Resistance Profiles of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli in various Clinical Specimens: A One-Year Clinical Surveillance at a Tertiary Care Hospital in Bangladesh
Article Information
Mst. Junnatul Ferdus*,1, Sanjida Khondakar Setu2, Abu Naser Ibne Sattar3, Samia Afreen Khan1, Sharmin Shanjana1, Sazzad Bin Shahid2
1Assistant professor, Z.H. Sikder women’s Medical college and Hospital, Dhaka, Bangladesh
2Associated Professor, Bangladesh Medical University, Bangladesh
3Professor and Chairman, Bangladesh Medical University, Bangladesh
4Professor and Head of the Department, Dhaka medical college and Hospital, Bangladesh
*Corresponding author: Mst. Junnatul Ferdus, Assistant professor, Z.H. Sikder women’s Medical college and Hospital, Dhaka, Bangladesh.
Received: 02 August 2025; Accepted: 11 August 2025; Published: 18 August 2025
Citation: Mst. Junnatul Ferdus, Sanjida Khondokar Setu, Abu Naser Ibne Satter, Samia Afreen Khan, Sharmin Shanjana, Sazzad Bin Shahid. Resistance Profiles of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli in various Clinical Specimens: A One-Year Clinical Surveillance at a Tertiary care Hospital in Bangladesh. Fortune Journal of Health Sciences. 8 (2025): 782-790.
View / Download Pdf Share at FacebookAbstract
Background: The global surge of carbapenem-resistant Gram-negative bacteria—particularly Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli—poses a major public health threat, especially in low- and middle-income countries like Bangladesh, where treatment options are limited.
Objective: This study aimed to assess the in vitro antimicrobial susceptibility of carbapenem-resistant K. pneumoniae, P. aeruginosa, and E. coli isolated from clinical specimens in a tertiary care hospital in Bangladesh, to inform antibiotic selection and infection control strategies.
Materials and Methods: Seventy-five non-duplicate carbapenem-resistant Gram-negative isolates were collected from urine, blood, wound swabs, sputum, tracheal aspirates, bronchoalveolar lavage, pus, throat swabs, tissue, and endotracheal tubes. Identification was performed using colony morphology on MacConkey and chromogenic agar, followed by standard biochemical tests. Antimicrobial susceptibility was determined using the Kirby-Bauer disk diffusion method in accordance with NCCLS guidelines.
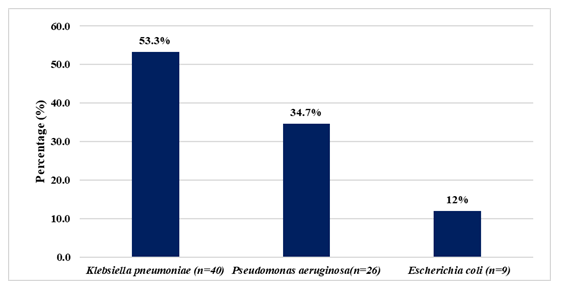
Results: K. pneumoniae was the most prevalent (53.3%), followed by P. aeruginosa (34.7%) and E. coli (12%). Urine (18.66%), tracheal aspirates (16%), wound swabs (13.33%), and blood (13.33%) were the most common sources. Notably, all isolates showed 100% resistance to amoxicillin, cefuroxime, ciprofloxacin, and amikacin.
Conclusion: The high prevalence of multidrug-resistant K. pneumoniae, P. aeruginosa, and E. coli reflects a critical healthcare challenge in Bangladesh. These findings underscore the urgent need for robust antimicrobial stewardship, improved infection control practices, and nationwide surveillance to combat antimicrobial resistance.
Keywords
Antimicrobial Resistance; Carbapenem-resistant Enterobacterales; Klebsiella pneumoniae; Pseudomonas aeruginosa; Escherichia coli; Bangladesh
Antimicrobial Resistance articles; Carbapenem-resistant Enterobacterales articles; Klebsiella pneumoniae articles; Pseudomonas aeruginosa articles; Escherichia coli articles; Bangladesh articles.
Article Details
Introduction
Bacterial antimicrobial resistance (AMR), defined as the ability of bacteria to withstand the effects of antibiotics that were once effective against them, has become a major global public health challenge in the 21st century [1]. It has emerged as the leading cause of deaths from infectious diseases worldwide, with the highest burden observed in low- and middle-income countries (LMICs) [2]. In low- and middle-income countries (LMICs), the rising prevalence of antimicrobial resistance (AMR) is driven by a combination of structural and systemic challenges. These include strained healthcare infrastructure, limited availability of diagnostic tools, high population density, poor access to clean water and adequate sanitation, and insufficient regulation of antibiotic distribution and usage [3]. Accurate information on the scale of the bacterial antimicrobial resistance (AMR) burden, regional trends, and the predominant pathogen–drug combinations is critical for guiding effective interventions. Without urgent action, the ongoing spread of AMR could substantially increase the lethality of many bacterial infections in the future [2]. Extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales, carbapenem-resistant Enterobacterales (CRE), and methicillin-resistant Staphylococcus aureus (MRSA) are among the most clinically significant antibiotic-resistant pathogens, posing serious challenges to treatment and infection control [4-7]. Due to the scarcity of effective therapeutic options for infections caused by these organisms, both the U.S. Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) have designated them as high-priority pathogens [8,9].
A major contributing factor to the current antimicrobial resistance crisis is the excessive and inappropriate use of antibiotics across multiple sectors, particularly in clinical settings, as well as in agriculture, animal husbandry, and the food production system [10]. Antimicrobial resistance (AMR), often described as the 'Silent Pandemic,' represents an urgent global health threat that requires immediate and effective action. It must be addressed as a present and escalating crisis, rather than being viewed as a challenge of the future [11]. In the absence of effective prevention and control strategies, antimicrobial resistance (AMR) is projected to become the leading cause of mortality worldwide by 2050 [12]. Global estimates indicate that antimicrobial resistance (AMR) was directly responsible for over 1.2 million deaths in 2019. If effective measures are not implemented, this number is projected to rise to around 10 million deaths annually by 2050 [12].
In response to the growing threat of antimicrobial resistance (AMR), various global health organizations and national governments have initiated efforts to address the issue. A key strategy is the 'One Health' approach, which emphasizes the need for coordinated, cross-sectoral collaboration at the global level. This approach engages multiple disciplines, including human health, veterinary medicine, agriculture, and environmental sciences. Organizations such as the Food and Agriculture Organization of the United Nations (FAO) and the World Organization for Animal Health (WOAH, formerly OIE) play critical roles by contributing their sector-specific expertise while working jointly to mitigate the impact of AMR [13]. Additionally, the World Health Organization (WHO) developed the Global Action Plan on Antimicrobial Resistance (GAP-AMR) as a strategic framework to address AMR. To support its implementation, WHO also launched the Global Antimicrobial Resistance and Use Surveillance System (GLASS), aimed at strengthening data collection and addressing knowledge gaps critical to achieving the objectives of the GAP-AMR. [14]. Among the various recently introduced strategies, enhancing public awareness of the antimicrobial resistance (AMR) pandemic stands out as a particularly effective preventative measure, necessitating clear and consistent communication with all relevant stakeholders [15].
The development of antimicrobial agents represents one of the most significant advancements in medical treatment. The advent of these drugs has played a crucial role in controlling infectious diseases and substantially lowering mortality rates, which were once the leading cause of death worldwide [16]. Since the introduction of the first antibiotic in 1910, average human life expectancy has increased by approximately 23 years [17]. Since then, more than 150 new antibiotics have been developed; however, their extensive and often inappropriate use has contributed to the emergence of increased resistance, including multidrug-resistant strains commonly referred to as 'superbugs.' This rise in resistance has led to higher mortality rates, primarily due to reduced clinical effectiveness associated with antibiotic misuse [18]. Antimicrobial resistance (AMR) refers to the ability of microorganisms—including viruses, parasites, fungi, and bacteria—to withstand antimicrobial treatments. Among these, bacterial antibiotic resistance poses a particularly serious challenge because bacteria can rapidly develop resistance to newly introduced antibiotics used to treat infections) [19]. An effective surveillance is critical for monitoring the prevalence of antimicrobial-resistant pathogens and for assessing the impact of interventions aimed at controlling their dissemination. Several studies have utilized surveillance data to quantify the burden of antimicrobial resistance (AMR) at national and international levels. As an illustration, the U.S. Centers for Disease Control and Prevention (CDC) released a 2019 report estimating infections and deaths associated with 18 AMR threats in the United States [20]. Similarly, Cassini et al. assessed the impact of eight bacterial pathogens and 16 pathogens–antibiotic combinations across the European Union (EU) and European Economic Area between 2007 and 2015 [21]. In Thailand, the burden of multidrug-resistant infections caused by six bacterial species in 2010 [22]. Additionally, an estimated global incidence rates of Escherichia coli and Klebsiella pneumoniae resistant to third-generation cephalosporins and carbapenems across 193 countries in 2014 [23].However, surveillance systems that depend primarily on clinically diagnosed infections may not accurately reflect the true burden of antimicrobial resistance (AMR), as they can be significantly influenced by factors such as healthcare-seeking behaviors and the accessibility and affordability of diagnostic services [24].
This study aimed to determine the prevalence of resistance among common Enterobacterales and non-fermenting Gram-negative bacteria from various clinical isolates from both hospitalized patients and community-dwelling adults in Bangladesh.
Materials and methods
This cross-sectional observational study was conducted over a one-year period, from March 2024 to February 2025, at a large academic medical center in Dhaka, Bangladesh. The study was approved by the Institutional Review Board of Bangabandhu Sheikh Mujib Medical University (BSMMU) (IRB Registration number: 5069). The primary objective was to assess the prevalence and characteristics of infections caused by carbapenem-resistant Gram-negative organisms (CR-GNOs) among both hospitalized patients and individuals residing in the community. Patients of all ages who were diagnosed with infections caused by CR-GNOs were included in the study. Clinical specimens were collected from 75 patients meeting the inclusion criteria. These specimens included various sample types such as urine, blood, sputum, wound swabs, and other relevant clinical materials, as requested by treating physicians. All samples were processed in the clinical microbiology laboratory of the medical center. Initial culture of specimens was performed by inoculating them onto MacConkey agar and chromogenic agar media to promote the growth of Gram-negative organisms and facilitate preliminary differentiation. Following incubation, bacterial colonies suggestive of Gram-negative pathogens were subjected to further analysis. Species identification and antimicrobial susceptibility testing were carried out using the Kirby-Bauer disc diffusion method, in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines [25]. Carbapenem resistance was determined based on zone diameters for carbapenem-class antibiotics, including imipenem and meropenem. Isolates that demonstrated reduced susceptibility or resistance were categorized as carbapenem-resistant according to CLSI [25]. Quality control strains (Escherichia coli ATTCC- 25922) were used during susceptibility testing to ensure accuracy and reliability of results. Data from both hospital and community-acquired infections were analyzed to understand the distribution and resistance patterns of CR-GNOs in different clinical contexts. Carbapenem sensitive isolates and grossly contaminated sample were excluded from the study.
Results
The incidence of carbapenem-resistant Enterobacterales (CRE) among clinical isolates obtained from patients in tertiary care hospitals was assessed over a one-year period. A total of 195 clinical specimens were initially deemed eligible for screening. Following the application of exclusion criteria, 75 samples were confirmed to harbor carbapenem-resistant Gram-negative bacteria (CR-GNB) and were included in the final analysis. Among the 75 CR-GNB isolates, Klebsiella pneumoniae were the most frequently identified, accounting for 40 cases (53.33%). This was followed by Pseudomonas aeruginosa with 26 isolates (34.66%), and Escherichia coli with 9 isolates (12%). These findings are illustrated in Figure 1. The predominance of Klebsiella pneumoniae highlights its significant role in carbapenem resistance within both hospital and community-acquired infections. The high frequency of Pseudomonas aeruginosa also reflects its intrinsic and acquired resistance mechanisms, contributing to therapeutic challenges. The relatively lower incidence of resistant Escherichia coli may suggest variation in resistance patterns based on local antimicrobial use and infection control practices.
The majority of carbapenem-resistant Gram-negative bacteria (CR-GNB) were isolated from urine samples, accounting for 14 cases (18.66%), followed by tracheal aspirates with 12 isolates (16%). Wound swabs and blood each contributed 10 (13.33%), while sputum samples yielded 9 (12%). Bronchoalveolar lavage and pus samples each accounted for 6 (8%), followed by throat swabs with 5 (6.66%), tissue samples with 2 (2.66%), and a single isolate from an endotracheal tube (1.33%).
Klebsiella pneumoniae, the most commonly isolated CR-GNB, were predominantly recovered from blood (10 isolates, 25%), sputum (9, 22.5%), and tracheal aspirates (8, 20%). Other sources included wound swabs (5, 12.5%), urine (4, 10%), throat swabs (3, 7.5%), and pus (1, 2.5%). Among the nine carbapenem-resistant Escherichia coli isolates, the majority (7, 77.77%) were recovered from urine samples, with one isolate each from wound swab and pus (11.11%). Of the 26 Pseudomonas aeruginosa. isolates, bronchoalveolar lavage was the most common source (6, 23.07%), followed by tracheal aspirate, pus, and wound swabs with 4 isolates each (15.38%). Additionally, two isolates each (7.69%) were obtained from throat swabs and tissue, and one isolate (3.84%) from an endotracheal tube. Detailed distribution is shown in Table 1.
Table 1: Distribution of CR-GNB in different clinical isolates (n = 75).
|
Clinical specimen |
Carbapenem resistance gram negative bacteria |
|||
|
Klebsiella pneumoniae n (%) |
Escherichia coli n (%) |
Pseudomonas aeruginosa n (%) |
Total n (%) |
|
|
Urine |
4 (10) |
7 (77.77) |
3 (11.53) |
14 (18.66) |
|
Wound swab |
5 (12.5) |
1 (11.11) |
4 (15.38) |
10 (13.33) |
|
Broncho alveolar lavage |
0 (0.0) |
0 (0.0) |
6(23.07) |
6 (8) |
|
Tracheal aspirate |
8 (20) |
0 (0.0) |
4 (15.38) |
12 (16) |
|
Throat swab |
3 (7.5) |
0 (0.0) |
2 (7.69) |
5 (6.66) |
|
Sputum |
9 (22.5) |
0 (0.0) |
0 (0.0) |
9 (12) |
|
Blood |
10 (25) |
0 (0.0) |
0 (0.0) |
10 (13.33) |
|
Tissue |
0 (0.0) |
0 (0.00 |
2 (7.69) |
2 (2.66) |
|
ET tube |
0 (0.0) |
0 (0.0) |
1 (3.84) |
1 (1.33) |
|
Pus |
1 (2.5) |
1 (11.11) |
4 (15.38) |
6 (8) |
|
Total |
40 (53.33) |
9 (12) |
26 (34.66) |
75 (100) |
*CR-GNB: Carbapenem resistant gram-negative bacteria.
Antimicrobial susceptibility testing of carbapenem-resistant Gram-negative bacterial isolates was performed using the Kirby-Bauer disc diffusion method in accordance with CLSI guidelines. Table 2 summarizes the resistance profiles observed among different bacterial genera. Among the 49 carbapenem-resistant Enterobacterales isolates, the highest levels of resistance were recorded against amoxicillin, cefuroxime, and ciprofloxacin, with all isolates (100%) exhibiting complete resistance to these agents. Resistance was also notably high against gentamicin (97.5%), ceftriaxone (95%), amikacin (90%), and cefotaxime (88.88%). In contrast, netilmicin showed the lowest resistance rate among this group, with only 22.22% of isolates being resistant. Specifically, all isolates of Klebsiella pneumoniae (n = 40, 100%) demonstrated complete resistance to ciprofloxacin, highlighting a concerning trend of resistance to fluoroquinolones. Similarly, all Escherichia coli isolates (n = 9, 100%) were found to be resistant to both amoxicillin and cefuroxime. In the group of carbapenem-resistant non-fermenters, Pseudomonas aeruginosa (n = 26) showed a distinct resistance pattern. All isolates (100%) were resistant to amikacin, suggesting limited effectiveness of aminoglycosides against these organisms. Additionally, a high proportion of isolates (n = 22, 84.62%) were resistant to ciprofloxacin and ceftazidime. Resistance to Cefepime was observed in 18 isolates (69.23%), while netilmicin resistance was comparatively lower, found in 14 isolates (44.66%). These findings underscore the significant therapeutic challenges posed by CR-GNB, especially given the high resistance rates to commonly used antibiotics. The data emphasize the urgent need for robust antimicrobial stewardship programs and the development of novel therapeutic options to manage infections caused by these multidrug-resistant pathogens.
Table 2: Antimicrobial resistant pattern of CR-GNB by disc diffusion method (n=75).
|
Antimicrobial agents |
Klebsiella pneumoniae n (%) |
Escherichia coli n (%) |
Pseudomonas aeruginosa n (%) |
Total n (%) |
|
Amoxicillin |
34 (85%) |
9 (100%) |
NA |
43(87.76%) |
|
Cotrimoxazole |
39 (97.5%) |
8 (88.88%) |
NA |
47(95.92%) |
|
Ciprofloxacin |
40 (100%) |
7 (77.77%) |
22 (84.62%) |
69 (92%) |
|
Ceftriaxone |
38 (95%) |
8 (88.88%) |
NA |
46 (93.88%) |
|
Gentamicin |
39 (97.5%) |
6 (66.66%) |
NA |
45 (91.84%) |
|
Amoxicillin-clavulanic acid |
32 (80%) |
4 (44.44%) |
NA |
36 (73.47%) |
|
Cefotaxime |
31 (77.5%) |
8 (88.88%) |
NA |
39 (79.59%) |
|
Ceftazidime |
32 (80%) |
7 (77.77%) |
22 (84.62%) |
41 (54.67%) |
|
Cefuroxime |
34 (85%) |
9 (100%) |
NA |
43 (87.75%) |
|
Cefepime |
15 (37.5%) |
4 (44.44%) |
18 (69.23%) |
37 (49.33%) |
|
Amikacin |
36 (90%) |
5 (55.55%) |
26 (100%) |
65 (86.67%) |
|
Aztreonam |
15 (37.5%) |
5 (55.55%) |
NA |
20 (40.82%) |
|
Netilmicin |
8 (20%) |
2 (22.22%) |
14 (44.66%) |
24 (32%) |
Discussion
In 2019, antimicrobial resistance (AMR) was associated with a substantial global health burden. An estimated 4.95 million deaths (95% uncertainty interval [UI]: 3.62–6.57 million) were linked to drug-resistant infections, including 1.27 million deaths (95% UI: 0.911–1.71 million) that were directly attributable to resistance across 88 pathogens–antibiotic combinations. These findings highlight AMR as a major contributor to global mortality, underscoring its significance as a critical public health concern on par with other leading infectious disease threats. [26]. By all available measures, bacterial antimicrobial resistance (AMR) represents one of the foremost global health challenges of the 21st century [27]. All six of the predominant bacterial pathogens contributing to the global burden of antimicrobial resistance (AMR) in 2019—Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa—have been classified as priority pathogens by the World Health Organization (WHO) due to their significant threat to public health and limited treatment options [28]. Although Klebsiella pneumoniae has been identified as an important pathogen causing a series of infections, including sepsis, pneumonia and urinary tract infections for many years; high healthcare costs, treatment failures, and high mortality rates in these infections have been increasingly reported in recent years, especially due to increasing carbapenem resistance [29].
Our study assessed the prevalence of antimicrobial resistance (AMR) among carbapenem-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa isolated from various clinical specimens collected in an urban setting in Bangladesh. Among all sample types, urine was the most commonly submitted, indicating a high burden of urinary tract infections associated with resistant pathogens. Klebsiella pneumoniae was the most frequently isolated organism, underscoring its critical role in the AMR landscape of Bangladesh. Notably, multidrug resistance (MDR) was more prevalent among non-fermenting Gram-negative bacilli, such as Pseudomonas aeruginosa, compared to members of the Enterobacterales order.
Our findings indicate that the distribution of carbapenem-resistant Gram-negative bacteria (CR-GNB) among 75 isolates. Of these, Klebsiella pneumoniae were the most frequently isolated pathogens, accounting for 53.33%, followed by Pseudomonas aeruginosa 34.66% and Escherichia coli 12% (Figure-1). Similar trends have been reported in earlier studies from Bangladesh, where Klebsiella pneumoniae emerged as the most frequently isolated CR-GNB [30]. This distribution mirrors data from other studies, where Klebsiella pneumoniae and Escherichia coli have consistently been the dominant carbapenem-resistant Enterobacterales, comprising 60.4% and 17.6% of isolates, respectively [31] further confirmed this trend, identifying Klebsiella pneumoniae as the most frequently isolated CR-GNB [32,33]. The increasing prevalence of Klebsiella pneumoniae in clinical settings highlights its growing role in the global AMR crisis.
Carbapenem-resistant Gram-negative bacteria (CR-GNB) were most frequently isolated from urine samples, followed by tracheal aspirates, wound swabs, blood, and sputum. Multiple studies conducted in India have demonstrated that urine is the most common source of carbapenem-resistant Enterobacterales (CRE), with lower prevalence noted in respiratory specimens and bloodstream infections [34,35]. Among Klebsiella pneumoniae isolates, the majority were recovered from blood samples, followed by sputum, tracheal aspirates, wound swabs, and urine, indicating their diverse clinical distribution. Study in New Delhi identified urine as the most frequently submitted clinical specimen, accounting for 29.33% of all samples. However, the highest burden of carbapenem-resistant Enterobacteriaceae (CRE) was observed in blood specimens, highlighting a significant concern for bloodstream infections caused by multidrug-resistant organisms [36]. Seven out of nine isolates of Escherichia coli were cultured from urine (Table-1) samples, suggesting a predominant association with urinary tract infections. Among culture-confirmed cases of urinary tract infections (UTIs), Escherichia coli was the most frequently isolated pathogen, accounting for 56% (1994 out of 3558 cases) [37]. Current study revealed, the majority of Pseudomonas aeruginosa isolates were obtained from bronchoalveolar lavage (BAL) fluid, indicating a strong correlation with lower respiratory tract infections. This distribution pattern underscores the organism's significant role in pulmonary infection. Pseudomonas aeruginosa was most frequently isolated from respiratory specimens (25.8%), followed closely by urine (22.5%) and wound swabs (22.5%). Study highlights the organism’s prominent role in lower respiratory tract infections [31], However, dissimilarity also documented in which Pseudomonas aeruginosa was commonly from urine samples (37.2%), followed by sputum (30.2%), pus (25.6%), and blood (4.66%) [38]. Such inconsistencies may be explained by differences in patient demographics, clinical settings, specimen collection methods, and local antimicrobial prescribing practices. For instance, a study conducted in a tertiary care hospital in Nepal found that Pseudomonas aeruginosa was most commonly isolated from urine specimens, followed by pus and sputum samples. This distribution pattern likely reflects differences in institutional workflows, patient case mix, and infection control practices specific to that healthcare setting [39].
The antimicrobial resistance profiles of carbapenem-resistant Gram-negative bacilli (CR-GNB), assessed via the Kirby–Bauer disc diffusion method, revealed critically high resistance rates among Enterobacteriaceae. Among 49 CRE isolates, there was complete resistance (100%) to amoxicillin, cefuroxime, and ciprofloxacin. Substantial resistance was also seen against gentamicin (97.5%), ceftriaxone (95%), amikacin (90%), and cefotaxime (88.9%), while netilmicin exhibited the lowest resistance rate at 22.2%. In particular, all Klebsiella pneumoniae isolates were resistant to ciprofloxacin. These findings align with recent regional studies reporting nearly universal resistance among carbapenem-resistant Klebsiella pneumoniae to β-lactam and β-lactam/β-lactamase inhibitor combinations, including amoxicillin-clavulanic acid (>96%) and cephalosporins (93–98%) [40,41]. Aminoglycoside resistance, while also high in carbapenem resistance Klebsiella pneumoniae (CRKP), typically remains lower for gentamicin relative to ciprofloxacin, although rates exceed 85% in many reports [42]. All Escherichia coli isolates in the current study demonstrated complete resistance (100%) to both amoxicillin and cefuroxime, consistent with findings from a tertiary-level hospital in Dhaka, where Escherichia coli isolates from urinary tract infection patients exhibited absolute resistance to these agents (Amoxicillin 100%, Cefuroxime 100%) [43]. This pervasive resistance profile underscores the prevalence of β-lactamase–mediated structural inactivation of these antibiotics, and aligns with regional antimicrobial resistance trends where extended-spectrum β-lactamase (ESBL)–producing Escherichia coli strains are dominant and confer high-level resistance to penicillins and early-generation cephalosporins [44]. Among carbapenem-resistant non-fermenters, all Pseudomonas aeruginosa. isolates exhibited resistance to amikacin. Additionally, 22 out of 26 isolates (≈85%) were resistant to both ciprofloxacin and ceftazidime, indicating extensive multi-drug resistance among these critical pathogens. This finding is consistent with surveillance data from Bangladesh, where environmental Pseudomonas aeruginosa showed 92% resistance to amikacin and 62% to ciprofloxacin [45], while clinical isolates demonstrated high resistance to ceftazidime (68%) and ciprofloxacin (74%) [46]. A broader review spanning 2006–2024 reported resistance rates exceeding 60% for fluoroquinolones and cephalosporins, with aminoglycoside resistance often surpassing 50% [47]. Another studies found ceftazidime and ciprofloxacin resistance rates of 100% and 83%, respectively [48]. All it highlights a significant challenge in treating Pseudomonas-related infections and support the need for robust antimicrobial stewardship, local resistance monitoring, and exploration of alternative agents like polymyxins or novel β-lactamase inhibitors to guide effective treatment strategies [49]. These trends underscore the growing threat of multidrug-resistant organisms (MDROs) in clinical settings, emphasizing the urgent need for effective antimicrobial stewardship and stringent infection control practices to curb resistance spread and ensure therapeutic efficacy [50,51].
Conclusion
The rise of carbapenem-resistant Gram-negative bacilli (CR-GNB), especially Enterobacteriaceae and Pseudomonas aeruginosa, poses a serious clinical threat due to their multidrug resistance. This study revealed high resistance to key antibiotics, including amikacin, ciprofloxacin, and ceftazidime, with Pseudomonas aeruginosa showing complete amikacin resistance. These findings align with global antimicrobial resistance trends, driven by antibiotic misuse and poor infection control. Immediate implementation of antimicrobial stewardship, infection prevention, and local antibiogram-guided therapy is crucial. Continuous surveillance and coordinated policy and clinical actions are essential to curb the spread of CR-GNB and preserve the efficacy of existing antibiotics for future clinical use.
AcknowledgmentsThe authors would like to extend their sincere thanks to the Microbiology Department for their invaluable assistance in obtaining data from the hospital’s microbiology laboratory. Their support was crucial to the successful completion of this research.
Financial Support and Sponsorship: Partial Funding for this study was provided by Bangabandhu Sheikh Mujib Medical University (BSMMU)
Conflicts of Interest: The authors declare no conflicts of interest.
References
- O’Neill, J. Tackling drug-resistant infections globally: final report and recommendations. London: Review on Antimicrobial Resistance, 11 (2016): 84-87.
- Murray CJ, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399 (2022): 629–655
- Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control 6 (2017): 1–8
- Álvarez A, Fernández L, Gutiérrez D, et al. Methicillin-resistant staphylococcus aureus in hospitals: latest trends and treatments based on bacteriophages. J Clin Microbiol 57 (2019): 1–8.
- Chotiprasitsakul D, Srichatrapimuk S, Kirdlarp S, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae: a 5-year experience at a tertiary care hospital. Infect Drug Resist 12 (2019): 461–468.
- Goto M, Nair R, Livorsi D, et al. 1164. County-Level Geographic Distribution of Extended-Spectrum Cephalosporin-Resistant Enterobacteriaceae Across Outpatient Settings of the Veterans Health Administration, 2000–2017. Open Forum Infect Dis. 5 (2018): 350–361.
- Rossi F, Girardello R, Cury AP, et al. Emergence of colistin resistance in the largest university hospital complex of Sao Paulo, Brazil, over five years. Braz J Infect Dis 21 (2017): 98–101
- Antibiotic resistance threats in the United States. Centers for Disease Control and Prevention, 9 (2019): 111–113
- Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. (2017): 1–7
- Llor C, Bjerrum L. Antimicrobial Resistance: Risk Associated with Antibiotic Overuse and Initiatives to Reduce the Problem. Ther Advdrug Saf 5 (2014): 6-10
- Founou RC, Blocker AJ, Noubom M, et al. The COVID-19 Pandemic: a Threat to Antimicrobial Resistance Containment. Future Sci 7 (2021): 8-16
- World Health Organization. Antimicrobial Resistance. (2023).
- World Health Organization. Monitoring and Evaluation of the Global Action Plan on Antimicrobial Resistance (2019).
- World Health Organization. Comprehensive Review of the WHO Global Action Plan on Antimicrobial Resistance Volume 1: Report WHO Evaluation Office (2021)
- Mostafa A, Abdelzaher A, Rashed S, et al. Is Health Literacy Associated with Antibiotic Use, Knowledge and Awareness of Antimicrobial Resistance Among Non-medical university Students in Egypt? A Cross-Sectional Study. BMJ Open 11 (2021): 3-15
- Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol. 1 (2010): 134-155
- Hutchings MI, Truman AW, Wilkinson B. Antibiotics: Past, Present and Future. Curr Opin Microbiol 51 (2019): 72–80
- Lobanovska M, Pilla G. Penicillin’s Discovery and Antibiotic Resistance: Lessons for the Future? Yale J Biol Med 90 (2017): 135–145
- Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial Resistance: a Global Multifaceted Phenomenon. Pathog Glob Health 109 (2015): 309–318
- US Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, (2019)
- Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibioticresistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 19 (2019): 56–66
- Lim C, Takahashi E, Hongsuwan M, et al. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. Elife. 5 (2016): 103-113
- Temkin E, Fallach N, Almagor J, et al. Estimating the number of infections caused by antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in 2014: a modelling study. Lancet Glob Health 6 (2018): 969–979
- Smith RM, Lautenbach E, Omulo S, et al. Human colonization with multi-drug-resistant organisms: getting to the bottom of antibiotic resistance. Open Forum Infect Dis 8 (2021): 1–15
- Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute. Twenty-fifth informational supplement, Clinical and Laboratory Standards Institute, Wayne, PA, (2015): M100
- Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 (2020): 1204–1222
- Temkin E, Fallach N, Almagor J, et al. Estimating the number of infections caused by antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in 2014: a modelling study. Lancet Glob Health 6 (2018): 969–979
- Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics (2017).
- Gaibani P, Lombardo D, Bussini L, et al. Epidemiology of Meropenem/Vaborbactam Resistance in KPC-Producing Klebsiella pneumoniaeCausing Bloodstream Infections in Northern Italy, 2018. Antibiotics (Basel). 10 (2021): 536-542
- Ferdous RN, Rahman MA, Hussain MA, et al. Prevalence of imipenem resistant gram-negative bacteria in a tertiary care hospital of Dhaka, Bangladesh. Bangladesh Journal of Medical Science 21 (2022): 145-150.
- Verma G, Singh N, Smriti S, et al. Modified Carbapenem Inactivation Method and Ethylenediaminetetraacetic Acid (EDTA)-Carbapenem Inactivation Method for Detection of Carbapenemase-Producing Enterobacterales and Pseudomonas aeruginosa. Cureus, 16 (2024): 11-16
- Gao B, Li X, Yang F, et al. Molecular epidemiology and risk factors of ventilator-associated pneumonia infection caused by carbapenem-resistant enterobacteriaceae. Frontiers in Pharmacology, 10 (2019): 262- 272
- Shortridge D, Carvalhaes C, Deshpande L, et al. Activity of meropenem/vaborbactam and comparators against Gram-negative isolates from Eastern and Western European patients hospitalized with pneumonia including ventilator-associated pneumonia (2014-19). J Antimicrob Chemother 76 (2021): 15-19
- Verma G, Singh N, Smriti S, et al. Modified Carbapenem Inactivation Method and Ethylenediaminetetraacetic Acid (EDTA)-Carbapenem Inactivation Method for Detection of Carbapenemase-Producing Enterobacterales and Pseudomonas aeruginosa. Cureus 16 (2024): 111-117
- Nayak G, Behera B, Mohanty S, et al. Analysis of in vitro activity of cefiderocol against carbapenem-resistant gram-negative bacilli by broth microdilution and disk diffusion method: a single-center study in Odisha, India. Infection and drug resistance (2022): 58-61
- Jaiswal SR, Gupta S, Kumar RS, et al. Gut Colonization with Carbapenem-resistant Enterobacteriaceae Adversely Impacts the Outcome in Patients with Hematological Malignancies: Results of A Prospective Surveillance Study. Mediterr J Hematol Infect Dis. 10 (2018): 17-21
- Zwane T, Shuping L, Perovic O. Etiology and Antimicrobial Susceptibility of Pathogens Associated with Urinary Tract Infections among Women Attending Antenatal Care in Four South African Tertiary-Level Facilities, 2015-2019. Antibiotics (Basel). 10 (2021): 669-675
- Khater ES and Abdo KM. Detection of carbapenem-resistant Pseudomonas aeruginosa in tertiary care hospital in Saudi Arabia. Microbes and Infectious Diseases 3 (2022): 693-702.
- Bhattarai S, Baral R, Shrestha R, et al. Isolation of Pseudomonas aeruginosa and its susceptibility pattern from various clinical samples in a tertiary care hospital. J Nepal Health Res Counc 19 (2021): 433–438
- Wang N, Zhan M, Wang T, et al. Long-Term Characteristics of Clinical Distribution and Resistance Trends of Carbapenem-Resistant and Extended-Spectrum β-Lactamase Klebsiella pneumoniae Infections: 2014-2022. Infect Drug Resist 16 (2023): 1279-1295
- Armin S, Fallah F, Karimi A, et al. Antibiotic Susceptibility Patterns for Carbapenem-Resistant Enterobacteriaceae. Int J Microbiol. (2023): 101-107
- Wang N, Zhan M, Wang T, et al. Long-Term Characteristics of Clinical Distribution and Resistance Trends of Carbapenem-Resistant and Extended-Spectrum β-Lactamase Klebsiella pneumoniae Infections: 2014-2022. Infect Drug Resist 16 (2023): 1279-1295
- Islam MR, Hoque MJ, Uddin MN, et al. Antimicrobial Resistance of E Coli Causing Urinary Tract Infection in Bangladesh. Mymensingh Med J 31 (2022): 180-185
- Mazumder R, Hussain A, Abdullah A, etal. International high-risk clones among extended-spectrum β-lactamase–producing Escherichia coli in Dhaka, Bangladesh. Front Microbiol 12 (2021): 13-19
- Sheikh Tayef, Rushafi Sikder, Munsi Md Shahiuzzaman et al. Antibiotic resistance in Pseudomonas aeruginosa: A systematic review of prevalence, patterns, and public health implications in Bangladesh (2006–2024).
- Akhtar S, Hoque ME, Kalam AK, et al. Genotypic detection of carbapenemase-producing Pseudomonas species and Acinetobacter species by multiplex PCR at a tertiary care hospital in South-East region of Bangladesh. Bangladesh J Med Microbiol 19 (2025):18–25
- Tay EF, et al. Distribution and antibiotic resistance patterns of Pseudomonas aeruginosa across pollution sources in the Buriganga River, Bangladesh. J Water Health. (2024)
- Saha P, Kabir RB, Ahsan CR, et al. Multidrug resistance of Pseudomonas aeruginosa: do virulence properties impact on resistance patterns? Front Microbiol 16 (2025): 33-39
- Setu SK, Sattar AN, Taufiq S, et al. A 3-year study of Infection Profile and Anti-Microbial Resistance of Pseudomonas aeruginosa Isolated from various clinical samples at a tertiary care hospital in Bangladesh. Fortune Journal of Health Sciences. 8 (2025): 401-407
- World Health Organization. Global Action Plan on Antimicrobial Resistance (2015)
- Laxminarayan R, et al. Antibiotic resistance the need for global solutions. Lancet Infect Dis. 13 (2013): 1057–1098.