Rituximab Treatment of Membranous Nephropathy after Failure of other Therapies- A Case report
Article Information
Elvana Rista1, Vilma Cadri2, Anila Duni3, Juna Musa4, Fjolla Hyseni5*, Ali Guy6, Ronny Khadra7, Alma Idrizi8
1Department of Nephrology, Hygeia Hospital Tirana, Albania
2Department of Nephrology, University Hospital Center “Mother Teresa”, Tirana, Albania
3Department of Nephrology, University Hospital of Ioannina, Ioannina, Greece
4Postdoctoral Research Fellow, Department of Surgery, Mayo Clinic, Rochester, Minnesota, USA
5Department of Urology, NYU Langone Health, New York, USA
6Clinical Assistant Professor, Department of Physical Medicine and Rehabilitation, New York University School of Medicine, NYU Medical Center, New York, USA
7College of Coastal Georgia, Brunswick, Georgia
8Department of Nephrology, University Hospital Center “Mother Teresa”, Tirana, Albania
*Corresponding Author: Dr. Fjolla Hyseni, Department of Urology, NYU Langone Health, New York, USA
Received: 24 January 2021; Accepted: 08 February 2021; Published: 01 March 2021
Citation: Elvana Rista, Vilma Cadri, Anila Duni, Juna Musa, Fjolla Hyseni, Ali Guy, Ronny Khadra, Alma Idrizi. Rituximab Treatment of Membranous Nephropathy after Failure of other Therapies- A Case report. Archives of Clinical and Medical Case Reports 5 (2021): 234-239.
View / Download Pdf Share at FacebookAbstract
Membranous Nephropathy (MN) also known as membranous glomerulonephritis is a slowly progressive kidney disease characterized of subepithelial immune complex deposits and subsequent thickening of glomerular basement membrane. MN is a common cause of proteinuria that may progress to ERDS. The decision to treat MN with immunosuppressive medications is complicated by the well-known natural history of disease and it depends on patient presentation and disease progression. Herein, we present a case of a 48-year-old male patient with weight gain, lower extremity edema, arterial hypertension, and nephrotic range proteinuria (13.5 gr).
The patient was diagnosed with MN 14 years ago with relapses and remission of disease over the years. First immunotherapy was stared with cyclosporine and methylprednisolone, but the patient occurred to have cyclosporine-induced toxicity.
Second immunotherapy was stared with methylprednisolone and cyclophosphamide that resulted in complete remission for 4 years. The disease relapsed again, and the patient was recommended the Rituximab treatment. Two doses of rituximab 1g were administered. In terms of total remission there was no indication for the third dose of rituximab.
Keywords
Membranous nephropathy; Immunotherapy; Rituximab
Membranous nephropathy articles; Immunotherapy articles; Rituximab articles
Membranous nephropathy articles Membranous nephropathy Research articles Membranous nephropathy review articles Membranous nephropathy PubMed articles Membranous nephropathy PubMed Central articles Membranous nephropathy 2023 articles Membranous nephropathy 2024 articles Membranous nephropathy Scopus articles Membranous nephropathy impact factor journals Membranous nephropathy Scopus journals Membranous nephropathy PubMed journals Membranous nephropathy medical journals Membranous nephropathy free journals Membranous nephropathy best journals Membranous nephropathy top journals Membranous nephropathy free medical journals Membranous nephropathy famous journals Membranous nephropathy Google Scholar indexed journals nephropathy articles nephropathy Research articles nephropathy review articles nephropathy PubMed articles nephropathy PubMed Central articles nephropathy 2023 articles nephropathy 2024 articles nephropathy Scopus articles nephropathy impact factor journals nephropathy Scopus journals nephropathy PubMed journals nephropathy medical journals nephropathy free journals nephropathy best journals nephropathy top journals nephropathy free medical journals nephropathy famous journals nephropathy Google Scholar indexed journals Immunotherapy articles Immunotherapy Research articles Immunotherapy review articles Immunotherapy PubMed articles Immunotherapy PubMed Central articles Immunotherapy 2023 articles Immunotherapy 2024 articles Immunotherapy Scopus articles Immunotherapy impact factor journals Immunotherapy Scopus journals Immunotherapy PubMed journals Immunotherapy medical journals Immunotherapy free journals Immunotherapy best journals Immunotherapy top journals Immunotherapy free medical journals Immunotherapy famous journals Immunotherapy Google Scholar indexed journals Rituximab articles Rituximab Research articles Rituximab review articles Rituximab PubMed articles Rituximab PubMed Central articles Rituximab 2023 articles Rituximab 2024 articles Rituximab Scopus articles Rituximab impact factor journals Rituximab Scopus journals Rituximab PubMed journals Rituximab medical journals Rituximab free journals Rituximab best journals Rituximab top journals Rituximab free medical journals Rituximab famous journals Rituximab Google Scholar indexed journals Computed tomography articles Computed tomography Research articles Computed tomography review articles Computed tomography PubMed articles Computed tomography PubMed Central articles Computed tomography 2023 articles Computed tomography 2024 articles Computed tomography Scopus articles Computed tomography impact factor journals Computed tomography Scopus journals Computed tomography PubMed journals Computed tomography medical journals Computed tomography free journals Computed tomography best journals Computed tomography top journals Computed tomography free medical journals Computed tomography famous journals Computed tomography Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Leukemia articles Leukemia Research articles Leukemia review articles Leukemia PubMed articles Leukemia PubMed Central articles Leukemia 2023 articles Leukemia 2024 articles Leukemia Scopus articles Leukemia impact factor journals Leukemia Scopus journals Leukemia PubMed journals Leukemia medical journals Leukemia free journals Leukemia best journals Leukemia top journals Leukemia free medical journals Leukemia famous journals Leukemia Google Scholar indexed journals Phospholipase articles Phospholipase Research articles Phospholipase review articles Phospholipase PubMed articles Phospholipase PubMed Central articles Phospholipase 2023 articles Phospholipase 2024 articles Phospholipase Scopus articles Phospholipase impact factor journals Phospholipase Scopus journals Phospholipase PubMed journals Phospholipase medical journals Phospholipase free journals Phospholipase best journals Phospholipase top journals Phospholipase free medical journals Phospholipase famous journals Phospholipase Google Scholar indexed journals
Article Details
1. Introduction
Membranous Nephropathy (MN) is one of the main causes of nephrotic syndrome in adults. MN is an antibody-mediated disease, caused by circulating autoantibodies against podocyte antigens [1]. In most cases it is primary.
Phospholipase A2 receptor (PLA2R) is the most important target antigen in membranous nephropathy [2]. Patients with PLA2R negative, might be positive for other antigens target like thrombospondin type-1 domain-containing (7A THSD7A), neural epidermal growth factor-like 1 (NELL-1), semaphorin 3B (Sema 3B) [3].
The clinical course varies from spontaneous remission, persistent nephrotic syndrome, ESRD and post-transplant recurrence. The decision to start immunosuppression therapy relies on the estimated risk of developing progressive kidney disease. Rituximab is the first line treatment in patients with moderate and high risk who have stable renal function.
2. Case Presentation
A 48-year-old male patient came to our attention with weight gain, lower extremity edema, arterial hypertension and nephrotic range proteinuria (13.5 gr). He states that he has been diagnosed with Membranous Nephropathy (MN). His medical history has started in March 2007, when he first presented with nephrotic syndrome. In July 2008 he underwent a renal biopsy that established the diagnosis of membranous nephropathy stage II-III, with presence of Ig G and C3; treatment with chlorambucil and corticosteroids was started and continued for 7 months, with complete remission until November 2011.
The patient was on angiotensin receptor blockers (ARB) therapy at all times, with well controlled blood pressure values below 125/80 mmHg, antilipemic and antiaggregant. In November 2011 the patient relapsed, proteinuria 6 gr was detected. In February 2012 proteinuria increased to 10 gr; therapy with cyclosporine 3mg/kg/d and methylprednisolone 16 gr/day was started. This therapy resulted in remission with proteinuria of 250 mg. After a year he relapsed again and in September 2013, he underwent renal biopsy to re-evaluate the situation, which showed again stage II-III membranous nephropathy and cyclosporine-induced toxicity was identified. In November 2014, he underwent treatment according to the modified Ponticelli scheme with methylprednisolone and cyclophosphamide for 6 months. Glomerular filtration rate (GFR) in this period was 49 ml/min. Complete remission for 4 years, and then proteinuria begins.
In 2018 the patient relapses again and resumes cyclosporine and methylprednisolone 32 mg. In February 2020 the patient was referred to our department with massive proteinuria, 13.5 gr/24 hours, hypoproteinemia, dyslipidemia and creatinine 1.5 mg/dl, GFR>60 ml/min. He was tested for PLA2R, which was negative. We recommended the Rituximab treatment. Two doses of rituximab 1g were administered, day 1 and 15. In May 2020, proteinuria 870 mg/24 hours, significant decrease in proteinuria (over 50%). In September 2020, sixth month after starting therapy, the patient shows up for follow-up to decide whether a third dose of rituximab was to be given. In examinations, proteinuria <250 mg/24 h, normal albumin and total protein, creatinine 1.3 mg / dl and GFR 96.7 ml/ min. In terms of total remission there was no indication for the third dose of rituximab. Last, but not least, our patient was infected with Covid-19, confirmed with RT-PCR of nasopharyngeal swab. Fortunately, he had mild disease treated with support therapy.
3. Discussion
Primary MN is an autoimmune disease, organ-specific, mediated by circulating autoantibodies to podocyte antigens. It is classified into primary MN, which comprises 75-80% of cases and into secondary due to autoimmune diseases, hepatitis B, malignancies, thyroiditis, drugs, alloimmune neonatal MN, de novo post-transplant. The natural course of MN patients is variable. About 1/3 of them goes into spontaneous remission, 1/3 remains proteinuric with stable renal function and 1/3 progresses to ESRD, especially those with very high proteinuria [4]. Because a good proportion of patients undergo spontaneous remission it is reasonable to wait 6 months, optimizing anti-proteinuric therapy. MN has been studied quite well the last couple of years and many changes have been made in the KDIGO guidelines in different aspects, starting from its diagnosis, prognosis and treatment. The diagnosis has been revolutionized by the discovery of PLA2R in 2009, published in the New England Journal of Medicine [2].
PLA2 R is a normal protein in podocytes. In MN the prevalence of finding that anti-PLA2R antibodies is 70-80%. In disease the PLA2R antigen is co-localized with IgG in subepithelial immune deposits. Over the years, renal biopsy has been the gold standard for diagnosis, but remains an invasive method. Circulating anti-PLA2R antibodies is a very important and specific datum, which has made it possible to diagnose primary MN, when there are clinical clues for it, without the need for renal biopsy, which should be reserved only in special cases such as unusual course of the disease, when anti-PLA2R antibodies levels are declining but nephrotic syndrome persists, or when the patient is receiving immunosuppression but has a rapid decrease in GFR or when antinuclear antibodies are positive [5, 6]. In addition, there is a strong association of anti-PLA2 R with the clinical activity of the disease [7, 8]. Clinicians now can additionally follow a serological marker of disease activity, not only the levels of albumin and proteinuria but also PLA2R which precedes and predicts clinical course of the disease, moreover that changes in its level are detected earlier compared to proteinuria. MN treatment is recently based on strong data following the findings of the MENTOR study, which compared the treatment of MN patients with rituximab and those treated with calcineurin inhibitors, cyclosporine. The study showed, there was a significant difference in the percentage of patients who underwent total remission of the disease for 24 months, compared to patients treated with cyclosporine [8].
In conclusion, our patient had remission in all treatment schemes, but relapsed were rapidly, except after the modified Ponticelli scheme which lasted about 4 years. In our view, taking into consideration patient history and evidence, the best choice in his case was treatment with rituximab. Anti-PLA2R was negative and we were not able to follow its clinical course through antibody levels, but the patient had excellent results after 3 months with significant decrease in proteinuria and total remission after sixth months. Over 60% of patients treated with rituximab have total remission of the disease, a result obtained in the MENTOR study. Recently, The Kidney Disease: Improving Global Outcomes guidelines (KDIGO), recommended rituximab as a drug of choice in relapsed disease.
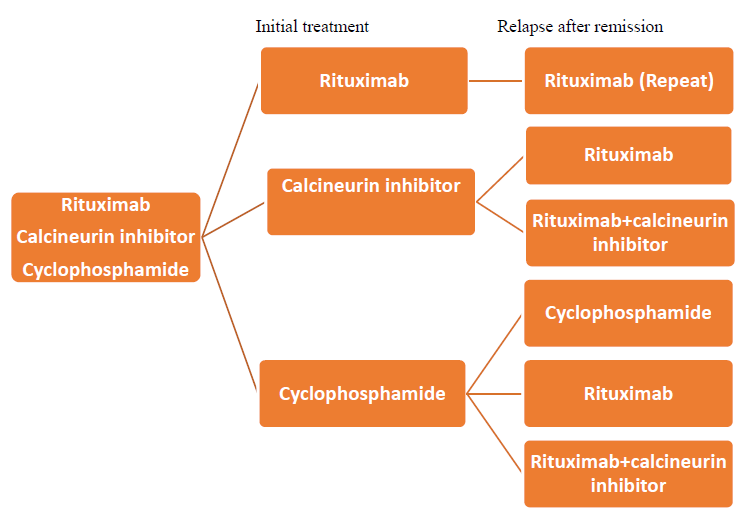
Figure 1: Relapse treatment scheme by KDIGO 2020
But in the meantime, in October 2020, important information is obtained from the Starmen study regarding the cases of remission under treatment with rituximab + tacrolimus versus those treated with cyclophosphamide + steroids. This study showed cyclophosphamide + steroids to be superior to the rituximab + tacrolimus treatment arm9. Cyclophosphamide has already been proven for years to be an effective treatmet for MN, but its side effects are important, so it should be reserved for high progression risk and resistant cases. It should be avoided if possible in smokers (because of risk for bladder and lung cancer) and patients of child-bearing age. However, the choice of a treatment scheme is made based on evidence, but also by the treating physician, depending on the experience and a good balance between the benefits and the risks of therapy.
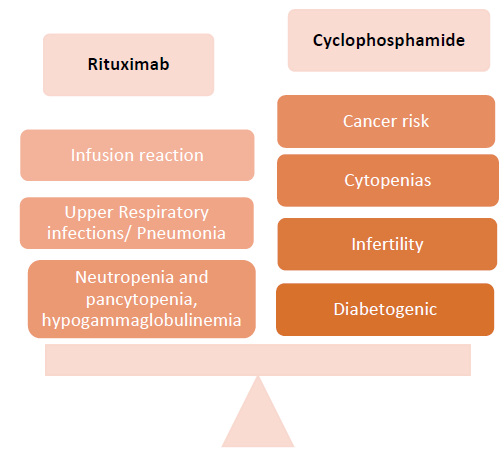
Figure 2: Safety issues of Rituximab compared with Cyclophosphamide
Results from new studies regarding the use of other drugs in MN such as: natural adrenocorticotropic hormone (ACTH), mycophenolate mofetil (MMF), obinutuzumab, plasma exchange are currently pending.
4. Conclusion
Rituximab (anti B-cell) is a very effective new treatment in the treatment of primary MN. The two studies that support this approach are GEMRITUX and MENTOR. Phospholipase A2 is a serological marker, which not only precedes and predicts the clinical signs, but also shows the activity of the pathology. Nephrologist should be familiar with all therapy options in order to offer patients the treatment with the best risk: benefit ratio, guided by clear algorithms published by KDIGO and Toronto Group.
References
- De Vriese AS, Glassock RJ, Nath KA, et al. A proposal for a serology-based approach to membranous nephropathy. Journal of the American Society of Nephrology 28 (2017): 421-430.
- Beck Jr LH, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. New England Journal of Medicine 361 (2009): 11-21.
- Sethi S, Debiec H, Madden B, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney International 97 (2020): 163-174.
- Trujillo H, Alonso M, Praga M. New ways of understanding membranous nephropathy. Nephron 144 (2020): 261-271.
- Bobart SA, De Vriese AS, Pawar AS, et al. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney International 95 (2019): 429-438.
- Rovin BH, Almaani S, Malvar A. Reimagining the kidney biopsy in the era of diagnostic biomarkers of glomerular disease. Kidney International 95 (2019): 265-267.
- Hoxha E, Thiele I, Zahner G, et al. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. Journal of the American Society of Nephrology 25 (2014): 1357-1366.
- Fervenza FC, Appel GB, Barbour SJ, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. New England Journal of Medicine 381 (2019): 36-46.
- Fernández-Juárez G, Rojas-Rivera J, van de Logt AE, et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney International (2020).
