Revisiting HRCT Features of ABPA
Article Information
Dr. Uma Debi*, Dr. Muniraju Maralakunte, Dr. Lokesh Singh, Dr. Mandeep Kumar Garg, Dr. Vikas Bhatia, Dr. Gita Devi, Dr. Anindita Sinha
Associate Professor, Radiodiagnosis and Imaging, PGIMER Chandigarh, India
*Corresponding Author: Dr. Uma Debi, Associate Professor, Radiodiagnosis and Imaging, PGIMER Chandigarh, India
Received: 23 April 2020; Accepted: 06 May 2020; Published: 02 June 2020
Citation: Uma Debi, Muniraju Maralakunte, Lokesh Singh, Mandeep Kumar Garg, Vikas Bhatia, Gita Devi, Anindita Sinha. Revisiting HRCT Features of ABPA. Archives of Clinical and Medical Case Reports 4 (2020): 520-526.
View / Download Pdf Share at FacebookAbstract
Allergic bronchopulmonary aspergillosis (ABPA) is an abnormal immune response (hyper-sensitivity) mounted against Aspergillus fumigatus in predisposed immunocompetent hosts. This entity is commonly seen in individuals with bronchial asthma and rarely in cystic fibrosis. Serological and radiological tests help to confirm the diagnosis. High resolution computed tomography (HRCT) is the radiological modality of choice for investigating ABPA subjects. The subjects with ABPA may present variably with no HRCT abnormalities, bronchiectasis, high attenuation mucus (HAM) and chronic fibrotic lung changes. The presence of HAM represents immunological severity and also indicates higher chances of relapse. HAM is pathognomonic for ABPA. HRCT findings of ABPA are reviewed in this article.
Keywords
Abnormal immune response; High resolution computed tomography; Asthma
Abnormal immune response articles, High resolution computed tomography articles, Asthma articles
Abnormal immune response articles Abnormal immune response Research articles Abnormal immune response review articles Abnormal immune response PubMed articles Abnormal immune response PubMed Central articles Abnormal immune response 2023 articles Abnormal immune response 2024 articles Abnormal immune response Scopus articles Abnormal immune response impact factor journals Abnormal immune response Scopus journals Abnormal immune response PubMed journals Abnormal immune response medical journals Abnormal immune response free journals Abnormal immune response best journals Abnormal immune response top journals Abnormal immune response free medical journals Abnormal immune response famous journals Abnormal immune response Google Scholar indexed journals immune articles immune Research articles immune review articles immune PubMed articles immune PubMed Central articles immune 2023 articles immune 2024 articles immune Scopus articles immune impact factor journals immune Scopus journals immune PubMed journals immune medical journals immune free journals immune best journals immune top journals immune free medical journals immune famous journals immune Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals High resolution computed tomography articles High resolution computed tomography Research articles High resolution computed tomography review articles High resolution computed tomography PubMed articles High resolution computed tomography PubMed Central articles High resolution computed tomography 2023 articles High resolution computed tomography 2024 articles High resolution computed tomography Scopus articles High resolution computed tomography impact factor journals High resolution computed tomography Scopus journals High resolution computed tomography PubMed journals High resolution computed tomography medical journals High resolution computed tomography free journals High resolution computed tomography best journals High resolution computed tomography top journals High resolution computed tomography free medical journals High resolution computed tomography famous journals High resolution computed tomography Google Scholar indexed journals tomography articles tomography Research articles tomography review articles tomography PubMed articles tomography PubMed Central articles tomography 2023 articles tomography 2024 articles tomography Scopus articles tomography impact factor journals tomography Scopus journals tomography PubMed journals tomography medical journals tomography free journals tomography best journals tomography top journals tomography free medical journals tomography famous journals tomography Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals endoscopy articles endoscopy Research articles endoscopy review articles endoscopy PubMed articles endoscopy PubMed Central articles endoscopy 2023 articles endoscopy 2024 articles endoscopy Scopus articles endoscopy impact factor journals endoscopy Scopus journals endoscopy PubMed journals endoscopy medical journals endoscopy free journals endoscopy best journals endoscopy top journals endoscopy free medical journals endoscopy famous journals endoscopy Google Scholar indexed journals CT scan articles CT scan Research articles CT scan review articles CT scan PubMed articles CT scan PubMed Central articles CT scan 2023 articles CT scan 2024 articles CT scan Scopus articles CT scan impact factor journals CT scan Scopus journals CT scan PubMed journals CT scan medical journals CT scan free journals CT scan best journals CT scan top journals CT scan free medical journals CT scan famous journals CT scan Google Scholar indexed journals Angiography articles Angiography Research articles Angiography review articles Angiography PubMed articles Angiography PubMed Central articles Angiography 2023 articles Angiography 2024 articles Angiography Scopus articles Angiography impact factor journals Angiography Scopus journals Angiography PubMed journals Angiography medical journals Angiography free journals Angiography best journals Angiography top journals Angiography free medical journals Angiography famous journals Angiography Google Scholar indexed journals Asthma articles Asthma Research articles Asthma review articles Asthma PubMed articles Asthma PubMed Central articles Asthma 2023 articles Asthma 2024 articles Asthma Scopus articles Asthma impact factor journals Asthma Scopus journals Asthma PubMed journals Asthma medical journals Asthma free journals Asthma best journals Asthma top journals Asthma free medical journals Asthma famous journals Asthma Google Scholar indexed journals
Article Details
1. Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is a dysregulated immune response (hyper-sensitivity reaction) mounted towards the fungi called Aspergillus fumigatus affecting the lungs which may manifest in form of pulmonary infiltrates and bronchiectasis [1]. Pulmonary aspergillosis has varied manifestations such as allergic bronchopulmonary aspergillosis (ABPA), aspergilloma (mycetoma), semi-invasive and invasive aspergillosis, depending upon the general immunity of the host as well as the local pulmonary immunity at the time of exposure to the fungus [1]. Impaired mucosal function with colonization of Aspergillus fumigatus can lead to a Type 1 hypersensitivity response resulting in ABPA in individuals who are likely to be atopic and immunocompetent [2-4]. ABPA is mostly seen in individuals with predisposing conditions like bronchial asthma and cystic fibrois (smaller proportion) [4, 5]. Denning et al, reported the worldwide prevalence of 4.3 million ABPA subjects in a cohort of 193 million asthmatic patients [13]. The patients classically presents with uncontrolled asthma, productive cough, hemoptysis, brownish mucoid plugs in sputum, wheeze and fever [1]. The environmental as well as polygenic factors are attributed to the patho-mechanisms triggering the pulmonary manifestation of ABPA on exposure to the Aspergillus spores [11]. Destruction of bronchial walls is attributed to the necrotizing, granulomatous inflammatory changes [3]. Establishment of ABPA diagnosis in a predisposed individual requires fulfilment of a set of an obligatory criteria including significantly reactive aspergillus skin test/ raised IgE levels against fungus (A Fumigatus) and raised total IgE levels with presence of other radiological and serological parameters [11]. Chest radiograph and computed tomography are the commonly used radiological modalities to assess the early and the late changes seen in ABPA. We review the CT findings of ABPA in this article.
2. HRCT Features of ABPA
The radiographic manifestations of ABPA is speculative by virtue of nature and stage of the disease. The radiographic findings are generally categorised as transient or permanent. The transient findings include nodules, consolidation, tram track opacities, finger in glove appearance, fleeting densities and permanent abnormalities described are ring shadows, bronchiectasis and pleuropulmonary fibrosis based on the temporal manifestations of the ABPA. In pre CT era, consolidation was believed to be the most common manifestation of the ABPA, in contrast the recent studies prove mucoid impaction as more common finding than the consolidation. In current practice high resolution computed tomography (HRCT) is the imaging modality of choice of evaluation of suspected ABPA patients. The common HRCT manifestations of ABPA are bronchiectasis with or without mucoid impaction, consolidation/s, mosaic attenuation of lungs, centrilobular nodules with or without tree in bud appearance and chronic pleuropulmonary fibrotic changes. Less commonly encountered HRCT manifestations are miliary nodules, perihilar opacities, pulmonary mass and pleural effusion [11].
In ABPA central and upper lobar bronchiectasis is encountered in contrast to the usual predilection of lower lobe involvement in usual bronchiectasis [6]. Central bronchiectasis has been arbitrarily assigned to inner two-thirds or central half of the lungs [7]. Bronchiectatic changes though usually central in ABPA, can be peripheral (Figure 1) as well in 26-39% cases [5]. Bronchiectasis based on the shapes (Figure 2) of the dilated bronchioles can be divided into three types: 1) Cylindrical (tram-track/signet ring appearance), 2)Varicoid bronchiectasis (beaded appearance), and 3) Cystic bronchiectasis (cluster of air filled cysts) [8]. The varicoid or cystic bronchiectasis is usually seen as a result of extensive damage to the bronchioles [4]. The recent expert committee considers the presence of central bronchiectasis as a complication of ABPA rather than a diagnostic criteria [11].
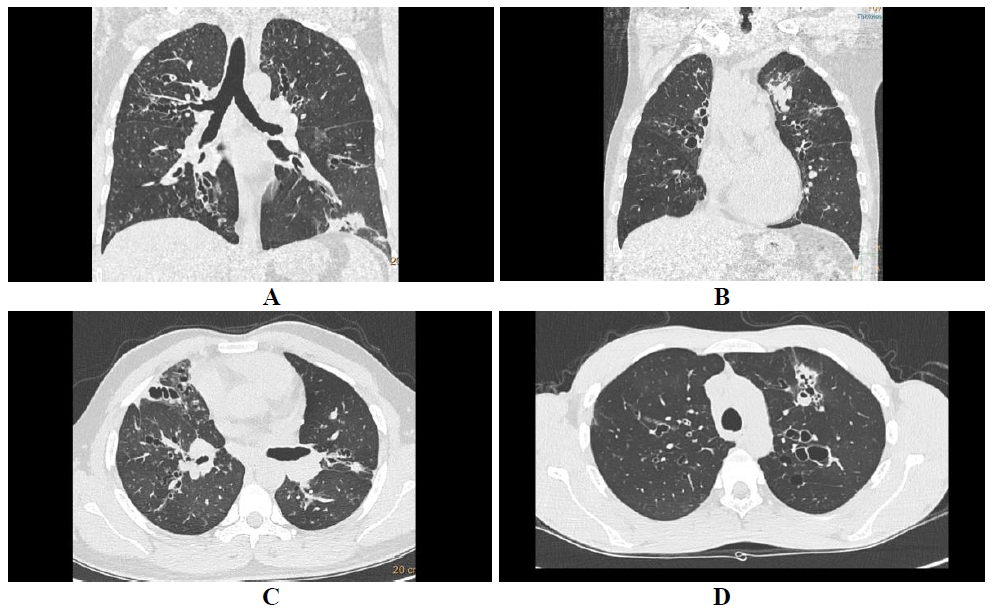
Figure 1: Central bronchiectasis. Coronal lung algorithm shows varicoid central bronchiectasis with patchy consolidation in left lower lobe (A) and another ABPA patient with central cystic bronchiectasis with bronchocele in left upper lobe (B).
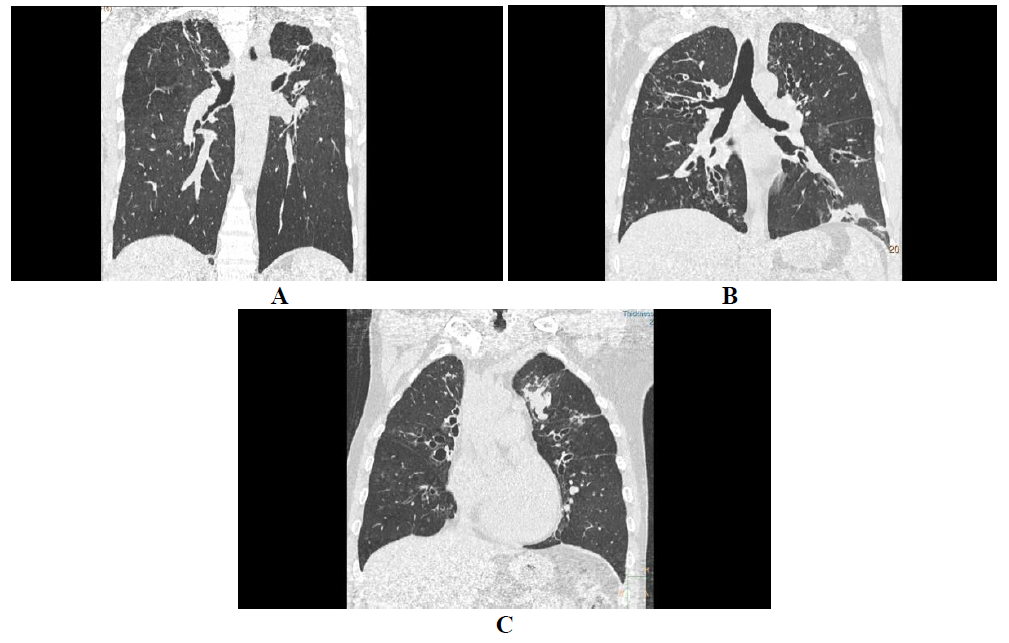
Figure 2: Central and peripheral bronchiectasis. HRCT axial sections of lung window shows central and peripheral bronchiectasis (A) with bronchocele in right central bronchi, and central bronchiectasis with air fluid level in anterior subsegmental bronchus (B).
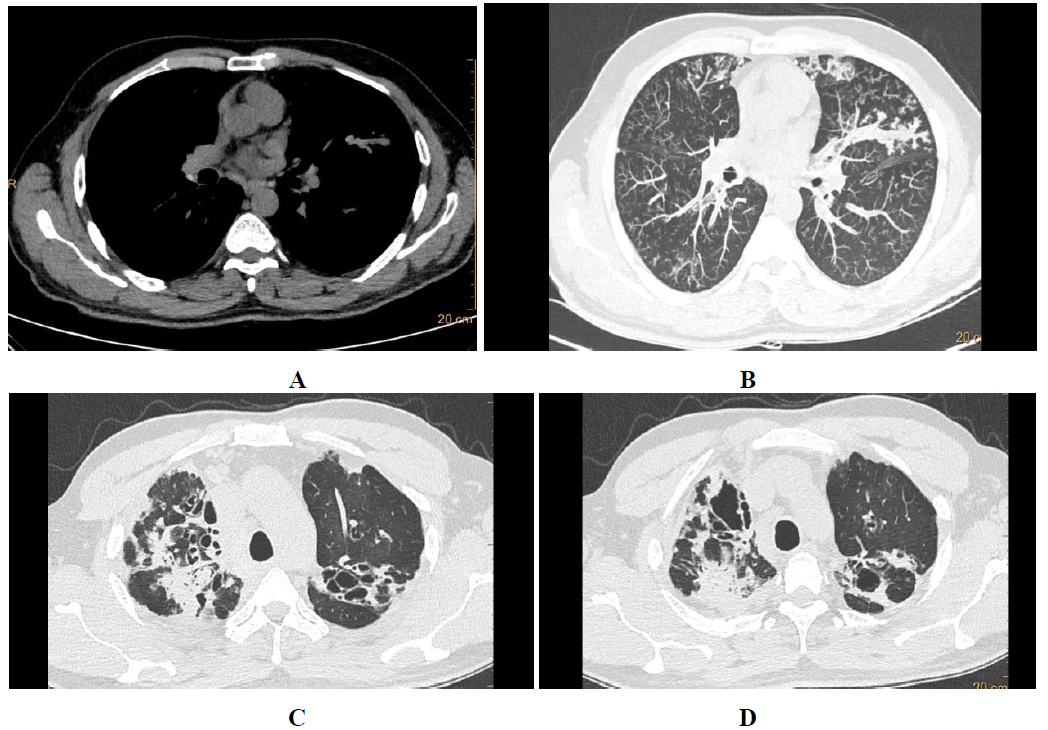
Figure 3: Bronchiectasis types. Coronal algorithms of lung window showing cylindrical (A), varicoid (B) and cystic (C) bronchiectasis with central and upper lobe predominance in ABPA.
High attenuation mucus (HAM) with attenuation values greater than the soft tissues (para-spinal muscles) and equalling that of calcification is seen in about 20-30% of patients with ABPA [3, 5, 9]. HAM is a specific feature and confirms ABPA as the root cause for bronchiectasis [5]. The high attenuation of the mucus is attributed to the fungal mineral components precipitated within the mucus [1]. The presence of HAM represents immunological severity and also indicates higher chances of relapse [5, 10].
The several researches have strived to classify ABPA on radiological basis, among them recently published International Society for Human and Animal Mycology (ISHAM) working group has classified ABPA based on computed tomography (Table 1), manifestations varying from no significant abnormality, to high attenuation mucus to chronic fibrotic pleuro-parenchymal changes. The HAM criteria closely correlate with the immunological severity.
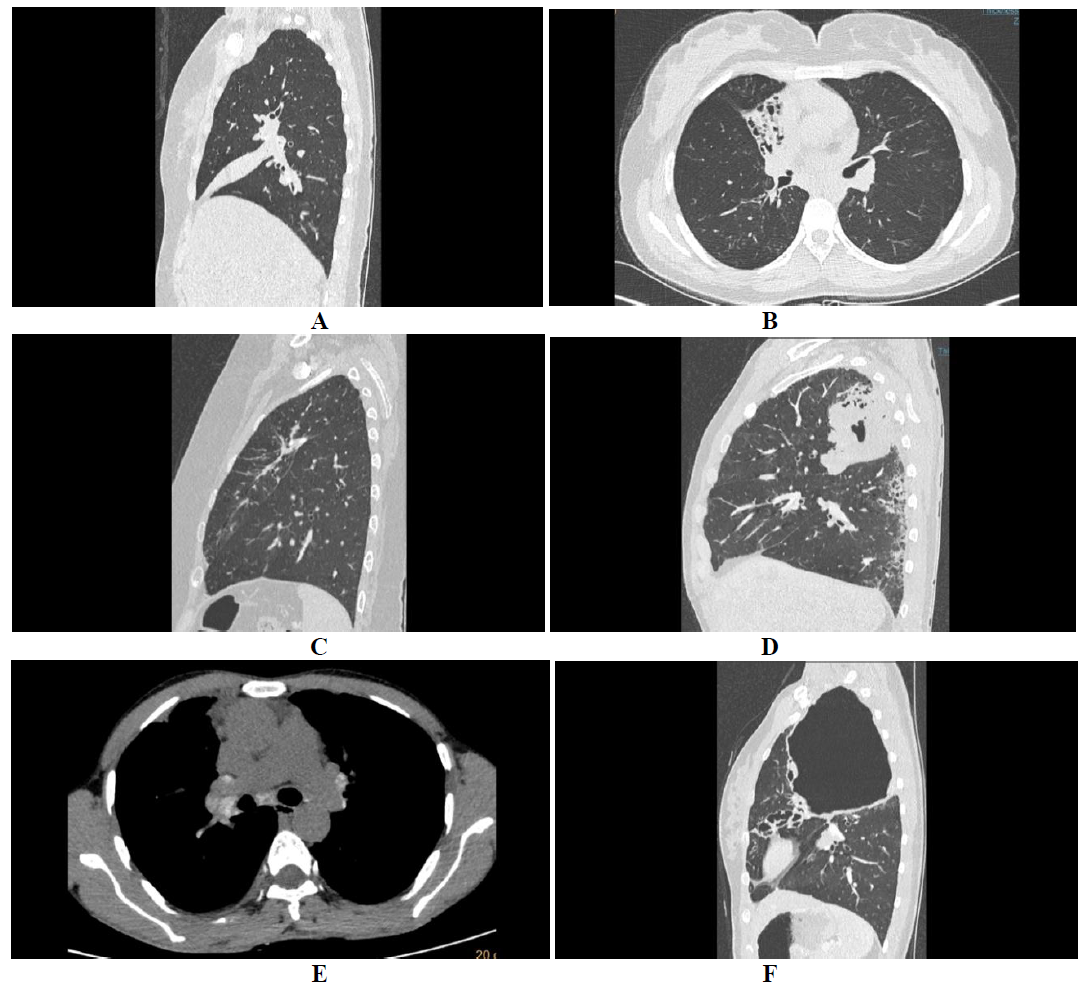
Figure 4: HAM and centri-acinar nodules. HRCT axial average algorithm showing central and peripheral bronchiectasis with high attenuation mucous (HAM) in bronchus of lingual (A), and MIP reformats of the same showing multiple centri-acinar nodules with tree in bud appearance (B).
|
Classifier |
Features |
|
ABPA – S (Serological ABPA) |
All diagnostic features of ABPA with no HRCT findings |
|
ABPA – B (Bronchiectasis) |
All diagnostic features of ABPA with bronchiectasis on HRCT |
|
ABPA – HAM (High attenuation mucus) |
All diagnostic features of ABPA with presence of high attenuation mucus on CT |
|
ABPA – CPF (Chronic pleuropulmonary fibrosis) |
ABPA with pulmonary fibrosis/ scarring, fibro-cavitary lesions, aspergilloma, pleural thickening and no HAM/ mucoid impaction |
Table 1: (Radiological classification of ABPA).
Serological ABPA is a stage of the disease with normal HRCT chest [5], whereas, bronchiectasis (Figure 1-3) and HAM (Figure 3A) stages are self-explanatory. Chronic pleuropulmonary fibrosis stage (Figure 3C) of ABPA should atleast have two other findings apart from bronchiectasis and HAM, like pulmonary fibrosis (Figure 4), fibrocavitory lesions, mycetoma and thickened pleura [11]. Chronic stages of the disease may present with atelectasis/collapse of lung (Figure 4A), consolidation (Figure 4B), fibrocalcific changes (Figure 4C), consolidation with central break down (fig 4 D), calcific mediastinal or hilar lymphadenopthay (Figure 4E), fibrotic bands (Figure 4F), cavities and cicatricial emphysema. At this stage, distinguishing ABPA from old pulmonary tuberculosis is difficult [9].
Asthmatics and patients with cystic fibrosis without ABPA may have overlapping CT features with that of patients with ABPA. Asthmatics may have a normal HRCT or may show hyper-inflation, bronchiolar wall thickening with narrowing of the bronchiolar lumen and centrilobular nodules representing peribronchiolar inflammation and mucus stasis in the bronchioles. Asthmatics might also show areas of bronchiolar ectasia, mosaic attenuation and air trapping on expiratory scans [3]. HRCT characteristics of two groups of subjects with underlying (n=44) asthma with ABPA (one group) and (n=38) asthma without ABPA (other group) were compared in a study by Ward et al. Bronchiectasis (95%), centrilobular nodules (93%), and mucoid impaction (67%) were more commonly seen in patients with ABPA, compared to the patients without ABPA who showed these characteristics in 29%, 28% and in 4% individuals respectively [12]. Cystic fibrosis related lung disease without ABPA, has several overlapping features with ABPA, but HAM due to its high specificity suggests that the lung disease is due to superimposed ABPA [5].
3. Conclusion
Pulmonary aspergillosis has varied manifestations. ABPA is the usual presentation in an immunocompetent individual exposed to the fungus. ABPA presents most commonly with central bronchiectasis. HAM is a specific feature of the disease and also helps in disease prognostication. Chronic fibrocavitory stage of the disease is difficult to differentiate from the sequelae of other chronic pulmonary infections like pulmonary tuberculosis.
References
- Panse P, Smith M, Cummings K, et al. The many faces of pulmonary aspergillosis: Imaging findings with pathologic correlation. Radiol Infect Dis 3 (2016): 192-200.
- Thompson BH, Stanford W, Galvin JR, et al. Varied radiologic appearances of pulmonary aspergillosis. RadioGraphics 15 (1995): 1273-1284.
- Silva CIS, Colby TV, Müller NL. Asthma and associated conditions: high-resolution CT and pathologic findings. Am J Roentgenol 183 (2004): 817-824.
- Greene R. The radiological spectrum of pulmonary aspergillosis. Med Mycol 43 (2005): 147-154.
- Agarwal R. Allergic bronchopulmonary aspergillosis: Lessons for the busy radiologist. World J Radiol 3 (2011): 178.
- Panchal N, Bhagat R, Pant C, et al. Allergic bronchopulmonary aspergillosis: the spectrum of computed tomography appearances. Respir Med 91 (1997): 213-219.
- Santis G, Hodson ME, Strickland B. High resolution computed tomography in adult cystic fibrosis patients with mild lung disease. Clin Radiol 44 (1991): 20-22.
- Agarwal R, Garg M, Gupta D, et al. Pictorial essay: Allergic bronchopulmonary aspergillosis. Indian J Radiol Imaging 21 (2011): 242.
- Shah A, Panjabi C. Allergic aspergillosis of the respiratory tract. Eur Respir Rev 23 (2014): 8-29.
- Agarwal R, Khan A, Gupta D, et al. An Alternate Method of Classifying Allergic Bronchopulmonary Aspergillosis Based on High-Attenuation Mucus. Kreindler JL, editor. PLoS ONE 5 (2010): 146-153.
- Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 43 (2013): 850-873.
- Ward S, Heyneman L, Lee MJ, et al. Accuracy of CT in the diagnosis of allergic bronchopulmonary aspergillosis in asthmatic patients. AJR Am J Roentgenol 173 (1999): 937-942.
- Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol 51 (2013): 361-370.
