Right Ventricular Infarction Secondary to Extensive Bilateral Pulmonary Emboli in the Setting of Negative D-dimer: A Case Report and Mini-Review
Article Information
Fariba Yazdanpanah1*, Bulent Zaim2, Charles Hunter3, Robyn Anderson4,5, Vivek Bahl2
1Department of Internal Medicine, University of Maryland Capital Region Medical Center, MD, USA
2Department of Cardiology, University of Maryland Capital Region Medical Center, MD, USA
3Department of Radiology, University of Maryland Capital Region Medical Center, MD, USA
4Department of Critical Care Medicine, University of Maryland Medical System, Baltimore, MD, USA
5Department of Critical Care Medicine, University of Maryland Capital Region Medical Center, Largo, MD, USA
*Corresponding author: Fariba Yazdanpanah, Department of Internal Medicine, University of Maryland Capital Region Medical Center,MD, USA.
Received: 04 May 2022; Accepted: 13 May 2022; Published: 20 June 2022
Citation: Fariba Yazdanpanah, Bulent Zaim, Charles Hunter, Robyn Anderson, Vivek Bahl. Right Ventricular Infarction Secondary to Extensive Bilateral Pulmonary Emboli in the Setting of Negative D-dimer: A Case Report and Mini-Review. Cardiology and Cardiovascular Medicine 6 (2022): 301-307.
View / Download Pdf Share at FacebookAbstract
Introduction: Acute pulmonary embolism (PE) is one of the presentations of venous thromboembolism (VTE) which can be potentially life-threatening by causing cardiovascular collapse. Commonly, a negative D-dimer assay is accepted for ruling out PE; however, there have been cases such as this case that challenge current practice.
Case presentation: This case report presents an 83-year-old female with sudden onset shortness of breath associated with low oxygen saturation on the physical exam. Initial workup revealed elevated levels of troponin-T and pro-B-type natriuretic peptide with preliminary normal D-dimer assay. At the start, the patient managed as Right Ventricular (RV) infarction with remarkable findings of RV dysfunction and pressure overload in transthoracic echocardiogram. Eventually chest CT angiogram documented extensive bilateral pulmonary emboli (PE), and interestingly, D-dimer became positive 5 days after the diagnosis of PE.
Conclusions: This case report is a rare case of initial negative D-dimer in the setting of extensive bilateral PE which caused right ventricular infarction. The literature review demonstrated only a few cases of PE in the setting of negative D-dimer. This unusual clinical scenario presents a diagnostic challenge in patients with low or moderate clinical probability for PE; even some current practices indicate stopping further diagnostic work-up if the D-dimer is negative in these groups. To mitigate negative outcomes in patients with low or moderate clinical probability, other strategies have been proposed to make an early diagnosis of PE such as triple combination modalities; however, they require additional analysis and consideration before they can be routinely recommended.
Keywords
Case Report; Mini-Review; Negative D-dimer; Pulmonary embolism; Right ventricular infarction; Venous thromboembolism
Article Details
1. Introduction
Acute Pulmonary Embolism (PE) is a potentially life-threatening presentation of Venous Thromboembolism (VTE). One of the mainstays in the diagnosis of PE is using the high-sensitivity D-dimer assay. Commonly, a negative D-dimer assay is accepted for ruling out PE; however, there have been cases such as this case that challenge the current practice. Our patient is an interesting, rare case of extensive bilateral PE with right ventricular infarction in light of intermediate clinical probability for PE and initially negative D-dimer assay which turned positive 5 days after the diagnosis of PE. Given that acute extensive PE can cause drastically poor outcomes by cardiovascular collapse, early diagnosis is very important to reduce morbidity and mortality, so current new studies are focusing on different diagnostic modalities to avoid misdiagnosis of PE in low or moderated clinical probability patients with negative D-dimer.
2. Case Presentation
An 83-year-old female presented to the emergency room with acute onset shortness of breath without chest pain for 3 hours. She denied leg swelling and previous episode (s) of deep venous thrombosis (DVT), immobilization, trauma, and long-distance trip. Past medical history was significant for hypertension and peripheral vascular disease.
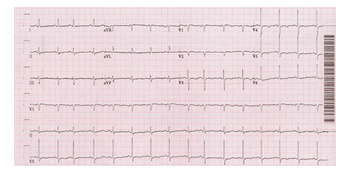
On examination, the oxygen saturation was 80–89% while breathing ambient air. The patient was tachycardic with a heart rate of 104 beats per minute at rest; otherwise, the physical exam was unremarkable. Initial lab tests revealed elevated levels of pro-B-type natriuretic peptide and troponin-T along with a normal level of the D-dimer assay tested via the quantitative immunoturbidimetric method. Electrocardiogram showed sinus tachycardia with premature atrial complexes and T-wave inversions in the anterolateral leads (Figure 1).
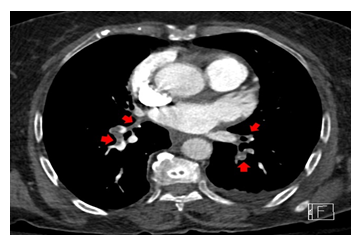
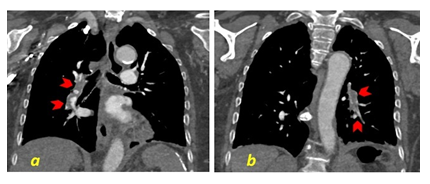
Chest X-ray findings were within normal limits. Transthoracic echocardiogram reported normal left ventricular ejection fraction at 55%, mild right ventricular dilation, and hypokinesia, with right ventricular systolic pressure of 39.7 mmHg. Although the D-dimer had been reported negative, because of the presenting symptom (shortness of breath), and paraclinical findings, PE workup was considered. Venous duplex ultrasound showed no evidence of acute DVT in the lower extremities, however, chest CT angiogram was consistent with extensive bilateral PE in the main pulmonary arteries extending into the segmental and subsegmental arteries (Figures 2, 3a, and 3b). It is noteworthy that repeated D-dimer tests became positive 5 days after the diagnosis of PE using the same primary quantitative method. Initially, the patient was managed with the non-ST elevation myocardial infarction protocol, including the therapeutic dose of anticoagulation and also oxygen therapy via nasal cannula for hypoxia. Upon confirming the PE, the treatment plan was refocused on right ventricle myocardial infarction in the setting of PE with the goal of optimizing right ventricular preload. Due to the patient’s age, medical background, and discussions with the patient and her family, the patient did not qualify for thrombolytic therapy either systemic or catheter-directed. Following the improvement of difficulty breathing and oxygen saturation, the patient was discharged on the therapeutic dose of direct oral anticoagulation (DOAC) along with scheduled follow-up appointments with hematologist/oncologist and cardiologist for subsequent evaluations.
3. Discussion and Conclusions
For the diagnosis of PE, different diagnostic modalities are being used including clinical probability, D-dimer assay, partial pressure of end-tidal CO2 (PetCO2), venous ultrasound of the lower extremities, ventilation-perfusion lung scan, and chest CT angiography [1]. Wells’ Criteria is one of the most commonly used tools for predicting the likelihood of PE based on clinical evaluation. According to Wells’ study, the chance of PE in patients with low (0-1), moderate (2-6), and high (>6) Wells’ scores is 1.3%, 16.2%, and 37.5% respectively. In addition, Wells' study shows that using the combined strategy of a clinical score with D-dimer has a negative predictive value of 99.5% (confidence interval (CI), 99.1%–100%) to diagnose PE [2]. The D-dimer assay is one of the most commonly used laboratory tests to rule out PE which is a protein fragment from the clot. Monoclonal antibodies to certain locations of the D-dimer protein, depending on the assay, have been developed and are added to the sample to bound to D-dimer. The D-dimer assay, by measuring these monoclonal antibodies, is a highly sensitive test in ruling out the PE, such that, the 2018 guidelines of the American Society of Hematology reported the sensitivity of D-dimer for diagnosis of PE as 97% (95% CI, 0.96%–0.98%) [3,4]. Additionally, D-dimer antigen levels are elevated in the acute phase of clot formation, and the half-life of these antigens is 4 to 6 hours. Continued fibrinolysis that occurs in PE causes the D-dimer level to remain elevated for about 7 days [3], so the assay could be falsely negative if the specimen is collected very early or later than 7 days after clot formation [5]. Since it is difficult to determine exactly when clot formation occurred, there is the potential to have a falsely negative D-dimer result and a misdiagnosis.
This case report demonstrates a patient with acute extensive bilateral PE along with right ventricular infarction in the setting of a moderate Wells’ score (score of 4.5) and initially negative D-dimer test, which turned positive 5 days after the diagnosis of PE. It is possible that, in our case, the initial D-dimer test was drawn too early in the disease process. Current literature reflects that the number of reported PE cases with negative D-dimer tests is only a few. Moreover, recommendations from different current sources state that “patients with low clinical probability for PE and negative results of a highly sensitive D-dimer can be considered to be without a diagnosis of PE and do not require chest CT angiography” [2,6]. In addition, a study published recently in NEJM known as the Pulmonary Embolism Graduated D-dimer (PEGeD) Study, combined varying cutoff levels of D-dimer, <1000ng/mL or <500ng/mL, based on patients’ low or moderate clinical probability respectively. The conclusion of the PEGeD Study also recommended against further diagnostic workup in patients with low or moderate clinical probability for PE because they had negative D-dimer levels [7]. Nevertheless, if currently accepted practices of combining clinical assessment with D-dimer alone are adequate to diagnose PE, then the work-up in this case and other reported patients with low or moderated clinical probability might have stopped and the diagnosis may have been missed since the initial D-dimer test was negative but this case report besides the review of reported cases brought this point to attention that in patients with low or moderate clinical probability, there is still a chance of being diagnosed with PE [1, 5, 8-10]. Then this question arises, what is the right choice when a patient’s clinical pretest probability for PE is low or moderate and the D-dimer assay is negative?
Due to the discrepancies between current recommendations and actual findings in practice, we suggest that the decision for the next step in patients with low or moderate clinical probability and negative D-dimer is multifactorial, and to improve the diagnostic yield of PE in these groups of patients clinicians need to consider new diagnostic strategies such as triple combination workup instead of the double combination of clinical probability and D-dimer assay. The triple combination workup proposed by Dhananjaya M. et al consisted of clinical probability, D-dimer, and CT pulmonary angiography. However, Youssf et al. state that the triple combination of clinical probability, D-dimer, and PetCO2 ≤28 mmHg could also increase the diagnostic accuracy of PE diagnosis [1,9]. This improvement can be explained by the fact that PE decreases alveolar CO2 content by obstructing the normal perfusion in the involved area of the lung. Therefore, the ventilation-perfusion ratio will increase and will create more alveolar dead space. Gas exhaled from this under-perfused lung contains low CO2 which leads to lower PetCO2 [9,11]. It is yet unclear which of these triple combination strategies is more practical, though Youssf et al.’s inclusion of PetCO2, which is a less-invasive test, may be preferred. Nonetheless, considering triple strategies in the diagnosis of PE in patients with low or moderate clinical probability requires revision to current common principles, and using these new combined modalities in practice which could be a valuable step in preventing potentially irreversible consequences from misdiagnosis of PE.
Declarations
Funding Information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with Ethical Standards
Conflicts of Interest
None to declare.
Ethical approval and Informed Consent to Participate
The patient’s identity is not disclosed anywhere. IRB or Ethics committee approval does not apply to the case report based on the authors’ institution. Written informed consent was obtained from the patient to participate in this case report.
Consent for Publication
The patient has provided informed consent for the publication of the case. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Availability of data and materials
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
Conception or design of the work and data collection: FY; drafting the article and revision: FY, VB; editing and conceptualization: BZ, CH, RA; All authors have read and approved the final manuscript.
References
- MD Meti K, Parakh R. D-dimer negative pulmonary embolism. Int J Adv Med 5 (2018): 429.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 135 (2001): 98.
- Schreiber DH. The role of D-dimer in the diagnosis of venous thromboembolism. Lab Med 33 (2002): 136-141.
- Lim W, Le Gal G, Bates SM, et al. American society of hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Advances 2 (2018): 3226-3256.
- Patel K, Patel R, Miller R, et al. A diagnostic dilemma: normal D-dimer in a patient with extensive pulmonary embolism. Chest 158 (2020): A2119-2120.
- Choosing wisely: An initiative of the ABIM Foundation, American College of Chest Physicians, American Thoracic Society. Five things physicians and patients should question.
- Kearon C, de Wit K, Parpia S, et al. Diagnosis of pulmonary embolism with D-dimer adjusted to clinical probability. N Engl J Med 381 (2019): 2125-2134.
- Dagkiran H, Atas R, Tomic I, et al. Severe pulmonary embolism with negative D-dimer-testing. Journal of Cardiol Ther (2015): 265-268.
- Youssf ARI, Ismail MFM, ElGhamry R, et al. Diagnostic accuracy of D-dimer assay in suspected pulmonary embolism patients. Egypt J Chest Dis Tuberc 63 (2014): 411-417.
- Dunn KL, Wolf JP, Dorfman DM, et al. Normal d-dimer levels in emergency department patients suspected of acute pulmonary embolism. J Am Coll Cardiol 40 (2002): 1475-1478.
- Kline JA, Meek S, Boudrow D, et al. Use of the alveolar dead space fraction (Vd/Vt) and plasma D-dimers to exclude acute pulmonary embolism in ambulatory patients. Acad Emerg Med 4 (1997): 856-863.