Results from an Early Economic Evaluation of the use of A Novel Point of Care Device for Diagnosis of Suspected Acute Coronary Syndrome Patient Within an Emergency Department in the National Health Service in England
Article Information
Mamta K Bajre1*, Adrian Towse2, Andrew Stainthorpe1, Julie Hart1
1Oxford Academic Health Science Network, UK
2Office of Health Economics, UK
*Corresponding author: Mamta K Bajre, Oxford Academic Health Science Network, UK.
Received: 09 November 2021; Accepted: 19 November 2021; Published: 26 November 2021
Citation: Bajre MK, Towse A, Stainthorpe A, Hart J. Results from an Early Economic Evaluation of the use of A Novel Point of Care Device for Diagnosis of Suspected Acute Coronary Syndrome Patient Within an Emergency Department in the National Health Service in England. Cardiology and Cardiovascular Medicine 5 (2021): 623-637.
View / Download Pdf Share at FacebookAbstract
Background The objective of this study was to undertake an early economic evaluation to analyse the potential costs and benefits associated with adopting a high sensitivity troponin (hs-cTn) at the Point of Care (POC) in the Emergency Department (ED) diagnostic pathway for suspected Acute Coronary Syndrome (ACS) patients in line with National Institute for Health and Care Excellence (NICE) Diagnostics Guidance (DG15) and NICE Clinical Guideline (CG95) as practised in the NHS in England.
Methods A decision tree analysis was undertaken to compare the current 60 to 90 minutes turnaround time for the standard laboratory hs-cTn test with an expected 20-minute turnaround time for a POC hs-cTn test. Three routes through the chest pain pathway were modelled based on the hs-cTn pathway used in Oxford University Hospitals (OUH) NHS Foundation Trust. Sensitivity analysis was performed.
Results The results indicate that if a hs-cTn POC test is used to diagnose patients in routes 1 to 3 of the diagnostic pathway for suspected ACS patients at ED, it potentially saves per patient costs of £33 in Routes 1 & 3 and £42 in Route 2. Moreover, it can also help in easing the pressure at ED as it enables diagnosis to be made between 55 to 70 minutes earlier across the 3 pathway routes. A hs-cTn POC test also has potential in achieving a ‘rule-in’ diagnosis for patients to speed up the treatment pathway for improved prognosis. The sensitivity analysis indicates that savings per patient increase as the nursing time for patient monitoring is varied between 70% and 90% . Furthermore, there is savings per patient even when the cost of the hs-cTn biomarker is varied by ~£10.
Conclusions Use of a hs-cTn test at POC can save between £33 and £42 per patient in ED when compared to the standard laboratory test. When such a POC t
Keywords
Acute myocardial infraction; Cardiac biomarker; Cost consequence; High sensitivity troponin; Point-of-care
Acute myocardial infraction articles; Cardiac biomarker articles; Cost consequence articles; High sensitivity troponin articles; Point-of-care articles
Article Details
1. Background
A feasibility study was conducted by Oxford Academic Health Science Network to explore the potential utility of a multi-biomarker point of care (POC) diagnostic test, including high sensitivity troponin (hs-cTn), for the diagnosis of patients with suspected ACS and/or complaining of chest pain, presenting in emergency department (ED) in line with NICE Diagnostics Guidance (DG15) and NICE Clinical Guideline (CG95) as practised within the NHS in England. Subsequent to this study NICE Diagnostics Guidance (DG15) was replaced by NICE Diagnostics Guidance (DG40) in August 2020, however, this has no implications for the results of the study. The feasibility study was conducted using the Lean Assessment Process (LAP) methodology [1]. LAP methodology is developed to align evidence generation with resources available at an early stage of a healthcare device development by establishing the feasibility (or not) of a potential technology using a preliminary assessment of design, value and evidence reliability. Results of the feasibility study found that all stakeholders interviewed agreed that there is an unmet need for hs-cTn biomarker tests for use at the POC and that a hs-cTn POC test had clinical utility in ED within the NHS. The stakeholders thought that hs-cTn is an effective blood test when used in conjunction with a full clinical assessment for the early diagnosis of Acute Myocardial Infarction (AMI). AMI is a subset of ACS and it is the damage to the cardiac muscle that is evidenced by elevated cardiac troponin levels in the setting of acute ischemia [2].
All stakeholders were of the view that rapid biomarker tests performed at the POC could lead to a faster turnaround time and patient management decisions, helping to alleviate pressures on the ED. Based on the results of the feasibility study, an early economic evaluation was performed to assess the costs consequences of adopting a hs-cTn biomarker test at the POC, in place of hs-cTn laboratory-based testing within the ED standard diagnostic care pathway, for the management of patients presenting with chest pain and symptoms suggestive of an ACS. hs-cTn laboratory-based tests recommended by NICE Diagnostics Guidance (DG15) were selected as Rapid Uptake Products by the Accelerated Access Collaborative and have been supported by the Innovation and Technology Payment programme [3]. This hypothetical early economic evaluation is undertaken to estimate the costs of implementing the hs-cTn POC in the ED pathway.
The objective of this early economic evaluation is:
- To assess the probable cost consequences of a POC hs-cTn test in the care pathway of patients suspected of ACS presenting with chest pain at ED within NHS
- To assess the possibility of improving the likelihood of achieving the 4-hour waiting time target performance in ED
2. Methods
2.1 Model Overview
The early economic model was developed to compare the indicative cost of testing a cardiac biomarker (hs-cTn) at the POC versus standard laboratory care testing for patients presenting with chest pain to ED within the NHS in England. Early economic evaluations are undertaken for a number of reasons [4]. In this case the new technology is hypothetical, albeit with characteristics that are credible and achievable for test developers, given the clinical need for the test identified in the feasibility study. This study helps to identify the associated cost of implementating POC in the ED pathway indicating to developers the likely willingness of the NHS to adopt such a test, were it to be successfully brought to market.
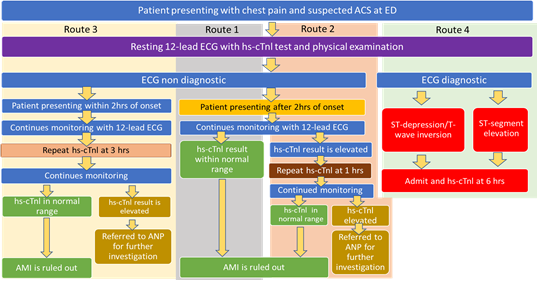
Standard laboratory care testing for hs-cTn has been introduced in most NHS hospital sites (around 80% according to the assay and laboratory testing equipment manufacturers) and is the current standard of care (SOC). Measuring hs-cTn via laboratory testing substantially reduced the time required for safe rule-out or rule-in of AMI. Troponin levels can be measured at either 1-hour post ED admission or 3 hours post ED admission for the early rule out of AMI, in both cases providing better patient care and information for clinicians detailed in Figure 1. NHS England is advocating hs-cTn testing via 1-hour and 3-hour testing pathways, as per NICE Diagnostics Guidance DG15 [5] in that these are cost-effective. As a consequence, this study used NICE Diagnostics Guidance DG15 and current practice in OUH to model four hypothetical routes through the ED chest pain pathway that patients presenting with chest pain might follow (see Figure 1), in order to identify the potential impact of such a POC test. This study then evaluated three of the routes (Routes 1,2 and 3), as the fourth route modelled involves an electrocardiogram( ECG) based diagnosis rather than hs-cTn based testing.
The three hypothetical routes modelled were for a POC hs-cTn test used in the ED setting. For patients presenting at ED after 2 hrs onset of symptoms, a hs-cTn test is performed on arrival at 0 hrs (Route 1) or a hs-cTn test is performed on arrival at 0 hrs and then the test is repeated after 1 h (Route 2). In Route 3, where patients present at ED within 2 hrs onset of symptoms, a hs-cTn test is performed on arrival at 0 hrs and repeated 3 hrs after the first test for hs-cTn. Decision-analytic models were developed and tested in Excel to compare POC testing to the SOC in the ED for the 3 routes through the pathway currently used in OUH and comparable to other trusts in the NHS in England based on DG15. Three different decision trees were produced to test the impact of POC testing on potential outcomes in the ED. The outcomes used in the study include the time taken for result turnaround and the costs associated with patient monitoring whilst a patient with chest pain is in the ED pending diagnosis.
The base-case model for all three routes assumes:
- 60 to 90 minutes turnaround time for the standard laboratory hs-cTn test. The turnaround time between a blood draw and the reporting of assay results is an important limiting factor to rapid decision making in the care pathway. Transporting the blood to a central laboratory and analysing it is a significant component of the turnaround time, resulting in reporting times for test results of between 60 to 90 minutes after the blood has been drawn. The reporting time may vary between hospitals;
- 20 minutes turnaround time for the POC hs-cTn test. A study suggests that a point-of-care troponin assay that can produce a result within 15 minutes after blood sampling had comparable discrimination ability to a hs-cTn assay for ruling out AMI after a single blood test [6].
We are assuming that the sensitivity and specificity of the two alternative tests are the same. This is a reasonable assumption as a study reported that in a large prospective multicentre study, the POC-hs-cTnI-TriageTrue assay provided high diagnostic accuracy in patients with suspected AMI with a clinical performance at least comparable to that of best-validated central laboratory assays. The POC test was validated with central laboratory hs-cTnT/I assay and a very safe and highly efficacious POC-hs-cTnI-TriageTrue 0/1-h algorithm was achieved [7].
2.2 Care pathways
In order to understand the effect of the intervention we modelled three pathways to assess the hypothetical impact of a POC hs-cTn test on current care pathways which are in line with the NICE Diagnostics Guidance (DG15) [5] The SOC pathways we modelled in the ED are illustrated in Figure 1. A patient arriving at an ED with a complaint of chest pain will be assessed with a 12 lead ECG and a blood sample will be taken promptly for hs-cTn testing along with other blood tests. A physical examination will also be performed, and the patient’s medical history is taken. Based on the results of this initial assessment and the ECG findings, a patient could be referred to a Cardiology Advanced Nurse Practitioner (ANP) to continue their care and monitoring ahead of the team receiving data to support a diagnosis. The accuracy of the hs-cTn test allows the results from type T or I troponin testing to be reviewed twice within four hours of patient presentation at the ED. Actual timings vary depending on local pathways. We use Route 1, Route 2 and Route 3 when referring to different routes through the pathway towards a diagnosis informed by tests including the hs-cTn test. They are summarised in Figure 1 and described in the next section.
Routes 1 and 2 are followed for the patient who presents at ED in or after 2 hours of the “onset of chest pain2 for suspected ACS and route 3 is followed for the patient who presents at ED within 2 hours of onset of chest pain for suspected ACS.
2.3 Three detailed chest pain pathways
The time saving benefits of POC testing in all 3 routes outlined here are based on the potential to make an earlier diagnosis based on the hs-cTn test results arriving earlier. The data presented in Figures 2, 3 and 4 is based on the situation where the hs-cTn test can be used as a pivotal test in decision-making. We assume any other significant test data arrives at the same time and therefore does not delay decision-making in the ED.
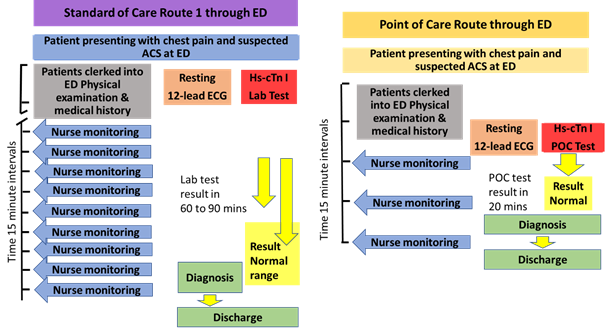
2.3.1 Route 1 detailed process
Admission to the ED including the initial assessment process takes approximately 30 minutes. The patient is assessed with the 12 lead ECG and blood is taken for the hs-cTn and other tests which are sent to the laboratory under the current SOC. The hs-cTn sample is analysed in the central laboratory and the result turn-around-time typically takes between 60 to 90 minutes in the standard care pathway from needle to result. However, if the hs-cTn is performed as the POC test in the ED, the result turnaround-time will be around 20 minutes. The more rapid access to the hs-cTn test results could allow diagnosis up to 30 to 80 minutes earlier with an average of 55 minutes. This could potentially avoid between two to five monitoring rounds to the patient by the Specialist Nurse and free-up an ED bed earlier. Average times are used in the model.
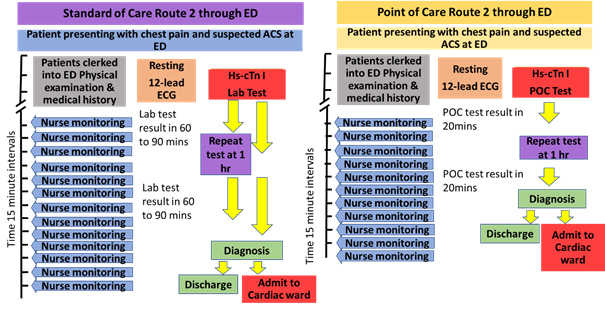
2.3.2 Route 2 detailed process
Route 2 also relates to patients arriving at the ED more than 2 hours after the onset of chest pain. As with Route 1, the patient is assessed with the 12 lead ECG and blood is taken for the hs-cTn and other tests which are sent to the laboratory in the current standard of care. Where the second hs-cTn test result is within normal range and other clinical factors support a diagnosis which safely rules out MI as the cause of the patient’s chest pain, then the patient might either be discharged from the ED to home or referred to an alternative investigative pathway outside the ED. Using POC testing, the hs-cTn test results would be available 20 minutes after testing. The patient will therefore have around 50 minutes ED contact time before the ED team will receive the first hscTn test result uder SOc. Where this first test is above the normal range, a second hs-cTn test will be requested 1 hour after the first test. In terms of the POC testing this means around 30 minutes after the results of the first test are known. Overall, the assessment of a patient presenting with chest pain to an ED using a POC hs-cTn test, from admission to diagnosis, might take a minimum of 140 minutes. The equivalent timing for the standard of care laboratory based hs-cTn test would be between 210 and 270 minutes depending on how quickly the lab tests might be returned to the ED. This suggests an average savings of 70 minutes (considering lab test report turnaround time of 60 minutes).
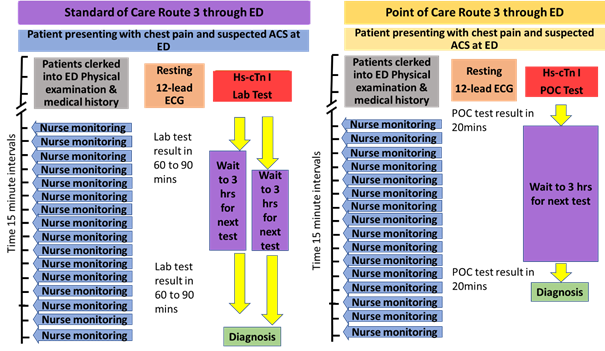
2.3.3 Route 3 detailed process
This route is followed by patients arriving at the ED less than 2 hours after the onset of chest pain.
Comparison of the timings is based on a 30-minute admission process before the initial hs-cTn test is requested. Both POC hs-cTn and standard care laboratory hs-cTn test results will have reported before the requisite second test and both hs-cTn test results are required to inform the diagnosis and a decision to discharge or admit. The second POC test will be available 20 minutes after it is initiated allowing a diagnosis and decision between 20 and 90 minutes (average 47.5 minutes) earlier than the laboratory hs-cTn test. The extra time required for the second standard of care hs-cTn test will mean the patient remains on the ED pending the decision to discharge or admit. This involves the Specialist Nurse in additional ongoing monitoring visits compared to that for the POC hs-cTn test.
2.4 Resource estimates used in the evaluation
This economic evaluation is based around a number of assumptions. First, we assume that a move to point of care hs-cTn testing in the ED will not require changes to the ED tariffs, so there will be no impact on hospital income. Second, we assume the main resource impacted by the use of POC hs-cTn testing are (i) the costs related to continuous monitoring by a band 6 specialist nurse and (ii) the cost of the POC hs-cTn test and disposable biomarker test cartridges. As noted, the comparator in this evaluation is the laboratory blood analysers used in the pathology laboratory and the same continuous monitoring by a band 6 nurse. A study suggests that monitoring of blood pressure, respiratory rate, and pulse oximetry should be performed at the time of initial presentation and these measurements may be repeated at every 15 minutes of intervals based on the changing clinical status of the patient [8]. We acknowledge that a nurse may not be with the patient 100% of the time but will complete monitoring on a regular (every 15 minutes) basis. Regular monitoring of suspected AMI patients by nurse makes it difficult, however, for the nurse to undertake other activities. We assume, therefore, that 80% rather than 100% of nurse time is spent with the patient for continuous monitoring, maintaining patient’s hydration and updating the records.
The costs considered for this early economic evaluation do not include the capital and maintenance costs of the POC analyser or for central laboratory, technologist time and all other direct costs associated with the tests in the standard and the POCT pathway. The evaluation only covers the costs of the laboratory hs-cTn test and an assumed cost of the POC test cardiac biomarker disposable cartridge. The cost of a band 6 nurse, on a per minute basis, is used and costing in the model is based on the difference in time interval from the arrival time to the discharge or admission time. In all three routes, 80% of the nursing time is considered. The costs of staff training to perform and report the POC test is also not included in the current analysis, although acknowledging there would be associated costs for the introduction of the test into the ED. The costs used in the model are detailed in Table 1 below.
|
Data |
Sources |
|
|
hs-cTn Lab Test cost |
£22.11 |
Campbell [9] |
|
hs-cTn POC Test cost |
£22.11 |
Considered same as the Lab cost (assumptions) |
|
Band 6 Nurse cost |
£0.60 per minute (A Band 6 nurse cost is £45 per hour) |
Unit costs of health and social care 2017-2018 (PSSRU) [10] |
|
Percentage of nursing time spent |
80% |
Only 80% of a full time nirse is considered for base case |
Table 1: Resource and cost assumptions used in the model
2.5 Basis for the decision tree analysis
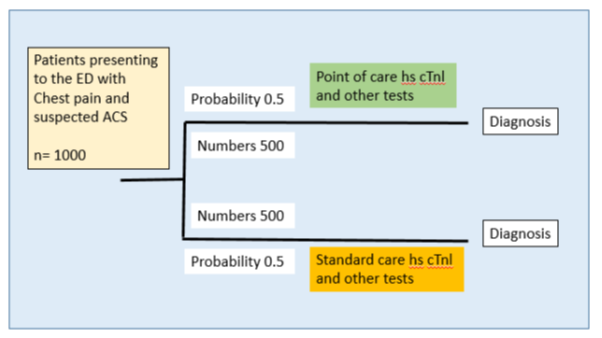
Each route is analysed as a decision tree towards achieving a diagnosis. It compares the impact of the POC testing compared to the SOC on relevant costs and outcomes for each route in the pathway. The decision tree covers the time a patient with chest pain/suspected AMI is in the ED pending a diagnosis and receiving care and monitoring for suspected AMI. The base population for the decision tree is considered as 1,000 patients. The two parts of the model are identical in structure (Figure 5) and it is assumed that after going to the ED, an equal number of patients (500 each) are considered for hs-cTn test in each arm and all tests are performed at a laboratory in one arm and as a POC test in the other. In all three models there is an identical decision node (Figure 5) where the initial hs-cTn test is performed, along with other assessments. It is assumed that all other diagnostic services and care would be the same and independent of the type of hs-cTn test performed. The key variable in the model is the time to diagnosis and the associated impact on per patient nursing care.
3. Results
This section details the base case results from all the 3 proposed routes in which the cost of standard care is compared to the cost of POC testing at ED and the sensitivity analysis performed.
3.1 Base case
The modelled data for route 1, suggests a possible savings of 27,500 minutes of nursing time that equates to a potential saving of £16,500 in ED nursing cost (Table 2). This is based on 55 minutes of reduced nursing time. This is considering the standard care hs-cTn test average turn-around-time of 75 minutes where as it is 20 minutes for the POC test. This leads to cost savings of £33.00 per patient.
|
Route 1 1 test only |
Total Nursing time spent (in minutes) |
Total Nursing time spent (in minutes per patient) |
Total nursing cost |
Total hs-cTn Test cost |
Total arm cost |
Total arm cost (per patient) |
|
A) Standard care (for 500 patients) |
67,500 |
135 |
£40,500.00 |
£11,055.00 |
£51,555.00 |
£103.11 |
|
B) POC (for 500 patients) |
40,000 |
80 |
£24,000.00 |
£11,055.00 |
£35,055.00 |
£70.11 |
|
Total Savings (A – B) |
27,500 |
55 |
£16,500.00 |
£0 |
£16,500.00 |
£33.00 |
Table 2: Base case result for route 1 when patient for patients needing 1 hs-cTn test in the ED.
The modelled data for route 2, suggests a possible savings of 35,000 minutes of nursing time which equates to a potential saving of £21,000 in ED nursing cost (Table 3). This is based on 70 minutes of reduced nursing time. This is considering the standard care hs-cTn test average turn-around-time of 75 minutes for each test at lab where as it is 20 minutes for each POC test. This leads to cost savings of £42.00 per patient.
|
Route 2 2 tests 1 hr apart |
Total Nursing time spent (in minutes) |
Total Nursing time spent (in minutes per patient) |
Total nursing cost |
Total hs-cTn Test cost |
Total arm Cost |
Total arm cost (per patient) |
|
A - Standard care (for 500 patients) |
105,000 |
210 |
£63,000.00 |
£22,110.00 |
£85,110.00 |
£170.22 |
|
B - POCT (for 500 patients) |
70,000 |
140 |
£42,000.00 |
£22,110.00 |
£64,110.00 |
£128.22 |
|
Total Savings (A – B) |
35,000 |
70 |
£21,000.00 |
£0 |
£21,000.00 |
£42.00 |
Table 3: Base case result for route 2 with 2 hs-cTn tests used in the ED setting.
The modelled data in route 3, suggests a possible savings of 27,000 minutes of nursing time that equates to a potential saving of £16,500 in ED nursing cost (Table 4). This is based on 55 minutes of reduced nursing time. This is considering the standard care hs-cTn test average turn-around-time of 75 minutes for each test at lab where as it is 20 minutes for each POC test. This leads to cost savings of £33.00 per patient.
|
Route 3 – 2 tests separated by 3 hours |
Total Nursing time spent (in minutes) |
Total Nursing time spent (in minutes per patient) |
Total nursing cost |
Total hs-cTn Test cost |
Total arm cost |
Total arm cost (per patient) |
|
A -Standard care (for 500 patients) |
157,000 |
315 |
94,500.00 |
£22,110.00 |
£116,610.00 |
£233.22 |
|
B - POCT (for 500 patients) |
130,000 |
260 |
£78,000.00 |
22,110.00 |
£100,110.00 |
£200.22 |
|
Total Savings (A – B) |
27,000 |
55 |
£16,500.00 |
£0 |
£16,500.00 |
£33.00 |
Table 4: Base case result for Route 3 with 2 hs-cTn tests used in the ED setting.
3.2 Deterministic Sensitivity analysis
One-way sensitivity analysis was performed on total nursing time spent on patient monitoring (Table 5) and on the cost of the hs-cTn biomarker for POCT (Table 6) in the routes 1, 2 and 3. The base case for the nursing time was considered at 80% of a full time nurse. Sensitivity analysis was performed at an interval of 10%, down to 70% and up to 90% for the total nursing time spent on the patients monitoring. Please see Table 5 below.
|
Percentage of nursing time spent |
Per patient savings route 1 |
Per patient savings route 2 |
Per patient savings route 3 |
|
70% |
£28.88 |
£36.75 |
£28.88 |
|
80% |
£33.00 |
£42.00 |
£33.00 |
|
90% |
£37.13 |
£47.25 |
£37.13 |
Table 5: Sensitivity analysis result for Route 1,2 & 3 on the nursing time in the ED setting.
Result of sensitivity analysis indicate that savings per patient goes down to £28.88 when only 70% of nursing time is spent and it increases to £37.13 when 90% of nursing time is spent on the patient compared to the based case of 80% of nursing time.
The base case for the hs-cTnl POC test cost was considered at £22.11. Sensitivity analysis was performed at an interval of ~£10, down to £10 and up to £30. Please see Table 6 below.
|
hs-cTnl POC Test cost |
Per patient savings route 1 |
Per patient savings route 2 |
Per patient savings route 3 |
|
£10.00 |
£45.11 |
£66.22 |
£57.22 |
|
£22.11 |
£33.00 |
£42.00 |
£33.00 |
|
£30.00 |
£25.11 |
£26.22 |
£17.22 |
Table 6: Sensitivity analysis result for Route 1,2 & 3 on cost for POC in the ED setting.
Result of sensitivity analysis indicate that savings per patient goes up to £57.22 when the cost of hs-cTnl POC Test goes down to £10 and it decreases to £17.22 when the cost of hs-cTnl POC Test goes up to £30 compared to the based case cost of £22.11.
The differences in nursing time and POCT cost has direct and measurable service impacts in both:
-Overall treatment cost and per patient nursing cost at ED
The savings per patient go as low as £28.88 and as high as £37.13 depending on the nursing time spent. Furthermore, the savings per patient go as low as £17.22 and as high as £57.22 when the cost of the hs-cTn biomarker is also varied in the analysis to the upper and the lower bound.
4. Discussion
This early economic evaluation study assesses the impact and potential benefits of introducing a novel hs-cTn test, at POC for patients presenting at the ED with chest pain and symptoms suggestive of an AMI. Investigation of patients with possible AMI is a common clinical scenario associated with significant health care resource burden. Strategies that reduce the time taken in reaching a diagnosis in chest pain can improve the use of resources and accelerate earlier discharge from the ED.
The cost analysis for the laboratory test (SOC) compared with a POC test for high sensitivity cardiac troponin for ruling out AMI at ED suggests that use of a POC test may potentially result in cost savings. If a hs-cTn POC is used to diagnose patients in routes 1 to 3 of care pathway, it may potentially save £33 in Route 1, £42 in Route 2 and £33 in Route 3 per patient. These time savings will also help in easing the pressure at ED as diagnosis is made between 55 to 70 minutes earlier across the 3 pathway routes, enabling early ruling out of AMI patients leading to earlier discharge and onward referral out of the ED for their required management. This will help hospitals avoid breaching the maximum 4-hour waiting time target for patients in the ED. Where the POC test has a ‘rule-in’ diagnosis for patients, the earlier timing should speed up the treatment pathway, potentially delivering improved prognosis. There are a number of caveats to the study. Currently, this hs-cTn POC test has not been used in a trial to assess patient outcome and there is no independent confirmation of the price (£22.11 ) of the POC test that we gained from one developer. We are assuming similar POC device accuracy to the lab test, although this does seem reasonable [11]. Also, the patient outcomes from the intervention and comparator tests were assumed to be the same for the purposes of this analysis.
As pointed out throughout the study, the potential of the hs-cTn test as the key diagnostic decision-making test in the three routes through the chest pain pathway includes the assumption that there will not be a requirement to wait for other blood test data before reaching a diagnosis. Studies show that the real-life impact of a POC test in the ED can vary greatly. Rapid turnaround time for test results are most beneficial in cases in which delays in test results are the primary determining factor holding up patient management decisions [11]. It is possible that the impact of POC hs-cTn testing will be limited, particularly in routes 1 and 2 by the need to wait for the results of other tests required for a safe diagnosis unless they are available on an equally swift turnaround time or conducted at POC. In the context of the 4-hour wait target in the ED [12] the move to POC for hs-cTn testing might be constructive in reaching the target for route 1 patients. However, this potential gain might be lost waiting for the laboratory to report on other blood tests. It is possible therefore that the benefits of the hs-cTn POC testing for cardiac biomarkers may only be realised if other changes are implemented alongside the introduction of POC testing to improve patient flow through early diagnosis. A POC hs-cTn test may also be helpful with help with early diagnosis of AMI at settings without access to a central laboratory like General Practices, smaller hospitals and ambulances. Further studies, such as service evaluations or pilot studies will be needed for validating any developed device and for data collection on its clinical performance in a health care setting. such a study would also need to include a formal economic evaluation using real-world data.
Such studies could also explore the potential impact of a POC test to improve patient care by facilitating faster diagnosis and implementation of evidence-based therapies or interventions for those patients who had experienced AMI [13], and the impact of a POC test on the 4-hour waiting time target for ED patients [14].
5. Conclusions
The main objective of this early economic evaluation is to determine the potential cost analysis of using a hypothetical hs-cTn POC biomarker test in current care ED pathways as compared with central laboratory testing (SOC). The preliminary cost data indicate that adopting a POC biomarker test for hs-cTn could save costs in ED when compared to the current standard of care. The early exploratory evaluation seems to suggest that introducing a POC test for hs-cTn results in quicker turnaround time (55 to 70 minutes per patient) and a potential saving of £33 - £42 per patient for all the three routes at the 75 minutes of result turnaround time where as it is 20 minutes for the POC test. The sensitivity analysis results indicate that savings per patient go as low as £28.88 and as high as £37.13 depending on the nursing time varied to 70% and 90% respectively. Furthermore, the savings per patient go as low as £17.22 and as high as £57.22 when the cost of the hs-cTn biomarker is also varied in the analysis to ~£10 and £30.
There are a number of caveats to the study. Currently, this hs-cTn POC test has not been used in a trial to assess patient outcome and there is no independent confirmation of the price (£22.11 ) of the POC test that we gained from one developer. We are assuming similar POC device accuracy to the lab test, although this does seem reasonable11 and therefore that patient outcomes from use of the intervention and comparator tests are assumed to be the same for the purposes of this analysis. However, there are a number of important assumptions made in the modelling, notably on the sensitivity and specificity of any POC test and the price. A clinical study would be needed to assess POC test accuracy, as compared to the current lab test. A formal economic evaluation would also be needed using the POC test. This could be incooporated into the study assessing the comparative efficacy of the POC test and the lab test.
References
- Melody N, Simone B, Peter B, et al. The Lean Assessment Process (LAP) - experiences of NIHR London IVD Cooperative working with early stage medical technologies. The International Federation for Medical and Biological Engineering (2017).
- Barstow C, Rice M, McDivitt JD. Acute Coronary Syndrome: Diagnostic Evaluation. Am Fam Physician 95 (2017): 170-177.
- NHS Accelerated Access Collaborative. 2018/19 Innovation and Technology Payment (ITP) Programme.
- Ijzerman MJ, Koffijberg H, Fenwick E, et al. Emerging use of early health technology assessment in medical product development. PharmacoEconomics 35 (2017): 727-740.
- Myocardial infarction (acute): Early rule out using high-sensitivity troponin tests (Elecsys Troponin T high-sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 assays) Diagnostics guidance DG15 (2014).
- Pickering JW, Than MP, Cullen L, et al. Rapid rule-out of acute myocardial infarction with a single high-sensitivity cardiac troponin T measurement below the limit of detection: a collaborative meta-analysis. Ann Intern Med 166 (2017): 715-724.
- Boeddinghaus J, Nestelberger T, Koechlin L, et al. Early Diagnosis of Myocardial Infarction With Point-of-Care High-Sensitivity Cardiac Troponin I. J Am Coll Cardiol 75 (2020): 1111-1124. Erratum in: J Am Coll Cardiol 75 (2020): 3001.
- Harjola VP, Parissis J, Brunner-La Rocca HP, et al. Comprehensive in-hospital monitoring in acute heart failure: applications for clinical practice and future directions for research. A statement from the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 20 (2018): 1081-1099.
- Campbell and Cochrane Economics Methods Group (CCEMG) and the Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre).
- Unit costs of health and social care (2018).
- Chapman AR, Lee KK, McAllister DA, et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndromes. Published correction appears in JAMA 319 (2018): 1168.
- Department of Health: The NHS Plan.
- Hugo Katus, André Ziegler, Okan Ekinci, et al. Early diagnosis of acute coronary syndrome, European Heart Journal 38 (2017): 3049-3055.
- Carlton EW, Ingram J, Taylor H, et al. Limit of detection of troponin discharge strategy versus usual care: randomised controlled trial. Heart 106 (2020): 1586-1594.