of Cardiac IKs Current in Repolarization Reserve Process During Late Sodium Current (INaL) Activation. Cardiology and Cardiovascular Medicine 3 (2019): 168-185.
View / Download Pdf Share at FacebookAbstract
The slow delayed rectifier K+ current (IKs) mediated by KCNQ1/KCNE1 channels contributes to the cardiac action potential in human and other species. Several studies have shown that IKs protects the heart from excessive action potential prolongation induced by the rapid delayed rectifier K+ current (IKr) inhibition. Moreover, several studies have shown that combined pharmacological inhibition of IKs and IKr currents increases cardiac parameters such as instability and dispersion of repolarization, and short term QT interval variability. It is known these effects promote a high risk of occurrence of « torsade de pointes ». However, the consequences of combined IKs current inhibition with an increase of the late sodium current (INaL) on cardiac repolarization has not been studied and reported in the literature. The aim of this work is to study the effects of pharmacological inhibition of IKs with chromanol 293B, a reference inhibitor of IKs current, during action potential prolongation promoted by INaL pharmacological activation by veratridine. These effects were also evaluated in the presence of doxorubicin, an anticancer drug increasing cardiac ventricular repolarisation and QT interval duration.
Methods
Involvement of IKs current in repolarization reserve process was studied in the presence of INaL current activated by veratridine on Guinea pig papillary muscle and isolated perfused heart using intracellular microelectrode and Langendorff techniques. Action potentials (AP) and Electrocardiogram (ECG) were recorded in the presence of veratridine alone and in the presence of veratridine and chromanol 293B or doxorubicin. The IKs current inhibition was previously evaluated on HEK-293/KCNQ1-KCNE1 stable cell line by chromanol 293B (supplemental Figure 1) and doxorubicin (Previous work).
Results
Our results show that the IKs current is in
Keywords
IKs / INaL currents; QT interval; Action potential prolongation; Repolarization reserve
IKs / INaL currents articles, QT interval articles, Action potential prolongation articles, Repolarization reserve articles
Article Details
Introduction
Torsades de pointes (TdPs) are polymorphic ventricular tachyarrhythmias which are commonly associated with prolongation of the QT interval of the electrocardiogram (ECG), and that may degenerate into ventricular fibrillation causing sudden death [1-3]. The prolongation of the QT interval of the ECG is associated with a reduction of cardiac ventricles repolarisation capacity [4, 5]. In the myocardium, several potassium currents contribute to the repolarisation and hence to the duration of action potential (AP) and QT interval [6]. When one of these repolarising currents is reduced, others may compensate this reduction. This is the concept of repolarisation reserve developed by several authors [4, 5, 7-9]. The delayed rectifier potassium current (IK) is the most important repolarising current terminating the ventricular action potential in several species such as human, dog and guinea pig [4, 5, 7, 8, 10]. This current (IK) results in two voltage and time dependent components: the rapidly activating current (IKr), and the slowly activating current (IKs) [11-14]. The current carried by hERG channels corresponds to IKr [15] whereas IKs results from the co-assembly of the subunits KCNQ1 and KCNE1 [16, 17]. Inhibition of IKr or IKs is implicated in the type 1 and 2 long QT syndrome (LQTS) which can be congenital [18, 19] or pharmacologically acquired [20, 21]. The pharmacological acquired form of the long QT syndrome results from the action of numerous cardiovascular and non-cardiovascular drugs that can reduce repolarisation capacity and cause prolongation of QT interval and TdPs [3]. Some of drugs responsible of TdPs in humans are able to inhibit IKr. These drugs include many pharmacological classes such as antidepressants, neuroleptics, antihistamines and antimicrobials [9, 22-26]. On the opposite, only few drugs have been shown to inhibit specifically IKs such as chromanol 293B, HMR1556 or L-735, 821 and the role of this current on the potential torsadogenic effect of new compound entities is probably underestimated in preclinical safety studies. Although IKs blockade alone induces only a small prolongation of action potential, recent studies have shown that the combination of IKs and IKr blockade markedly increases the incidence of TdPs, short-term QT interval variability and action potential prolongation [27-30]. These results suggest that IKs may play a significant protective role in limiting the occurrence of TdPs in the presence of IKs blockers. Inhibition of IKs and IKr coupled with an activation of the late sodium persistent current INaL has also been demonstrated to significantly increase the dispersion of repolarization, beat to beat variability (or instability) and the occurrence of TdPs in rabbits [31]. Nevertheless, the effects of combined pharmacological IKs inhibition coupled with INaL activation on prolongation and dispersion of cardiac repolarization has not yet been explored in the literature. Doxorubicin is an anticancer drug administrated during chemotherapeutic treatment of several tumours and haematological malignancies [32]. This compound has been reported to acutely prolong QT interval and to increase dispersion of repolarization [33]. Futhermore, we have recently shown that the QT prolongation observed in Guinea-pig isolated hearts in the presence of doxorubicin results from specific inhibition of IKs [34].
The purpose of the present work was to evaluate the consequences of the reduction of the repolarization reserve induced by specific IKs inhibition on the prolongation and the dispersion of cardiac repolarization associated with INaL activation. To this aim, we have investigated the effects of combined chromanol 293B (a specific IKs blocker) [35, 36] and veratridine (an INaL activator) [37] i) on the action potential duration and shape in Guinea pig papillary muscles and ii) on QT interval duration and dispersion of repolarisation in isolated Langendorff-perfused heart. We have also investigated the consequences of a reduction of the repolarization reserve by doxorubicin on the repolarization changes induced by veratridine. Effects of chromanol 293B (supplemental figure 1) were confirmed and effects of doxorubicin (previous work) were evaluated on IKs current using HEK-293/KCNQ1-KCNE1 stable cell line.
Material and methods
All experimental procedures were performed in accordance with “The provision of the European Convention” on the protection of vertebrate animals which are used for experimental and other scientific purposes, and with “the Appendices A and B”, made at Strasbourg on March 18, 1986 (Belgian Act of October 18, 1991).
Cell culture and transfectionThe coding sequences of hKCNQ1 and hKCNE1 genes were subcloned into pIRES-2-eGFP and pIRES-1- CD8 vectors respectively (Invitrogen, Cergy-pontoise, France). Both channel subunits were expressed in HEK-293 cells (American type culture collection, Manassas, VA, USA) by stable transfection process using Calcium Phosphate cationic method (ThermoFisher Scientific, France) with the protocole as described by the manufacturer. HEK-293 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS) and 1% (v/v) penicillin/ streptomycin in atmosphere 95% air / 5% CO2. HEK-293 cells were pricked out two times per week and seeded in T75 cm3 flask to maintain cell culture and in 100 mm diameter Petri dishes to transfect cells at 300 000 cells per Petri dish density. 10 µg and 1 µg of pIRES-2-eGFP-KCNQ1 and pIRES-1-CD8-KCNE1 vectors respectively were used for the HEK-293 cells transfection. Then, first selection of transfected cells was performed using MACS (Magnetic Cell Sorting of Human leukocytes CD8 Microbeads, Miltenyi Biotec) specific kit as described by the manufacturer. Selected cells were grown in DMEM medium supplemented by 1mg/mL of Geneticin (G418, Sigma Aldrich) during one month with medium replacement every two days. Stable clones were isolated using cloning cylinders (Sigma Aldrich), and grown in DMEM meduim supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS), 1% (v/v) penicillin / streptomycin and 200 µg/mL of G418 in atmosphere 95% air / 5% CO2.
ElectrophysiologyIn order to observe cardiac Iks current inhibition by chromanol 293B on HEK-293/KCNQ1-KCNE1 stable cell line, electrophysiology experiments were performed using the patch perforated configuration with amphotericin B at 0.8 mg/mL into the pipette medium. Cells were seeded at a density 15000 cells / 35 mm dishes. Each current was calculated by using an axopatch 200B amplifier (Axon Instrument, Sunnyvale, CA, USA), low-pass filterd 3 kHz and digitized at 10 kHz using a 12-bits analog to digital converter digidata (1322 series, Axon Instrument, Sunnyvale, CA, USA). Patch clamp pipettes were pulled using vertical puller PC-10 (Narashighe, London, UK) from borosilicate glass capillaries with a resistance between 3-5 M?. The bath solution contained (in mM) 140 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 0.3 NaH2PO4, 10 D-Glucose and 5 HEPES adjusted to pH 7.4 with NaOH. The pipette solution contained (in mM) 20 KCl, 110 K-Aspartate, 5 Na2-Phosphocreatine, 1 MgCl2, 5 EGTA and 10 HEPES and 0.8 mg/mL Ampho B adjusted to pH 7.2 with KOH. All experiments were performed at room temprature (22 °c). During patch recordings, cells were clamped at – 80 mV and then depolarized from – 70 mV to 100 mV during 3.5 sec by 10 mV increament steps following by 3.5 sec at – 40 mV repolarising potential for each depolarization step to record tail current. Pharmacological current inhibition by chromanol 293B was performed using a repetitive unique depolarization step at 100 mV from – 80 mV during 3.5 sec following by 3.5 sec at – 40 mV repolarising potential. In these protocols, the pulse cycling rate was 10 s and the current amplitudes were calculated at the pic of the tail current and the end of the first depolarization steps. Cells were continuously superfused with a microsuperfusion system. Current amplitudes were expressed in current densities. Results are expressed as mean ± standard error of the mean (SEM) from IKs Itail amplitudes.
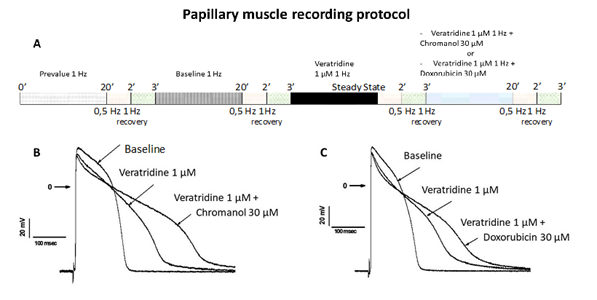
Guinea pig papillary muscle action potential recordingsThe Guinea pig papillary muscles were prepared as follows. Guinea pigs (Darkin Hartley, Charles River laboratories) weighing between 370 and 460 g were anesthetized with intraperitoneal (i.p.) injection of sodium pentobarbital (90 mg/kg; CEVA Santé Animale, 54.7 mg/mL). In addition, heparin (250 IU, Choay) was also injected in i.p. A sternotomy followed by a pericardiotomy allowed the heart to be quickly removed and transferred to Tyrode’s solution previously equilibrated for 20 minutes with a 95% O2-5% CO2 gas mixture and containing (in mM): 113.10 NaCl, 21.90 NaHCO3, 1.81 NaH2PO4, 1.20 MgCl2, 2.45 CaCl2, 4.60 KCl, 5 Glucose, (pH 7.4 ± 0.5, adjusted with HCl or NaOH). The heart was removed from the wash solution and placed for dissection in Tyrode’s solution and maintained at room temperature. Ventricles were isolated and papillary muscles were quickly removed from the right ventricle. Papillary muscles were transferred in a Plexiglas tissue chamber and maintained by stainless steel minutiae pins. The preparation was then continuously superfused by the Tyrode’s solution. This solution was gassed with 95% O2-5% CO2 and flowed with a constant rate of 5 mL/min allowing a rapid renewal. Temperature in the Plexiglas tissue chamber was checked regularly during the experiment and maintained at +36.0 ± 0.5 °C. Preparation was stimulated at 1 Hz and superfused by the Tyrode’s solution for about 45 minutes to allow stabilization before starting measurements. As soon as the contraction of the preparation was observed, the absence of spontaneous automatism was checked. When the preparation was stable, action potentials were recorded at 1 Hz. Intracellular electrical activity was recorded from cells on the surface of papillary muscles by means of microelectrodes (resistance ranged from 10 to 30 M?). These electrodes were drawn from borosilicate glass having an inner diameter of 1.16 mm and an outer diameter of 2.0 mm and containing an inner filament (GC200F- 15, Phymep, France) filled with 2.7 M KCl. Microelectrodes were connected by an Ag/AgCl holder to a high-input impedance amplification system. The preparation was electrically stimulated through a bipolar Ag electrode with 2 ms-rectangular pulses and with an intensity of 1.5 times the threshold voltage to limit variations in latency between stimulus and action potential upstroke. Intracellular signals were displayed on a dual-beam oscilloscope, digitized at a basal sampling frequency of 32 kHz and amplified. These signals were analyzed with a microcomputer. An interactive software program (Acquis1, version 4.0) provided acquisition of data and on-line measurement of action potential parameters. An Ag electrode was used as a common reference. After the stabilization period, preparations were superfused with tyrode (prevalue), vehicle (baseline) and compounds (veratridine 1 µM, chromanol 293B 30 μM, doxorubicin 30 μM) using the experimental protocols described in figure 1A, n=6 for each group).
Isolated Langendorff-perfused Guinea pig hearts experimentsThe isolated heart was prepared as follows. Guinea pigs (Darkin Hartley, Charles River laboratories) weighing between 400 and 570 g were anesthetized with intraperitoneal injection of sodium pentobarbital (90 mg/kg; CEVA Santé Animale, 54.7 mg/mL). In addition, heparin (250 IU, Choay) was also injected i.p. A sternotomy followed by a pericardiotomy allowed the heart to be quickly removed. Then, it was rinsed in Krebs-Henseleit solution at 4 °C and containing (in mM): 124.60 NaCl, 24.90 NaHCO3, 0.30 NaH2PO4, 1.10 MgSO4, 4.00 KCl, 11.10 Glucose, 2.00 Na-pyruvate, and 1.80 CaCl2 (pH 7.4 ± 0.5, adjusted with HCl or NaOH). A perfusion cannula was inserted in the aorta for retrograde perfusion. Hearts were mounted in a thermostatic chamber and perfused at a pressure of 63 ± 3 mm Hg with a Krebs Henseleit solution. This solution was continuously warmed (37 ± 1 °C), buffered (pH 7.4 ± 0.05) and gassed with 95% O2-5% CO2. The perfusion pump (Minpulse 3, Gilson) was regulated by a pressure transducer coupled with an amplifier (MLT 0699, AD Instruments). A cannula with a fluid filled balloon was inserted in the left ventricle through the mitral orifice, and the balloon was connected to a pressure transducer coupled with an amplifier (BridgeAmp, AD Instruments) for monitoring left ventricular pressure and cardiac frequency. Hearts were allowed for a minimal 35 minutes stabilization period before recordings. Minimal diastolic pressure was adjusted to 10-15 mm Hg during the stabilization phase. The hearts were not electrically stimulated and followed their spontaneous rhythm. Two ECG electrodes connected to an ECG amplifier (Animal BioAmp, AD Instruments), were held lightly against the epicardium, one on the apex and the other on the right atria, to generate a bipolar electrocardiogram with well defined P waves, QRS complex, and T waves from which measurements of QT interval were done. ECG signals were digitized at 2 kHz and recorded on a PowerLab physiological data-acquisition system (PowerLab 8/30, AD Instruments). After the stabilization period, hearts were perfused with Krebs-Henseleit (prevalue), vehicle (baseline), and compounds (veratridine 300 nM, chromanol 293B 10 μM, doxorubicin 10 μM), alone during 10 minutes, in order to evaluate their effects on ECG parameters. Chromanol 293B and doxorubicin were also perfused in combination with veratridine after 5 minutes of pre treatment with veratridine alone (see the experimental protocols described in figure 3A, n=6 hearts for each group). In the isolated perfused heart experiments, mean values of each parameter were obtained from values of 10 ECG complexes. The differences of veratridine, doxorubicine and chromanol 293 B concentrations used during Guinea pig papillary muscle and isolated perfused heart experiments could be explain by the difference of access to the tissue by the drug. In papillary muscle experiments, drugs diffuse and are diluted in the volume of the cuve while in isolated perfused heart they are directly perfused into the heart.
DrugsFor all experiments, veratridine and chromanol 293B (Sigma-Aldrich), were prepared as stock solution in DMSO, and doxorubicin (Apin Chemical) was prepared as stock solution in distilled water. After dilution to the desired final concentrations, the DMSO and distilled water concentration was 0.1%. At this concentration, in the control experiments, DMSO and distilled water were shown to have no effects in both action potential and ECG parameters (data not shown).
Data analysisIn each experiment (action potential recordings and isolated perfused heart), values are expressed as mean and standard error of the mean (SEM), and percentage variations from baseline. In the action potential experiments, for the comparison between baseline and treatment, one-way ANOVA followed by Dunnett test if necessary, was performed on absolute values (symbol *). To compare treatments between them in each group of action potential experiments, a Student paired t test was performed on absolute values (symbol +). In the Guinea pig isolated perfused heart experiments, for the comparison between baseline and treatment, one-way ANOVA followed by Dunnett test if necessary, was performed on absolute values (symbol *). In each group, to compare treatment at 5 minutes to treatment at 10 minutes, one-way ANOVA followed by Dunnett test if necessary, was performed on absolute values (symbol +). The comparison of groups was realised using two-way ANOVA followed by Bonferroni test if necessary, on absolute variations from baseline (symbol !). All statistical tests were performed using SigmaStat 3.1 software.
Results
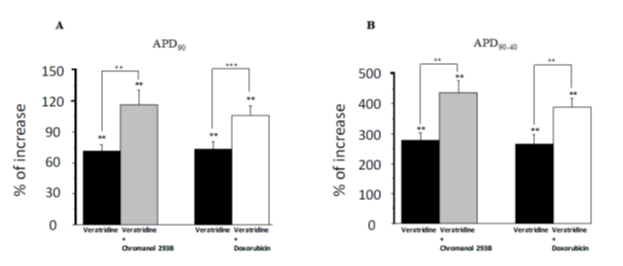
Effects on papillary muscle action potentialsUsing experimental protocol described in Figure 1A, effects were evaluated on the following AP parameters, presented in the Table 1: action potential duration at 40% (APD40), 50% (APD50), 70% (APD70), 90% (APD90), action potential triangulation (APD90-40), resting potential (RP), action potential amplitude (Amplitude) and maximal upstroke velocity (dV/dtmax). The investigation of the effects of all drugs was mainly focused on two AP parameters, APD90 and APD90-40 since they constitute predictive factors of vulnerability to TdP occurrence [38]. Typical APs, recorded in control conditions and in the presence of channel modulators are presented in Figures 1B and C. Superfusion of veratridine until steady state (59.2 ± 3.0 min) induced significant increase in APD90 and in APD90-40 (+70.3 ± 6.3 % and +272.3 ± 24.8 % respectively, p< 0.01 vs baseline, Figure 1B). Chromanol 293B superfused alone during 20 min did not significantly modify AP parameters (data not shown). Addition of chromanol 293B, after veratridine superfusion, potentiated the effects on APD90 and on APD90-40 (+114.0 ± 14.1 % and +429.9 ± 39.7 % respectively, p< 0.01 vs veratridine, Figure 1B). In the other group, before doxorubicin addition, veratridine significantly increased APD90 and APD90-40 (+72.6 ± 7.0 % and +257.2 ± 32.6 %, respectively, p< 0.01 vs baseline, figure 1C), as observed previously. Doxorubicin superfused alone (20 min) did not significantly modify AP parameters (data not shown). Addition of doxorubicin significantly potentiated the effects of veratridine on APD90 and on APD90-40 (+104.7 ± 9.5 %, p< 0.001 and +382.2 ± 30.4 %, p< 0.01 respectively, vs veratridine, Figure 1C). Effects on APD90 and APD90-40 by veratridine alone or combined to chromanol 293B or doxorubicin at 1 Hz stimulation rate are presented in Figure 2 A and B. In these groups, preparations were also stimulated at 0.5 Hz and veratridine alone showed reverse-frequency dependent effects on action potential duration and prolongation whereas the additional increase in APD90 and APD90-40 induced by the combination of veratridine and chromanol 293B or doxorubicin was not significantly different between the two stimulation rates (data not shown). The absence of reverse use dependence effects by doxorubicin and chromanol 293B addition to veratridine at low frequency confirm IKs current involvement which is not affected by this process compare to IKr current. Effects of drugs on AP parameters are summarized in the Table 1.
|
Parameters |
Chromanol 293B 30 µM |
Doxorubicin 30 µM |
||||
|
Baseline |
Vera |
Vera + chro |
Baseline |
Vera |
Vera + Dox |
|
|
RP (mV) |
-91.3 ± 0.8 |
-91.8 ± 0.8 |
-92.7 ± 0.8 |
-91.6 ± 0.7 |
-89.6 ± 0.6* |
-89.6 ± 0.7* |
|
APD40 (ms) |
142.93 ± 8.8 |
170.3 ± 11.2* |
191.6 ± 12.0**+ |
116.7 ± 6.0 |
123.7 ± 6.6 |
122.8 ± 11.7 |
|
APD50 (ms) |
163.0 ± 9.6 |
211.0 ± 13.1** |
258.3 ± 17.6**++ |
127.2 ± 5.5 |
160.7 ± 5.3** |
172.9 ± 11.1** |
|
APD70 (ms) |
167.7 ± 10.5 |
267.6 ± 13.4** |
340.6 ± 19.8**++ |
144.2 ± 5.1 |
217.1 ± 5.5** |
255.9 ± 10.1**++ |
|
APD90 (ms) |
178.9 ± 10.7 |
304.7 ± 13.2** |
382.9 ± 20.1**++ |
156.8 ± 5.1 |
270.6 ± 6.4** |
320.9 ± 6.9**+++ |
|
APD90-40 (ms) |
36.1 ± 2.2 |
134.4 ± 6.7** |
191.3 ± 11.9**++ |
41.1 ± 2.8 |
146.8 ± 10.4** |
198.2 ± 12.2**++ |
|
Amplitude (mV) |
135.1 ± 0.4 |
127.6 ± 0.6** |
126.3 ± 0.7** |
133.7 ± 0.6 |
125.4 ± 1.9** |
123.8 ± 1.5** |
|
dV/dtmax (V/s) |
186.6 ± 27.2 |
153.5 ± 20.7 |
146.5 ± 21.5 |
216.5 ± 14.0 |
194.6 ± 9.4 |
195.0 ± 8.7 |
Table 1: action potential parameters obtained during action potential recording experiments at 1 Hz on guinea pig papillary muscles. RP: resting potential, APD40; APD50; APD70; and APD90: action potential duration at 40, 50, 70 and 90% of repolarisation, APD90-40: action potential triangulation. This parameter calculated by the difference between APD90 and APD40 corresponds to triangulation degree, an indicator risk of Early After Depolarization (EAD) and TdPs. Amplitude: action potential amplitude, dV/dtmax: maximum upstroke velocity. Statistics: * p<0.05, ** p<0.01 one way ANOVA and Dunnett test between baseline and treatments. + p<0.05, ++ p<0.01, +++ p<0.001 student paired t test between treatments in each group.
Figure 1: Action potential studies using intracellular microelectrode on Guinea pig papillary muscle. A: Diagrammatic representation of the experimental recording protocol used in Guinea pig papillary muscle experiments. 20’, 2’, and 3’ represent the time recording for each tested condition. B: Typical action potential recordings at 1 Hz stimulation frequency in control conditions and in the presence of veratridine alone or combined with chromanol 293B. C: Typical action potential recordings at 1 Hz stimulation frequency in control conditions and in the presence of veratridine alone or combined with doxorubicin.
Figure 2: Effects of veratridine 1 µM, chromanol 30 µM and doxorubicin 30 µM on action potential duration at 90% (APD90) and action potential triangulation (APD90-40), at 1 Hz. A and B: After baseline step, preparation were superfused with veratridine until steady state, then with veratridine + chromanol or doxorubicin (n=6). Data are expressed as percentage variations from baseline ± SEM: ** = p< 0,01 vs baseline (ANOVA followed by Dunnett’s test performed on absolute values from baseline); ++ =p< 0,01 for the effects of veratridine + chromanol vs veratridine; and +++ =p< 0,001 for the effects of veratridine + doxorubicin vs veratridine (Student paired t test performed on absolute values from baseline).
Effects on electrophysiological parameters on isolated perfused Guinea pig hearts
The effects of IKs and INaL channel modulators were investigated on the ECG parameters recorded on isolated perfused Guinea pig heart, and more particularly on the QT and Tpeak-Tend (Tp-e) intervals. Since none of the evaluated drugs affect heart rate, QTc variations were similar to QT variations. Tp-e interval, measured between the peak and the end of the T wave, corresponds to the transmural dispersion of cardiac repolarisation [39-41]. This interval is also expressed with the ratio Tp-e / QT which is a better predictive indicator of TdPs vulnerability than QT and Tp-e considered independently [42].
|
Parameters |
RR interval (ms) |
PR interval (ms) |
QRS interval (ms) |
QT interval (ms) |
Tpeak-Tend interval (ms) |
|
Baseline |
261.8 ± 6.6 |
59.2 ± 2.3 |
18.8 ± 0.3 |
148.3 ± 3.4 |
16.4 ± 2.6 |
|
Vera 5’ |
263.2 ± 9.2 |
60.1 ± 2.7 |
19.7 ± 0.7 |
162.1 ± 7.4* |
35.3 ± 4.8** |
|
Vera 10’ |
261.2 ± 5.2 |
64.9 ± 3.5**+ |
19.8 ± 1.2 |
170.6 ± 5.9** |
47.6 ± 8.0**+ |
|
Washout |
263.7 ± 5.8 |
59.1 ± 3.0 |
19.6 ± 0.8 |
145.7 ± 3.4 |
20.7 ± 3.5 |
|
Baseline |
277.4 ± 10.2 |
56.9 ± 2.1 |
17.0 ± 1.0 |
157.0 ± 3.8 |
17.5 ± 1.9 |
|
Chro 5’ |
283.3 ± 12.4 |
57.0 ± 2.1 |
18.1 ± 1.0 |
163.3 ± 4.4** |
16.9 ± 1.0 |
|
Chro 10’ |
281.3 ± 13.2 |
57.7 ± 2.0 |
18.3 ± 1.0 |
164.8 ± 4.0** |
18.4 ± 2.3 |
|
Washout |
272.3 ± 11.0 |
57.9 ± 1.9 |
17.5 ± 1.1 |
155.9 ± 3.2 |
20.1 ± 3.7 |
|
Baseline |
281.1 ± 9.9 |
62.0 ± 1.2 |
17.1 ± 0.8 |
155.0 ± 2.7 |
15.1 ± 1.5 |
|
Dox 5’ |
289.4 ± 10.6 |
63.3 ± 1.0 |
17.3 ± 0.7 |
156.2 ± 2.9 |
15.5 ± 1.9 |
|
Dox 10’ |
301.1 ± 13.5* |
63.6 ± 1.5 |
17.0 ± 1.0 |
157.1 ± 2.6 |
18.4 ± 2.6**++ |
|
Washout |
293.5 ± 13.4* |
62.9 ± 1.4 |
17.4 ± 1.0 |
155.8 ± 3.2 |
20.3 ± 2.1** |
|
Baseline |
270.6 ± 6.6 |
62.6 ± 1.2 |
17.8 ± 0.7 |
153.5 ± 3.5 |
18.7 ± 3.6 |
|
Vera 5’ |
302.5 ± 28.5 |
64.8 ± 1.5 |
18.0 ± 1.2 |
170.7 ± 5.7* |
46.5 ± 6.5* |
|
Vera + Chro |
281.6 ± 6.3 |
71.1 ± 2.0**+ |
19.8 ± 1.8 |
202.8 ± 8.6**+++ |
82.0 ± 13.6**++ |
|
Washout |
270.9 ± 3.4 |
62.9 ± 1.5 |
16.2 ± 0.8 |
152.7 ± 4.1 |
21.2 ± 3.5* |
|
Baseline |
248.4 ± 9.4 |
60.6 ± 1.4 |
16.9 ± 0.8 |
139.0 ± 4.5 |
16.3 ± 1.2 |
|
Vera 5’ |
242.9 ± 10.3 |
63.4 ± 1.2* |
18.4 ± 0.4 |
151.1 ± 5.9** |
39.4 ± 3.5** |
|
Vera + Dox |
251.1 ± 8.7 |
68.0 ± 1.6**++ |
21.1 ± 1.0**+ |
178.5 ± 6.8**+++ |
75.1 ± 5.7**++ |
|
Washout |
245.8 ± 7.5 |
61.2 ± 1.7 |
17.0 ± 0.8 |
136.2 ± 4.5 |
21.3 ± 2.2* |
Table 2: ECG parameters / intervals obtained during isolated perfused Guinea pig heart experiments. RR: RR interval, PR: PR interval, QRS: QRS interval, QT: QT interval, Tpeak-Tend (Tp-e): Tpeak-Tend interval. Statistics: * p<0.05, ** p<0.01 one way ANOVA and Dunnett test between baseline and treatments. + p<0.05, ++ p<0.01, +++ p<0.001 Student paired t test between treatments at 5’ and 10 ‘ or Vera + Chro or Vera + Dox in each group. QT and Tp-e interval statistical comparisons between the different groups were illustrated on the corresponding histogramms (Figure 4).
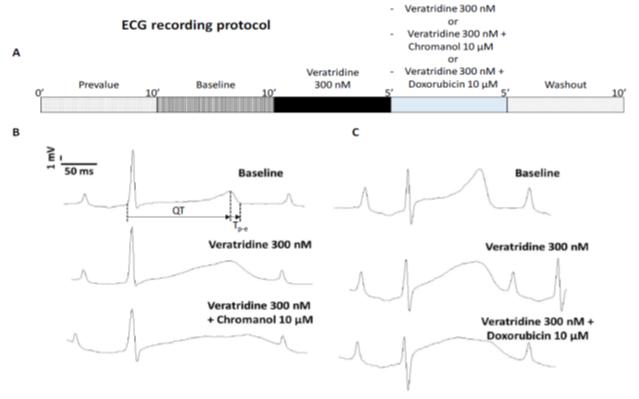
As shown in the experimental protocol illustrated in Figure 3A, chromanol 293B or doxorubicin were tested for 5 min combined with veratridine, after 5 min pre-treatment with this last compound. Effects of drugs on ECG parameters are summarized in Table 2. Typical traces of ECG recordings in control conditions and in the presence of veratridine alone or combined with chromanol 293B or doxorubicin, are shown in Figure 3B and C. In panel B, classical ECG traces are presented in the control conditions (Baseline). An increase of QT and Tp-e intervals is observed on the ECG trace after veratridine 300 nM perfusion alone. This QT and Tp-e intervals increase is potentiated after addition of chromanol 293B 10 µM to veratridine. Same results are observed with doxorubicin. In panel C, the QT and Tp-e intervals increase observe after veratridine 300 nM perfusion alone is potentiated when doxorubicin 10 µM is added.
Figure 3: Electrocardiogramm recordings in Guinea pig isolated Langendorff perfused heart model. A: Diagrammatic representation of the experimental recording protocol used in Guinea pig isolated Langendorff perfused heart experiments. B: Representative bipolar electrocardiographic signals recorded during the experiments on isolated perfused Guinea pig heart: The prevalue step corresponds to the control solution superfusion without vehicle. After 10 minutes of baseline corresponding to the control solution added with the vehicle, veratridine 300 nM was perfused during 5 minutes followed by a 5 minutes perfusion of veratridine 300 nM + chromanol 10 µM (n=6). C: The prevalue step corresponds to the control solution superfusion without vehicle. After 10 minutes of baseline corresponding to the control solution added with the vehicle, veratridine 300 nM was perfused during 5 minutes followed by a 5 minutes perfusion of veratridine 300 nM + doxorubicin 10 µM (n=6).
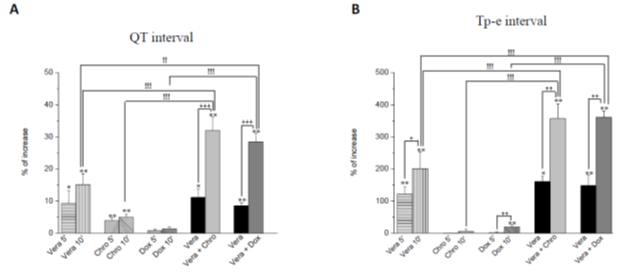
Perfusion of each drug alone: Perfusion of veratridine during 10 minutes induced significant increases in QT interval (+9.3 ± 4.0%, p< 0.05 vs baseline at 5 minutes and +15.0 ± 3.4%, p< 0.01 vs baseline at 10 minutes, Figure 4A), and in Tp-e interval (+115.2 ± 23.1%, p<0.01 vs baseline at 5 minutes and +190.2 ± 51.4%, p<0.01 vs baseline at 10 minutes, figure 4B). Perfusion of chromanol 293B during 10 minutes induced a slight but significant increase in QT interval (+4.0 ± 0.8% and +5.0 ± 0.9% at 5 and 10 minutes respectively, p< 0.01 vs baseline, Figure 4A) but did not statistically affect Tp-e interval (Figures 4B). Doxorubicin perfused during 10 minutes did not affect QT interval but induced a significant increase in Tp-e interval (+21.9 ± 6.2%, p< 0.01 vs baseline at 10 minutes, Figure 4B).
Figure 4: Effects of veratridine 300 nM, chromanol 10 µM and doxorubicin 10 µM on QT, Tpeak-Tend intervals of ECG signals. Each drug (n=6) was perfused during 10 minutes after baseline. Veratridine was also perfused alone 5 minutes followed by a 5 minutes of veratridine in combination with chromanol 293B or doxorubicin. Data are expressed as percentage variations from baseline ± SEM. * =p< 0,05 and ** =p< 0,01 vs baseline (ANOVA followed by Dunnett’s test performed on absolute values from baseline). !! =p< 0,01 and !!! =p< 0,001 for the effects of veratridine combinated to chromanol or doxorubicin vs veratridine, chromanol and doxorubicin alone after 10 minutes perfusion (ANOVA followed by Bonferroni’s test performed on absolute variations from baseline). + =p< 0,05, ++ =p< 0,01 and +++ = p< 0,001 to compare effects between 5 and 10 minutes in each group (Student paired t test were performed on absolute values from baseline).
Perfusion of drug combination: When compared to veratridine perfused alone (during 10 min), addition of chromanol 293B to veratridine (5 min after 5 min veratridine perfusion) significantly potentiated the effects observed on QT interval (+32.1 ± 4.1%, p< 0.001 vs time-matched values in the veratridine group, Figure 4A) and Tp-e interval (+338.5 ± 45.9%, p< 0.001 vs time-matched values in the veratridine group, Figure 4B). Doxorubicin, as chromanol 293B, potentiated the effects of veratridine on QT and Tp-e intervals (QT: +28.4 ± 2.6%, p< 0.01 vs time-matched values in the veratridine group, figure 4A; Tp-e interval: +360.7 ± 20.0%, p< 0.001 vs time-matched values in the veratridine group, Figure 4B). The analysis of the Tp-e/ QT ratio showed identical results as Tp-e interval effects (datas not shown).
IKs current inhibition by chromanol 293B
In order to check cardiac Iks current inhibition by chromanol 293B on HEK-293/KCNQ1-KCNE1 stable cell line, electrophysiology experiments were performed using the patch perforated configuration with four drug concentrations 1, 3, 10 and 30 µM (Supplemental Figure 1). Our data indicate that the cumulative perfusion of these chromanol 293B concentrations has an inhibitory effect of Iks current with a significant decrease of its amplitude (Supplemental Figure 1A). The current amplitude on the prepulse at + 100 mV potential decrease from 3.5 nA to 0.6 nA in the presence of 30 µM of chromanol 293B. From the four cumulative perfused chromanol 293B concentrations, a dose response curve is obtained with the percentage of current inhibition in function of Log chromanol 293B concentration. The curve is fitted with pharmacological Hill equation, and an IC50 = 6.51 µM and a Hill number n=0.75 are determined (Supplemental Figure 1B). Iks current traces recorded in the control condions and in the presence of 10 µM of chromanol 293B are presented in the supplemental figure1 panel C. These data indicate that chromanol 293B is able to inhibit Iks current and could be potentialy used to study the role of this current in repolarisation reserve process in the intracellular microelectrode and isolated perfused heart studies. Effects of doxorubicin was also evaluated on Iks current with the same experimental conditions in previous published works with an IC50 = 4.78 µM and an inhibition close to 75% at 30 µM.
Supplemental Figure 1: Pharmacological experiments on human IKs cardiac potassium repolarizing current stably expressed on HEK-293 cells. Cells were patched using perforated patch configuration. Cells were clamped at – 80 mV potential and then, cells were depolarized from – 70 mV to 100 mV by repetitive unique step during 3.5 s followed by 3.5 s at the repolarising step of – 40 mV. After steady state in control condition four cumulative concentrations of chromanol 293B, 1, 3, 10 and 30 µM were perfused on cells until steady state for each of them. (A) Typical IKs currents traces recorded in control condition and after perfusion of each tested chromanol 293B concentration and washout (n=4). (B) % of inhibition in function of the Log [chromanol 293B] fitted by Hill equation to calculate the IC50 of chromanol 293B inhibition on IKs current. (C) Typical IKs current traces recorded using an I/V step protocol in control condition and in the presence of 10 µM of chromanol 293B. ±
Discussion
KCNQ1 and KCNE1 protein subunits constitute the cardiac channel responsible of IKs potassium repolarizing current [16, 17]. This current is a part of delayed ventricular potassium component and is associated to the IKr current, in the ventricular repolarization. Pharmacological inhibition of IKs current could potentially have an arrhythmic effect, as loss of function mutations in the congenital long QT syndrome (LQT1 or LQT5) where this current is involved [43, 44]. This pharmacological inhibition results in drug side effects and could be evaluated in preclinical safety pharmacology test. Moreover, it has been shown that the cardiac IKs potassium repolarizing current plays a key role in the ventricular repolarisation reserve process [4, 5]. In this context, first, we checked the IKs current inhibition by chromanol 293B, a selective inhibitor and by doxorubicin, a chemotherapeutic molecule (previous published works [34]), on HEK 293 cell line stably expressing KCNQ1 / KCNE1 subunits that constitute the channel responsible of IKs potassium repolarizing current. Results on these previous works have demonstrated QT interval prolongation by IKs inhibition without effects on IKr repolarizing current showing the impotance of preclinical assessment on IKs current inhibition. Then, in a second time, using these two inhibitors, we studied the role of the IKs current in repolarisation reserve process on action potentials and ECG parameters using intracellular microelectrode in Guinea pig papillary muscle and Guinea pig isolated Langendorff perfused heart techniques.
In the pharmacological study, our data confirm that chromanol 293B inhibits IKs current in a dose dependant manner. The current amplitude is drastically decreased by the perfusion of cumulative concentrations of chromanol 293B with a partial reversible effect. The estimated IC50 is 6.51 µM, a result close to those reported in the literature from several mamal species including human [35, 36]. Effects of doxorubicin were evaluated on IKs current in previous published work [34]. The estimated IC50 was 4.78 µM. Taken together, these data indicate that chromanol 293B and the chemotherapeutic agent doxorubicin could be used as inhibitors of IKs current to study the role of this current in the repolarisation reserve process.
Then, we performed intracellular microelectrode and isolated Langendorff perfused heart experiments to study the role of IKs ventricular repolarizing current in the repolarization reserve process. It has been shown that QT-interval prolongation itself is antiarrhythmic whereas it becomes proarrhythmic when it is associated with interrelated proarrhythmic factors including TRIaD (Triangulation Reverse use dependance Instability and Dispertion). These factors include action potential triangulation, reverse- use dependence, electrical instability of the action potential and dispersion of repolarization [45-47]. They are considered as biomarkers to predict the development of “torsade de pointes” arrhythmia.
In the present work, proarrhythmic conditions were induced by veratridine in both models. This alkaloid is known to activate a late persistent sodium current INaL [37] which corresponds to incomplete or slowed INaL inactivation [48]. As expected, veratridine (at 1 µM in action potential papillary muscle experiments and 300 nM in isolated perfused heart experiments) induced triangulation of the action potential associated to an increase of ADP90-40 and a significant increase of action potential and QT interval duration. Furthermore, this agent showed reverse-frequency on action potential duration and caused a rise in dispersion of repolarisation (Tp-e interval, [49]). The increase in AP-duration and reverse-use dependence have already been reported for veratridine in the same preparation [50] as well as in rabbit heart [31]. Both triangulation and dispersion were described on rabbit heart [51]. By contrast, chromanol 293B did not modify these parameters when tested alone. This result is in conformity with the experiment realized in dog, rabbit and human hearts [8, 10, 52]. It has also been reported that chromanol 293B did not increase the AP duration and triangulation in rabbit heart [27]. In the same way, we have tested the chemotherapeutic agent doxorubicin used to treat cancer. The present results indicate that the perfusion of this drug alone does not affect QT interval. This observation differ with our previous data recorded from the same preparation [34] and could be explained by differences in perfusion duration (10 min vs 45 min) and the concentrations used (10 µM vs 30 µM). As already reported in clinical pharmacological studies [33], doxorubicin slightly amplified QT interval dispersion.
We also demonstrate here the participation of the slow outward potassium current in action potential triangulation and dispersion of repolarization induced after the activation of the sustained component of the fast sodium current. The block of IKs by chromanol 293B in the presence of veratridine significantly increased action potential duration and triangulation and enhanced the index of dispersion of repolarization. Similar effects have been observed in the presence of doxorubicin under the same experimental conditions. As we previously showed, the chemotherapeutic agent doxorubicin blocks IKs in isolated Guinea pig papillary muscle [34]. Taken together these data demonstrate the participation of the slow potassium current IKs in the functional efficiency of repolarisation reserve. In situations where APD is prolonged, IKs activation may limit excessive APD lengthening by a negative feedback mechanism providing more safety of the repolarisation process [5, 8]. It has been shown that IKs is able to protect heart against the proarrhythmic reverse rate-dependent action potential triangulation induced by IKr blockade [7, 27, 30].
For the first time, we clearly show here that doxorubicin at concentrations which do not modify the cardiac action potential duration, is able to reduce the repolarisation reserve probably by blocking the IKs current. On the other hand, this work further confirms that IKs limits the excessive prolongation of action potential and QT interval, and protects from the triangulation and dispersion processes caused by the late persistent sodium current. INaL may be the consequence of pathological gating or regulation abnormalities such as LQT3 [53], LQT4 [54] and LQT-CAV3 (LQT8) [55] syndromes. The sustained sodium current is also observed in acquired heart diseases as heart failure and post- infarction remodelled myocardium. INaL enhancement is generally associated with prolonged repolarisation and reduced repolarisation reserve [48].
It can be concluded that the pharmacological block of IKs does not prolong APD, but can decrease the safety of repolarisation reserve process and therefore increase the proarrhythmic risk, especially in the pathological situations where the late persistent sodium current is activated.
Conflict of interests
Authors declare to do not have conflict of interest.
Authorship
Contribution to this work: RP, CS and HMM did experiments and analysis of the data. RP, HMM, JFF, MLG and PB did the design of experiments and interpretation of the data. RP, JFF, MLG, PB and HMM write the drafting version of the manuscript. All of the authors approve the final version of the submitted manuscript and are agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Financial supports
HMM was supported by ANRT fellowship, the CNRS “Centre National de le Recherche Scientifique”, the University of Poitiers and by the CRO Physiostim company; RP, CS and MLG were supported by the CRO Physiostim company; JFF and PB were supported by the CNRS “Centre National de le Recherche Scientifique”, the University of Poitiers.
References
- Arunachalam K, Lakshmanan S, Maan A, Kumar N, Dominic P. Impact of Drug Induced Long QT Syndrome: A Systematic Review. J Clin Med Res 10 (2018): 384-390.
- Etchegoyen CV, Keller GA, Mrad S, Cheng S, Di Girolamo G. Drug-induced QT Interval Prolongation in the Intensive Care Unit. Curr Clin Pharmacol 12 (2017): 210-222.
- Fenichel RR, Malik M, Antzelevitch C, Sanguinetti M, Roden DM, Priori SG, Ruskin JN, Lipicky RJ, Cantilena LR, Independent Academic Task F. Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol 15 (2004): 475-495.
- Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med 259 (2006): 59-69.
- Roden DM, Yang T. Protecting the heart against arrhythmias: potassium current physiology and repolarization reserve. Circulation 112 (2005): 1376-1378.
- Locati ET, Bagliani G, Padeletti L. Normal Ventricular Repolarization and QT Interval: Ionic Background, Modifiers, and Measurements. Card Electrophysiol Clin 9 (2017): 487-513.
- Biliczki P, Virag L, Iost N, Papp JG, Varro A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br J Pharmacol 137 (2002): 361-368.
- Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, Nagy Z, Bogats G, Lathrop DA, Papp JG et al. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation 112 (2005): 1392-1399.
- Kui P, Orosz S, Takacs H, Sarusi A, Csik N, Rarosi F, Cseko C, Varro A, Papp JG, Forster T et al. New in vitro model for proarrhythmia safety screening: IKs inhibition potentiates the QTc prolonging effect of IKr inhibitors in isolated guinea pig hearts. J Pharmacol Toxicol Methods 80 (2016): 26-34.
- Volders PG, Stengl M, van Opstal JM, Gerlach U, Spatjens RL, et al. Probing the contribution of IKs to canine ventricular repolarization: key role for beta- adrenergic receptor stimulation. Circulation 107 (2003): 2753-2760.
- Chen L, Sampson KJ, Kass RS. Cardiac Delayed Rectifier Potassium Channels in Health and Disease. Card Electrophysiol Clin 8 (2016): 307-322.
- Cheng JH, Kodama I. Two components of delayed rectifier K+ current in heart: molecular basis, functional diversity, and contribution to repolarization. Acta Pharmacol Sin 25 (2004): 137-145.
- Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res 78 (1996): 689-696.
- Sanguinetti MC, Jurkiewicz NK. Lanthanum blocks a specific component of IK and screens membrane surface change in cardiac cells. Am J Physiol 259 (1990): H1881-H1889.
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 97 (1999): 175-187.
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384 (1996): 78- 80.
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384 (1996): 80-83.
- Aizawa Y, Ueda K, Scornik F, Cordeiro JM, Wu Y, Desai M, et al. A novel mutation in KCNQ1 associated with a potent dominant negative effect as the basis for the LQT1 form of the long QT syndrome. J Cardiovasc Electrophysiol 18 (2007): 972-977.
- Napolitano C, Priori SG, Schwartz PJ, Bloise R, Ronchetti E, et al. Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice. JAMA 294 (2005): 2975-2980.
- Naik A. Long QT syndrome revisited. J Assoc Physicians India 55 (2007): 58-61.
- Saenen JB, Vrints CJ. Molecular aspects of the congenital and acquired Long QT Syndrome: clinical implications. J Mol Cell Cardiol 44 (2008): 633-646.
- Bischoff U, Schmidt C, Netzer R, Pongs O. Effects of fluoroquinolones on HERG currents. Eur J Pharmacol 406 (2000): 341-343.
- Heist EK, Ruskin JN. Drug-induced proarrhythmia and use of QTc-prolonging agents: clues for clinicians. Heart Rhythm 2 (2005): S1-S8.
- Saxena P, Zangerl-Plessl EM, Linder T, Windisch A, Hohaus A, Timin E, Hering S, Stary- Weinzinger A. New potential binding determinant for hERG channel inhibitors. Sci Rep 6 (2016): 24182.
- Woosley RL, Chen Y, Freiman JP, Gillis RA: Mechanism of the cardiotoxic actions of terfenadine. JAMA 269 (1993): 1532-1536.
- Woosley RL, Sale M. QT interval: a measure of drug action. Am J Cardiol 72 (1993): 36B-43B.
- Guerard NC, Traebert M, Suter W, Dumotier BM. Selective block of IKs plays a significant role in MAP triangulation induced by IKr block in isolated rabbit heart. J Pharmacol Toxicol Methods 58 (2008): 32-40.
- Lengyel C, Varro A, Tabori K, Papp JG, Baczko I. Combined pharmacological block of I(Kr) and I(Ks) increases short-term QT interval variability and provokes torsades de pointes. Br J Pharmacol 151 (2007): 941-951.
- Michael G, Dempster J, Kane KA, Coker SJ. Potentiation of E-4031-induced torsade de pointes by HMR1556 or ATX-II is not predicted by action potential short-term variability or triangulation. Br J Pharmacol 152 (2007): 1215-1227.
- So PP, Hu XD, Backx PH, Puglisi JL, Dorian P. Blockade of IKs by HMR 1556 increases the reverse rate-dependence of refractoriness prolongation by dofetilide in isolated rabbit ventricles. Br J Pharmacol 148 (2006): 255-263.
- So PP, Backx PH, Dorian P. Slow delayed rectifier K+ current block by HMR 1556 increases dispersion of repolarization and promotes Torsades de Pointes in rabbit ventricles. Br J Pharmacol 155 (2008): 1185-1194.
- Carter SK, Blum RH. New chemotherapeutic agents--bleomycin and adriamycin. CA Cancer J Clin 24 (1974): 322-331.
- Nousiainen T, Vanninen E, Rantala A, Jantunen E, Hartikainen J. QT dispersion and late potentials during doxorubicin therapy for non-Hodgkin's lymphoma. J Intern Med 245 (1999): 359-364.
- Ducroq J, Moha ou Maati H, Guilbot S, Dilly S, Laemmel E, Pons-Himbert C, Faivre JF, Bois P, Stucker O, Le Grand M. Dexrazoxane protects the heart from acute doxorubicin-induced QT prolongation: a key role for I(Ks). Br J Pharmacol 159 (2010): 93-101.
- Bosch RF, Gaspo R, Busch AE, Lang HJ, Li GR, Nattel S. Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc Res 38 (1998): 441-450.
- Busch AE, Suessbrich H, Waldegger S, Sailer E, Greger R, Lang H, Lang F, Gibson KJ, Maylie JG. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflugers Arch 432 (1996): 1094-1096.
- Zong XG, Dugas M, Honerjager P. Relation between veratridine reaction dynamics and macroscopic Na current in single cardiac cells. J Gen Physiol 99 (1992): 683-697.
- Valentin JP, Hoffmann P, De Clerck F, Hammond TG, Hondeghem L. Review of the predictive value of the Langendorff heart model (Screenit system) in assessing the proarrhythmic potential of drugs. J Pharmacol Toxicol Methods 49 (2004): 171-181.
- Antzelevitch C. T peak-Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest 31 (2001): 555-557.
- Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 4 (2007): 964-972.
- Kanters JK, Haarmark C, Vedel-Larsen E, Andersen MP, Graff C, Struijk JJ, Thomsen PE, Christiansen M, Jensen HK, Toft E; T(peak)T(end) interval in long QT syndrome. J Electrocardiol 41 (2008): 603-608.
- Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, Yan GX. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 41 (2008): 567-574.
- Ackerman MJ. The long QT syndrome: ion channel diseases of the heart. Mayo Clin Proc 73 (1998): 250-269.
- Ackerman MJ. The long QT syndrome. Pediatr Rev 19 (1998): 232-238.
- Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation 103 (2001): 2004-2013.
- Hondeghem LM, Hoffmann P. Blinded test in isolated female rabbit heart reliably identifies action potential duration prolongation and proarrhythmic drugs: importance of triangulation, reverse use dependence, and instability. J Cardiovasc Pharmacol 41 (2003): 14-24.
- Shah RR, Hondeghem LM. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm 2 (2005): 758-772.
- Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac "late sodium current.". Pharmacol Ther 119 (2008): 326-339.
- Tokatli A, Kilicaslan F, Alis M, Yiginer O, Uzun M. Prolonged Tp-e Interval, Tp-e/QT Ratio and Tp-e/QTc Ratio in Patients with Type 2 Diabetes Mellitus. Endocrinol Metab (Seoul) 31 (2016): 105-112.
- Honerjager P, Reiter M. The relation between the effects of veratridine on action potential and contraction in mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol 289 (1975): 1-28.
- Milberg P, Reinsch N, Wasmer K, Monnig G, Stypmann J, Osada N, Breithardt G, Haverkamp W, Eckardt L. Transmural dispersion of repolarization as a key factor of arrhythmogenicity in a novel intact heart model of LQT3. Cardiovasc Res 65 (2005): 397-404.
- Lengyel C, Iost N, Virag L, Varro A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (I(Ks)) fails to lengthen rabbit ventricular muscle QT(c) and action potential duration. Br J Pharmacol 132 (2001): 101-110.
- Bennett PB, Yazawa K, Makita N, George AL, Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature 376 (1995): 683-685.
- Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett V: Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A 101 (2004): 17533-17538.
- Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 114 (2006): 2104-2112.