Relationship of Type-2 Diabetes and Systemic Arterial Hypertension
Article Information
Hanadi Marouf1*, Ubaid Khan2, Behdad Dehbandi3
Review Article
1Consultant Family Medicine, Primary Healthcare Corporation, Qatar
2Department of Medicine, King Edward Medical University Lahore, Pakistan
3Abipromedical Medical Center, Riga, Latvia
*Corresponding author: Hanadi Marouf, Consultant Family Medicine, Primary Healthcare Corporation, Qatar
Received: 28 April 2021; Accepted: 06 May 2021; Published: 22 May 2021
Citation:
Hanadi Marouf, Ubaid Khan, Behdad Dehbandi. Relationship of Type-2 Diabetes and Systemic Arterial Hypertension. Archives of Internal Medicine Research 4 (2021): 114-124.
View / Download Pdf Share at FacebookAbstract
Systemic arterial hypertension is considered a significant cardiovascular risk factor and is found in up to 2/3rd of patients with T2DM. Multiple clinical trials and research studies have been done to check the benefits of controlled BP in the patients with T2DM. Studies showed that people with higher BP targets in diabetic patients have general recommendations of systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg. The multiple interlinked pathways are participated in the pathophysiological mechanisms that contributing to the increased risks of cardiovascular complications, including hypertension in diabetic patients. The therapeutic implications include pharma-cological and non-pharmacological interventions to control systemic arterial hypertension. Non-pharmacological approaches consist of lifestyle modifications, DASH diet, and sodium restriction. The pharmacological interventions include angiotensin inhibitors, calcium channel blockers, and thiazide-type diuretics, and these drugs have proven effective against hypertension in diabetic patients. In addition, the management of blood pressure is another significant factor in controlling hypertension, and this helps in monitoring the effectiveness of the treatment. Furthermore, Telehealth and Renal Denervation are two practical approaches that have been used in RCTs for BP control in hypertensive patients and have shown effective results.
Keywords
Hypertension, Type-2 Diabetes, Blood Pressure, Treatment
Article Details
1. Introduction
Type-2 diabetes mellitus affects more than seven million people leads to the 2.8 million hospitalizations and more than the 300,000 deaths reported annually. Studies showed that improved glycemic control could lower the progression and onset of the microvascular complications, but this glycemic control on CVD complications has not been determined yet [1]. Studies also reported that impaired glucose metabolism in an individual is likely related with the increased risk of systemic arterial hypertension and CVD complications [2]. HTN is present in approximately 50% of T2DM patients and the risk of peripheral vascular disease, cardiac disease, retinopathy, nephropathy, and stroke has increased. The patients who have type-2 diabetes are at more risk of developing systemic arterial hypertension than non-diabetic individuals [3] T2DM increases the peripheral and central arterial stiffness, leading to the increase of blood pressure [2].
Data reported that systemic arterial hypertension in type-2 diabetic patients is more harmful in developing countries due to a lack of adequate preventive and management programs for cardiovascular diseases. The “American Diabetes Association” and the “European Association for the Study of Diabetes” demonstrated that proper management of CVD risk factors including control of “high blood pressure and management of obesity”, maybe even more beneficial in patients with T2DM due to their more risk of developing CVD morbidity and mortality [4]. This literature review describes the relationship of type-2 diabetes with systemic arterial hypertension, potential risk factors, epidemiology, treatment, and management of hypertension in diabetic individuals.
1.1 Epidemiology
Studies reported that systemic arterial hypertension is more prevalent in men than in women in non-diabetic individuals. However, women with T2DM and the impaired glucose tolerance have higher incidence rate of HTN than men. Furthermore, type-2 diabetic females possess a greater risk of death from CVD than males, and the reason underlying this cause is still unclear [5]. Data also showed that the prevalence of hypertension is varied in different ethnic groups. When compared, the incidence rate of HTN with T2DM is more prevalent in “African-Americans than Caucasians” between the age of 45 and 75 years [6]. A higher rate of “obesity, genetic predisposition, and environmental factors” are considered responsible factors in these populations. In the “African-American” populations prevalence of HTN and T2DM than other ethnic groups have been observed [3].
On the other hand, a recent report of the “NHANES 1999-2008” data showed that the American-Mexican populations have an increased prevalence rate of DM and HTN and a less risk of co-existent HTN and DM when compared with the “African-Americans and Caucasian participants” [6]. Several factors contribute to the increased coexistence of T2DM and hypertension. In one study conducted by “The multicenter Treatment Options for DM in Adolescents and Youth (TODAY) trial,” among adolescents who suffered from diabetes aged between 10–17 years old, indicated that the prevalence of hypertension increased from “11.6% at baseline to 33.8%” [7]. Furthermore, it is reported that there are particular subpopulations in which the coexistence of type-2 diabetes and systemic arterial hypertension can pose some serious risks. Women in their gestation period are more at risk for pre-eclampsia due to hypertension caused by diabetes. Besides this, the higher incidence of childhood type-2-diabetes is alarming and worrisome, as CVD risk factors early in life can cause accelerated atherosclerosis with aging [8].
1.2 Common causes of hypertension due to type-2 diabetes
Diabetic nephropathy is one of the most reported cause
of hypertension in the diabetic patients. Those patients
who have T2DM can develop abnormal renal functioning. Type-2 diabetes (T2DM) and insulin resistance (IR) can stimulate the SNS and the renin-angiotensin system and promote Na+1 retention. Also, T2DM is associated with the enhanced proliferation of vascular smooth muscle cells. Increased BP and high glucose level in the blood can damage the endothelial cells of the heart and ultimately cause oxidative damage [8].
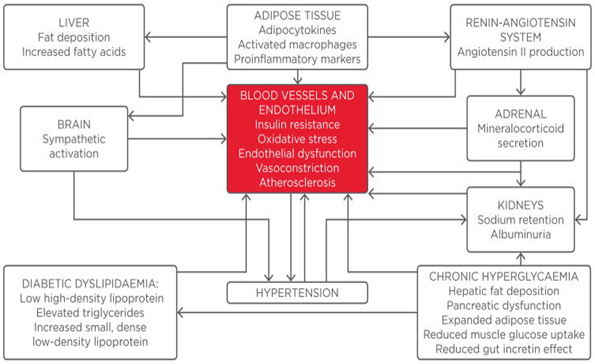
2. Pathophysiology
T2DM and systemic arterial hypertension possess several pathophysiologic mechanisms such as the inappropriate activation of the "renin-angiotensin-aldosterone system" (RAAS), impaired insulin-mediated vasodilation, escalated sympathetic nervous system activation, impaired immune responses, and dysfunctioning of renal handling of Na+1 retention [9]. Also, increased visceral adiposity and obesity are considered contributing factors behind hypertension and type-2 diabetes' co-existence [10]. Studies showed that oxidative stress and inflammation in the adipose tissues cause more angiotensinogen production (AGT) and angiotensin-II, which ultimately triggers the RAAS activation.
Moreover, scientific studies revealed that the overexpression of the angiotensinogen in white adipose tissues (WAT) results in the elevated blood pressure [11]. AGT and Ang-II proved to have a local and systemic effects on the blood pressure regulation. Angiotensin-II exerts its effect via activation of the Ang-II type-1 receptor. When this receptor is activated, several physiological responses in non-adrenal tissues have been reported, such as the production of ROS, endothelial dysfunctioning, IR, and hypertension. Hence, it is reported that RAAS activation has a significant effect on the co-existence of diabetes mellitus and hypertension [12].
Studies reported another mechanism is the increased production of aldosterone hormone and signaling cascade via mineralocorticoid receptor (MR); these two signaling pathways play an integral role in hypertension's pathogenesis. Studies revealed that corticosteroids contributed to cardiovascular disease in diabetic patients through the activation of the MR [13]. On the other hand, adipose tissues produce a lipid-soluble factor that further stimulates aldosterone production from the region of adrenal zona glomerulosa. A study reported that "Complement-C1q TNF-related protein-1" (CTRP1) is an adipokine it facilitates the production of aldosterone in the rodent model of IR and obesity. When the aldosterone produced in the tubules of kidneys it enhances sodium retention, and leads to the plasma volume expansion and increased BP [14].
Furthermore, aldosterone strives for non-genomic actions via MR activation that contributes to systemic arterial hypertension by changing cellular redox states, altering signaling cascades, and endothelial-mediated vascular relaxation. However, adipose tissues promote the systemic increase in blood pressure via the local production of RAAS components [15].
2.1 Role of overweight and insulin resistance in D2M and HTN
In one Strong Heart Study, baseline measurements were predicted for hypertension, including elevations in the baseline systolic BP, waist circumference, left ventricular mass, and diabetes mellitus. Furthermore, another longitudinal study was observed and revealed that parental HTN has an age-independent impact on both male and female offspring in BP elevations, blood glucose levels, and triglyceride levels [16].
Moreover, this study demonstrated that insulin resistance was the underlying pathophysiologic factor contributing to hypertension. A longitudinal study indicated an increase in BMI and the waist circumference was attributed to an increased risk of developing “blood pressure, left ventricular hypertrophy, impaired blood glucose level, and diabetes
mellitus” [17].
2.2 Role of hormonal and neuroendocrine factors in D2M and HTN
A sedentary lifestyle and excessive calorie intake promote insulin resistance and due to this, metabolic signaling response of insulin in the liver, muscle cells, and adipose tissues becomes impaired. This altered signaling cascade of insulin metabolic pathway promotes the enhanced expression of the vascular adhesion molecule, inflammation, oxidative damage, and decreased vascular bioavailable NO. Studies showed that reduced bioavailability of NO decreases endothelial-mediated vascular relaxation and facilitates vascular stiffness [18]. However, IR and obesity are linked with the inappropriate stimulation and activation of the RAAS and the sympathetic nervous system. Research findings indicated that RAAS activation in obesity condition, increase the propensity of IR, DM, hypertension, and CVD [19].
Current evidence suggested that Ang-II and aldosterone act via non-genomic pathways and promote IR through the activation of serine kinases. An increase in the serine kinase activation promotes the insulin signaling molecule phosphorylation. The “insulin receptor substrate protein-1” ultimately leads to the impairment of “phosphoinositol-3-kinase and protein kinase-B activation and diminished insulin metabolic signaling, and as a result, defective NO-mediated vascular relaxation” [20, 21].
2.3 Oxidative stress and HTN
Research studies demonstrated that oxidative stress and damage plays an integral role in developing IR, diabetes mellitus, and hypertension. Several cells in the body in which ROS produces include the vascular smooth muscle cells via xanthine oxidase (XO), endothelial cells, mitochondrial respiratory chain, and nitric oxide synthase [22]. These ROS cause the damage of endothelial functioning by tissue injury directly, the impairment of NO-mediated vasodilation, and the decreased bioavailability of NO. In addition, it is indicated that xanthine oxidase (XO) and mitochondrial oxidative stress lead to the excess production of ROS in the diabetic individuals and cause hypertension. Studies revealed that ROS overproduction causes inflammation in the body by activating inflammatory cascades such as “NF-kB” and damages the tissues [23]. Inflammation is attributed to the adhesion molecules' enhanced activity, cytokines such as “tumor necrosis factor, interleukin-6, acute phase reactants, including C-reactive protein”.
Furthermore, “mechanical stretch, a characteristic phenomenon in systemic arterial hypertension,” led to the stimulation of “p47phox” and “Rac1”, thus guides to the “NADPH oxidase activation”. The hormone aldosterone and Ang-II can also turn on the NADPH oxidase and set off oxidative stress and cause damage [24].
2.4 Diabetic cardiomyopathy
Diabetic cardiomyopathy is a diseased condition in diabetes mellitus patients and it is characterized by defective diastolic function. One study reported that this illness is observed in chemically-induced-inusliopenic and genetically pre-disposed insulin-resistant rodents. Studies revealed that this a myopathic state in which potassium and sodium channels function become altered [25].
Also, calcium-ATPase channels and sodium-calcium exchanger function and abnormalities of protein kinase C metabolism have been observed. Due to these observed complications, patients with type-2 diabetes are likely at more risk of developing systemic arterial hypertension [26].
3. Therapeutic Implications for Systemic Arterial Hypertensive Patients Non-pharmacological Approach
3.1 Lifestyle modifications
Studies reported that lifestyle modifications such as dietary alterations, diet low in salt, more physical activity daily, restriction on alcohol use had shown potential results to reduce blood pressure. Low saturated fat intake and more dietary fiber use showed significant results in BP and reduction in diabetes [27].
4. Dietary Approach to Stop Hypertension (DASH)
The “Dietary Approach to Stop Hypertension” (DASH) is a nutritional linked strategy employed by the US “National Heart, Lungs and Blood Institute” (NHLBI). It is a nutritional and non-pharmacological approach to control hypertension. The DASH diet includes foods rich in vegetables, fruits, low saturated fat products, whole grains; diets low in sweets and refined grains have proven beneficial metabolic and cardiovascular results in diabetic patients [28]. The DASH diet helps reduces BP and the waist circumference and helps maintain body weight and blood glucose levels. This nutritional strategy plays a vital role in the lowering of LDL cholesterol and improves HDL cholesterol. Besides, the DASH diet is proven effective in the reduction of inflammatory molecules in diabetes mellitus patients [29].
4.1 Sodium restriction
Studies reported that excessive use of sodium contributed to the risk of CVD and hypertension. Different clinical trials have shown that reduction of sodium in diet effectively lowers the BP. The systolic and diastolic BP reduced on an average “1mm Hg in normal people and 2-4 mm Hg” in hypertensive individuals. In another study, it is reported that oxidative stress reduction has been observed due to less sodium consumption on the cardiovascular remodeling in an insulin resistance model in the rats [30]. However, it is concluded that less use of dietary sodium has potential effects in lowering BP, hypertension, and other cardiovascular events [31].
4.2 Increased aerobic physical activity
Physical inactivity is reported as a significant underlying cause of cardiovascular diseases. Moreover, healthy dietary patterns, enhanced aerobic physical activity daily is an essential factor in these patients. Studies reported that the 30-to-45 minutes of brisk walking 3-to-5 days weekly had improved lipid profiles, blood pressure, and an insulin resistance [32].
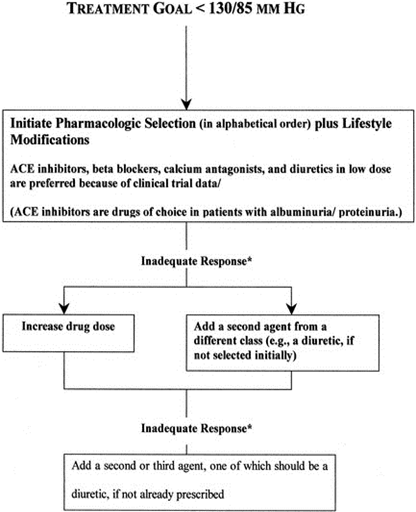
5. Pharmacological Therapy
5.1 Angiotensin receptor blockers (ARBs)
The “Ang-II converting enzyme inhibitors” decreases the Ang-II activity, and as a result, decreased blood pressure, vasodilation, and improvement on the cardiac and renal tissues have been observed. In one "Heart Outcome Prevention Evaluation" study, the ACE inhibitor's potential effects vs. placebo/control on cardiovascular complications were compared. In this study, 25% risk reduction was observed in cardiovascular complications [33]. In a "MICRO-HOPE" study, beneficial effects of Ramipril were observed on cardiovascular disease patients and diabetes mellitus patients.
Moreover, RAAS blockades play a vital role in the lowering of BP in the hypertensive patients, improves insulin resistance in the diabetic patients, controls the oxidative damage, and improves the vascular function [34].
5.2 Calcium channel blockers (CCBs)
CCBs are proven effective against hypertension and have been broadly studied. Studies reported that ACE-I and CCBs have remarkably reduced cardiovascular complications in diabetic hypertensive patients. In another study, it is indicated that amlodipine significantly reduces cardiovascular complications in diabetic patients compared to beta-blockers [36].
5.3 Thiazide-type diuretics
Thiazide-type diuretics have been widely used against systemic arterial hypertension for a long time. In one study, it is reported that the diuretic chlorthalidone was proven beneficial in reducing CVD morbidity and mortality [37]. Another thiazide-type diuretic indapamide reduces the stroke and a risk of heart failure in old hypertensive patients [3, 38].
5.4 BP control in diabetic patients
Clinical studies showed that the patients who are treated in such a way to have lower BP targets are at lower risk of developing CVD complications and the diabetic nephropathy. Combination therapy with different medications plays an essential role in BP control in hypertensive patients, but some drug-induced side effects have also been reported. Different clinical trials have been done to determine the significance of BP in patients with diabetes mellitus [39]. In one HOPE study, it is demonstrated that reducing BP is linked with the less risk of developing cardiovascular complications. In an ADVANCE clinical trial study, it is shown that there is a lower risk of developing CVD complications in lower BP group individuals and less mortality rate. Studies suggested that a maximal BP control in the diabetic patients is obtained with systolic BP between “130-to-135 mmHg and diastolic BP of 80-to-85 mm Hg” [3].
5.5 Future perspectives
Treatment of hypertension in diabetic patients remains challenging and demands advanced therapeutic approaches. Telehealth and renal denervation are two of one of the emerging measures for the treatment of hypertensive patients [40]. The use of Telehealth helps in the exchange of information quickly through electronic communications in order to improve patient’s health status. A randomized controlled trial was conducted to measure the Telehealth outcomes for hypertension in diabetic individuals, and it showed the decrease in the systolic BP in patients was observed [40]. The stimulation of the “renal sympathetic nerves” imparts a vital role in the pathogenies of HTN. The “Renal denervation is a spercutaneous catheter-based strategy” that disrupts the renal nerves via the process of the radiofrequency ablation. In one RCT, the efficacy of renal denervation was evaluated in the hypertensive patients [41]. BP measurements were significantly reduced by “32-12 mm Hg (baseline of 178/96mm Hg) in the hypertensive patients”, and this reduction persisted after six months [41, 42].
Co.nclusion
Individuals with co-existing hypertension and diabetes mellitus are more likely to develop cardiovascular disease and DM hypertensive microvascular complications. Recent research studies and randomized controlled trials have recommended the target “systolic and diastolic BP of 140 mmHg and <90 mmHg” respectively. In diabetic hypertensive patients, several anti-hypertensive medications such as ACE-I and CCBs are required for treatment purposes. Choice of the drug, side effects of the drug, and cost are essential factors that need to be considered for hypertension management. Lifestyle modifications, DASH diet, restricted sodium intake, and enhanced physical activity have potential effects, not on BP control but also lipid profile, glycemic control, and CVD risk. Further research is needed to understand better the pathogenetic mechanisms and advanced therapeutic implications for diabetic hypertensive patients.
References
- Gress TW, Nieto F J, Shahar E, et al. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. New England Journal of Medicine 342 (2000): 905-912.
- Schram M T, Henry R M, Van Dijk R A, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension 43 (2004): 176-181.
- Lastra G, Syed S, Kurukulasuriya L R, et al. Type 2 diabetes mellitus and hypertension: an update. Endocrinology and Metabolism Clinics 43 (2014): 103-122.
- Colosia A D, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes, metabolic syndrome and obesity: targets and therapy 6 (2013): 327.
- Chen G, Mcalister F A, Walker RL, et al. Cardiovascular outcomes in Framingham participants with diabetes: the importance of blood pressure. Hypertension 57 (2011): 891-897.
- Liu X, Song P. Is the association of diabetes with uncontrolled blood pressure stronger in Mexican Americans and blacks than in whites among diagnosed hypertensive patients? American journal of hypertension 26 (2013): 1328-1334.
- Carson AP, Howard G, Burke G L, et al. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 57 (2011): 1101-1107.
- Lago R M, Singh P P, Nesto R W. Diabetes and hypertension. Nature Clinical Practice Endocrinology & Metabolism 3 (2007): 667-667.
- Sarwar N, Gao P, Seshasai S, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375 (2010): 2215-2222.
- Rizvi A A. Addressing hypertension in the patient with type 2 diabetes mellitus: pathogenesis, goals, and therapeutic approach. European medical journal. Diabetes 5 (2017): 84.
- Fox C S. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study. Trends in cardiovascular medicine 20 (2010): 90-95.
- Boustany CM, Bharadwaj K, Daugherty A, et al. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 287 (2004): R943-R949.
- Sowers J R. Diabetes mellitus and vascular disease. Hypertension 61 (2013): 943-947.
- Jeon J H, Kim K Y, Kim J H, et al. A novel adipokine CTRP1 stimulates aldosterone production. The FASEB Journal 22 (2008): 1502-1511.
- Mehta P K, Griendling K K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. American Journal of Physiology-Cell Physiology, 292 (2007): C82-C97.
- Mitsumata K, Saitoh S, Ohnishi H, et al. Effects of parental hypertension on longitudinal trends in blood pressure and plasma metabolic profile: mixed-effects model analysis. Hypertension 60 (2012): 1124-1130.
- Whaley-Connell A, Sowers J R. Indices of obesity and cardiometabolic risk. Hypertension 58 (2011): 991-993.
- Zhang H, Wang Y, Zhang J, et al. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arteriosclerosis, thrombosis, and vascular biology 31 (2011): 2063-2069.
- Sowers J R, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Annals of internal medicine 150 (2009): 776-783.
- Whaley-Connell A, Sowers J R. Aldosterone and risk for insulin resistance. Hypertension 58 (2011): 998-1000.
- Kim J-A, Jang H-J, Martinez-Lemus L A, et al. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. American Journal of Physiology-Endocrinology and Metabolism 302 (2012): E201-E208.
- Taniyama Y, Griendling K K. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 42 (2003) 1075-1081.
- Cooper S A, Whaley-Connell A, Habibi J, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. American Journal of Physiology-Heart and Circulatory Physiology, 293 (2007): H2009-H2023.
- Barhoumi T, Kasal DA, Li MW, et al. T Regulatory lymphocytes prevent angiotensin ii–induced hypertension and vascular injury. Hypertension 57 (2011): 469-476.
- Fang Z Y, Prins J B, Marwick T H. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocrine reviews 25 (2004): 543-567.
- Aneja A, Tang WW, Bansilal S, et al. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. The American journal of medicine 121 (2008): 748-757.
- Tuomilehto J, Lindström J, Eriksson J G, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 344 (2001): 1343-1350.
- Sacks F M, Svetkey L P, Vollmer W M, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. New England journal of medicine 344 (2001): 3-10.
- Azadbakht L, Fard N R P, Karimi M, et al. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes care 34 (2011): 55-57.
- Cook N R, Cutler J A, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). Bmj 334 (2007): 885.
- Rugale C, Oudot C, Desmetz C, et al. Sodium restriction prevents cardiovascular remodeling associated with insulin-resistance in the rat. Annales de Cardiologie et D'angeiologie (2013): 139-143.
- Whelton S P, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Annals of internal medicine 136 (2002): 493-503.
- Hansson L, Lindholm L H, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. The Lancet 353 (1999): 611-616.
- Investigators H O P E S. Effects of an angiotensin-converting–enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. New England Journal of Medicine 342 (2000): 145-153.
- Sowers J R, Epstein M, Frohlich E D. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension 37 (2001): 1053-1059.
- Furberg C D, Wright J T, Davis B R, et al. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Journal of the American Medical Association, 288 (2002): 2981-2997.
- Manrique C, Johnson M, Sowers J R. Thiazide diuretics alone or with β-blockers impair glucose metabolism in hypertensive patients with abdominal obesity. Am Heart Assoc (2010).
- Muramatsu T, Matsushita K, Yamashita K, et al. Comparison between valsartan and amlodipine regarding cardiovascular morbidity and mortality in hypertensive patients with glucose intolerance: NAGOYA HEART Study. Am Heart Assoc (2012).
- Cooper-Dehoff R M, Gong Y, Handberg E M, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. Jama 304 (2010): 61-68.
- Wakefield B J, Holman J E, Ray A, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemedicine and e-Health 17 (2011): 254-261.
- Esler M. Symplicity HTN-2 Investigators Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376 (2010): 1903-1909.
- Fagard R H, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 51 (2008): 55-61.