Relation between Mean Velocity Index, Time Constant and Critical Closing Pressure in Patients with Subarachnoid Hemorrhage
Article Information
Vasilios Papaioannou1,2*, Karol Budohoski2, Michal Placek2, Zofia Czosnyka2, Peter Smielewski2, Marek Czosnyka2
1Department of Intensive Care Medicine, Alexandroupolis hospital, Democritus University of Thrace, Alexandoupolis, Greece
2Academic Neurosurgery Unit, Brain Physics lab, Addenbrooke’s hospital, Cambridge CB20QQ, UK
*Corresponding author: Vasilios Papaioannou, Department of Intensive Care Medicine, Alexandroupolis hospital, Democritus University of Thrace, Greece.
Received: 19 January 2022; Accepted: 27 January 2022; Published: 02 February 2022
Citation: Vasilios Papaioannou, Karol Budohoski, Michal Placek, Zofia Czosnyka, Peter Smielewski, Marek Czosnyka. Relation between Mean Velocity Index, Time Constant and Critical Closing Pressure in Patients with Subarachnoid Hemorrhage. Archives of Clinical and Biomedical Research 6 (2022): 119-133.
View / Download Pdf Share at FacebookAbstract
Background: In patients suffering from Subarachnoid Hemorrhage (SAH), Delayed Cerebral Ischemia (DCI) is partly associated with Vasospasm (VS) and impaired cerebral autoregulation. We investigated the pattern of changes of different Transcranial Doppler (TCD)-derived indices of cerebrovascular dynamics during VS, in patients dichotomized by the presence of DCI.
Methods: A retrospective analysis was performed using recordings from 32 SAH patients, diagnosed with VS through bilateral TCD measurements. Patients were divided in 2 groups, depending on development of DCI. Cerebral autoregulation was estimated using the moving correlation coefficient Mxa, calculated from spontaneous fluctuations of Cerebral Blood Flow Velocity (CBFV) and Arterial Blood Pressure (ABP). We also measured cerebral arterial time constant (tau) as the product of resistance (Ra) and Compliance (Ca) and Critical Closing Pressure (CrCP), using two different methods of assessment (CrCPAaslid and CrCPVarsos).
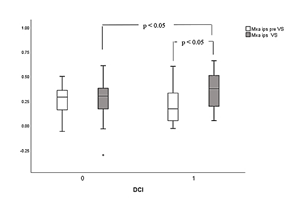
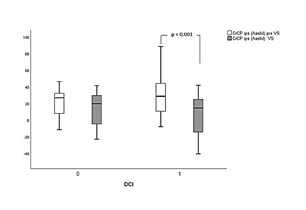
Results: In the whole population (N=32), VS caused shortening of tau (ipsilateral to spasm: 0.17 ± 0.08 vs before: 0.25 ± 0.17 sec, p = 0.04), decrease in CrCPAaslid (ipsilateral to spasm: 9.69 ± 23.28 vs before: 27.23 ± 23.31 mmHg, p = 0.01) and interhemispheric asymmetry with lower values on ipsilateral side (p < 0.01). Ipsilateral CrCPAaslid during VS was negatively correlated with Mxa (r =-0.43, p=0.01), whereas CrCPVarsos exhibited positive correlation with tau (r=0.74, p < 0.001). In patients with DCI (N=19), VS caused increase in Mxa (ipsilateral to spasm: 0.36 ± 0.18 vs before: 0.26 ± 0.23, p = 0.04), decrease in CrCPAaslid (ipsilateral to spasm: 6.61 ± 24.5 vs before: 17.24 ± 19.4 mmHg, p = 0.04) and lower values on ipsilateral side (p < 0.01).
Conclusions: During VS, tau and CrCP were reduced in
Keywords
Critical closing pressure; Delayed cerebral ischemia; Subarachnoid hemorrhage; Time constant; Transcranial Doppler; Vasospasm
Article Details
1. Introduction
Vasospasm (VS) and Delayed Cerebral Ischemia (DCI) constitute major complications following Subarachnoid Hemorrhage (SAH). DCI has been shown to occur in approximately 40% of patients suffering from SAH and is more common in those who develop VS in large cerebral arteries, since arterial narrowing has a delayed onset with a peak between 5 and 14 days, post ictus [1]. However, the maximum rate of DCI in patients with VS is around 50%, whereas up to one-third of patients with DCI do not exhibit large artery vasospasm [1,2]. In this respect, different studies have found that a combination of VS and dysfunction of cerebral autoregulation during the first 4-5 days post-SAH, correlate with the occurrence of DCI [3-5]. Many experimental studies have demonstrated that during the acute phase of SAH, global cerebral ischemia, blood-brain barrier disruption, cortical spreading depolarizations, microvascular spasm with endothelial dysfunction, as well activation of an inflammatory cascade might constitute novel mechanisms that could increase tissue vulnerability to secondary insults [1,2,6]. It has been suggested that macrovascular spasm leads to distal compensatory vasodilatation with shortening of autoregulatory plateau, which could signal impaired autoregulation upon testing, whereas microvascular spasm might induce a shift of the plateau to the right, towards higher arterial blood pressure (ABP) [7].
Surrogate markers of Cerebral Blood Flow (CBF), such as non-invasive Transcranial Doppler (TCD), are frequently used to monitor development of VS, as well as integrity of autoregulation, through estimation of the dynamic changes that take place between ABP and CBF Velocity (CBFV) [8]. In this respect, different physiological parameters can be estimated, such as the index Mx evaluating the low frequency autoregulatory response [9], or other ‘high frequency’ components of autoregulation, like cerebro-vascular Resistance (Ra) and Compliance (Ca) [10-12]. Furthermore, secondary indices describing cerebrovascular dynamics have recently received attention, such as cerebral arterial time constant (tau) that is the product of resistance and compliance [13] and Critical Closing Pressure (CrCP), which estimates the theoretical lowest value of ABP that is needed for maintaining CBF [14-16].
The clinical significance of such indices has been tested in patients suffering from SAH, where shortening of tau [17] and decreased CrCP [18,19] in both temporal and spatial assessments, have been found, during VS. In a recent study, we also showed that both Mx and tau are altered in patients with VS who experience DCI, whereas Slow Waves (SWs) that reflect dynamic oscillations in cerebral blood volume related to autoregulatory vasodilatation and vasoconstriction with an associated frequency range of 0.05 to 0.005 Hz, were predictive of DCI [20]. The primary aim of this study was to assess how VS affects Mx, tau and CrCP that describe both low- and high frequency components of cerebral autoregulation, in patients suffering from SAH. Thus, both temporal (pre-VS vs during VS) and spatial (ipsilateral to VS side vs contralateral) comparisons were performed. Secondarily, we explored the potential differences of VS-associated changes in cerebrovascular dynamics between subgroups of patients with and without DCI, in both temporal and spatial settings. We also evaluated the potential correlations between different metrics used, in order to better understand their interrelations during VS and how their interplay might be affected by the development of DCI.
2. Material and Methods
2.1 Study population
We analyzed retrospectively digitally recorded and prospectively collected data from all patients admitted to the Department of Neurosurgery in Addenbrooke’s hospital between June 2010 and January 2012 with a diagnosis of SAH, examined with TCD to assess state of autoregulation and detect VS [4,5,20]. Written approval of the study was given by both patients and the local Addenbrooke’s Research Ethics Committee. Thirty-two conscious patients with SAH (mean age: 52.4 ± 10, 12 males and 20 females) who developed TCD-detected VS were included in the study. Overall patient characteristics are presented in Table 1. None from our patients had Intracranial Pressure (ICP) monitoring or Extraventricular Drain (EVD). Cerebral VS was defined as mean blood flow velocity in the middle cerebral artery (CBFVMCA) higher than 120 cm/sec and Lindegaard Ratio (LR) (LR = CBFVMCA/ CBFVICA) > 3 [21,22], whereas its median onset was 6 days after SAH. Nineteen patients developed DCI within 21 days of ictus. Delayed cerebral ischemia was defined as a drop of ≥ 2 points on the Glasgow Coma Scale (GCS) lasting more than 2 hours, after excluding intracranial hemorrhage, acute hydrocephalus, seizures, metabolic derangements or infection, as has been previously described [4,5,23].
|
Variables |
Overall |
DCI |
Non-DCI |
p value |
|
(N=32) |
(N=19) |
(N=13) |
||
|
Age, years (± SD) |
52.4 ± 10 |
54.15 ± 11.8 |
50 ± 7.6 |
0.27 |
|
Sex (male/female) |
20-Dec |
11-Aug |
9-Apr |
0.74 |
|
WFNS (mean) |
2 ± 1.3 |
2 ± 1.3 |
2 ± 1.3 |
0.97 |
|
Modified Fisher scale |
3 ± 0.8 |
3 ± 0.7 |
3 ± 1 |
0.94 |
|
GCS admission |
12 ± 3 |
12 ± 3 |
12 ± 4 |
0.7 |
|
GCS discharge |
14 ± 3 |
13 ± 3 |
14 ± 1 |
0.16 |
|
GOS |
4 ± 1 |
4 ± 1 |
4 ± 1 |
0.55 |
|
Aneurysm location |
||||
|
1.AcomA |
9 |
6 |
3 |
0.53 |
|
2.MCA |
8 |
4 |
4 |
0.93 |
|
3.PcomA |
9 |
6 |
3 |
0.12 |
|
4.PICA |
4 |
2 |
2 |
0.46 |
|
5.BA |
1 |
0 |
1 |
0.35 |
|
6.ICA |
1 |
1 |
0 |
0.32 |
|
VS site |
||||
|
1.Right |
14 |
9 |
5 |
0.44 |
|
2.Left |
11 |
6 |
5 |
0.67 |
|
3.Bilateral |
7 |
4 |
3 |
0.7 |
|
Clipping/coiling |
20/12 |
7-Dec |
5-Aug |
0.4 |
|
Rebleeding |
1 |
0 |
1 |
0.3 |
|
DCI: delayed cerebral ischemia, WFNS: World Federation of Neurosurgical Societies, GCS: Glasgow Coma Scale, GOS: Glasgow outcome scale, AcomA: anterior com-municating artery, MCA: middle cerebral artery, PcomA: posterior communicating artery, PICA: posterior inferior cerebellar artery, BA: basilar artery, ICA: internal carotid artery, VS: vasospasm. See ref [20]. |
||||
Table 1: Characteristics of all patients and subgroups with and without DCI.
All patients were treated with oral nimodipine 60 mg every 4h, as well as with aggressive hypertensive, hypervolemic and hemodilutional therapy for the management of VS [24]. Neurologic status upon admission was assessed using GCS and the World Federation of Neurosurgical Societies (WFNS) scale, whereas modified Fisher scale was used for grading the amount of subarachnoid blood [25]. The Glasgow Outcome Scale (GOS) was used for assessing outcome at discharge from hospital.
2.2 Monitoring and data analysis
Arterial blood pressure was monitored non-invasively with Finapres 2300 (Ohmeda, Amsterdam, the Netherlands) with the hand kept at the heart level in all patients. Patients were supine with the head of the bed raised 300 to 450. Bilateral TCD examinations of extracranial internal carotid arteries and MCA were performed, using 2MHz probes with Doppler Box (DWL Compumedics Germany). The raw data signals were recorded at sampling frequency of 200 Hz using ICM+ software (Cambridge Enterprise, Cambridge, UK, https://icmplus.neurosurg.cam.ac. uk/). Mean values of signals were calculated by averaging values in a 10-second time window and then secondarily averaging over the whole monitoring period (30-40 minutes). Heart rate was calculated using the peak of the first harmonic of the spectra of ABP. Only sessions with minimum of 30 minutes of simultaneous ABP and bilateral CBFVMCA recordings were included in the analyses. Average periods of 3 days of TCD recordings before and during VS, as well as differences between ipsilateral and contralateral to the VS sides were compared for all patients and between subgroups with and without DCI. Both location of VS on TCD and lateralization of ischemic symptoms were used for assessing hemispheric differences of various measured cerebrovascular properties. When bilateral VS was present, the analysis included averaging of both sides.
2.3 Calculation of different cerebrovascular parameters
2.3.1 Cerebrovascular resistance (CVR)
CVR that is the resistance of small cerebral arteries and arterioles can be estimated using TCD mean CBFV and cerebral perfusion pressure (CPP = ABP-ICP), according to the following equation [11]:
CVR = mean CPP/mean CBFVMCA *Sa [mmHg/(cm/sec*cm2)] 1
where Sa denotes the cross-sectional area of the insonated vessel and has units of cm2. Since vessel’s actual diameter is unknown, an assumption is made that it remains constant during measurement time. In this case, it can be omitted from the equation giving rise to a simplified formula, which represents values of CVR per 1 cm2 of cross-sectional area with units mmHg*sec/cm. In cases where ICP does not change during measurements and remains much smaller than ABP, equation 1 can be written as:
CVRa or Ra = mean ABP/mean CBFVMCA [mmHg*sec/cm] 2
Since ICP was not monitored in any of the patients included in this study, CVR was measured according to equation 2 [11,13].
2.3.2 Compliance of the cerebral arterial bed (Ca)
Ca is the change in arterial blood volume related to changes in arterial blood pressure. Relative changes in Ca can be estimated according to the relationship between the pulsatile changes in cerebral arterial blood volume (CaBV), which is derived from TCD blood flow velocity and the pulsatile changes in ABP [11-13]:
Ca = AMPCaBV*Sa/AMPABP [(cm2*(cm/sec)*sec)/mmHg] or [cm3/mmHg] 3
Sa is the cross-sectional area of the insonated vessel, where AMPCaBV and AMPABP are the fundamental harmonic amplitudes of the pulse changes of CaBV and ABP respectively, using a Fast Fourier transformation of their original time series. Pulsatile changes of cerebral arterial blood volume (ΔCaBV) can be estimated using the methodology first described by Avezaat and Eijnhoven [26], where ΔCaBV during a cardiac cycle is calculated as an integral of the difference between arterial pulsatile inflow and venous outflow of cerebral blood. For more details, see References [11,12]. Due to the unknown cross-sectional area of the insonated vessel, values of Ca cannot be calibrated in units of cm3/mmHg and comparisons between patients are impossible, whereas only relative changes with time can be monitored. In this case, the TCD derived CaBV signal can be normalized (divided) by the unknown cross-sectional area of the insonated vessel, therefore, units are not units of volume (cm3) but cm [13].
2.3.3 Cerebral arterial time constant (tau)
The time constant of cerebral arterial bed is a TCD-derived index indicating how fast ‘arterial blood stabilizes after a change in ABP’ [11,13]. In other words, it can reflect ‘the time of the filling of arterial bed distal to the level of insonated vessel, following cardiac systole’[13,17]. In that case, although CBFVMCA is measured withing a large artery, tau describes the distal vascular network as the product of Ca and CVR:
Tau = Ca*CVR = (AMPCaBV*Sa/AMPABP)*(mean ABP/ mean CBFVMCA * Sa) [sec] 4
It seems that substituting equations 2 and 3 into equation 4 enables the unknown Sa of the arterial insonated vessel to be excluded, allowing the time constant to be measured in seconds. Furthermore, tau enables comparisons between different patients and allows for assessing whether alterations in compliance are balanced by changes in resistance and vice versa [11].
2.4 Mean velocity index (Mx)
The TCD-derived Mean Velocity Index (Mx) can be measured for assessing cerebral autoregulation. It is calculated as a Pearson’s moving correlation coefficient between 30 consecutive samples of averaged (over 10 seconds) CPP and mean CBFVMCA with an update every 10 seconds [9]. In this study, ABP instead of CPP was measured, giving rise to the Mxa index. A passive transmission of ABP fluctuations to mean flow velocity reflects impaired cerebral autoregulation and therefore, the calculated Mxa will be positive. A zero or negative Mxa signifies none or inverse association between ABP and CBFVMCA, something that is associated with preserved autoregulation [9]. In sedated TBI patients, it has been suggested that a threshold of impaired auto-regulation must lie for Mx between 0.15 and 0.25, whereas values greater than 0.3 indicate definitely disturbed autoregulation [27]. However, in patients suffering from SAH, values of Mxa during VS greater than 0.44 ipsilateral and 0.34 contralateral to vasospastic side were associated with impaired autoregulation [28].
2.5 Critical closing pressure (CrCP)
CrCP reflects ‘a lower critical threshold for ABP, since when ABP falls below this threshold the small brain vessels collapse and CBF stops’ [19]. Aaslid suggested that the intercept point of a regression line between systolic and diastolic ABP plotted along the x axis and systolic and diastolic CBFVMCA plotted along the y axis can be used for estimation of CrCP [29]. Mitchel used the first harmonics of the pulse waveforms of arterial pressure and CBFV as an alternative method for assessing CrCP [30]. Never-the-less, all aforementioned methods suffer from many limitations, mostly due to the possibility of obtaining negative values of CrCP. Varsos introduced a novel method for CrCP estimation, based on a model of cerebrovascular impedance, which never produces negative values [31]. For more detailed information, see Reference [11]. In this study, both methods of Aaslid (CrCP Aaslid) and Varsos (CrCP Varsos) were implemented and compared for estimating the CrCP in every patient. CrCPAaslid estimation was based on the following equation [12,29]:
CrCPAaslid=ABPsys-[(ABPsyst-ABPdia/CBFVMCAsys-CBFVMCAdia)*CBFVMCAsys] 5
whereas CrCPVarsos was estimated using a non-invasively version (using ABP instead for CPP, as well as Ra instead for CVR), as has been described previously [31]:
CrCPVarsos = ABP*[1-1/√[(Ra*Ca*HR*2π)2+1]] 6
where HR is heart rate and π =3.14.
3 Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 24 package (Armonk, NY, USA). Patients were dichotomized into DCI and non-DCI groups (groups 1 and 0 respectively). The assumption of normal distribution was confirmed using Kolmogorov-Smirnoff test. Temporal comparisons between TCD-derived indices before (pre-VS) and during VS, as well as spatial comparisons between ipsilateral and contralateral sides were performed using t-paired test for the overall group of patients. The independent t-test was used for comparisons between groups with and without DCI. Values were averaged per monitoring session, before and during VS, based on the TCD onset of VS for each patient. Bivariate correlations between different measured variables were estimated using the Pearson r coefficient. Data are presented as mean ± SD, whereas the level of significance (p value) was set at 0.05.
4. Results
Table 1 summarizes the baseline characteristics for the included patients divided by the presence of DCI. The two cohorts did not differ in terms of age, WFNS and Fisher scales, GCS upon both admission and discharge, as well as GOS. In addition, no statistical differences were found between aneurysm location and site of vasospasm. ABP values did not differ significantly between pre VS and VS period of measurements, neither for the whole population nor between and within groups.
4.1 Temporal and spatial comparisons for the whole group of patients
Statistically significant differences of measured TCD-derived variables before and during VS, as well between ipsilateral and contralateral side to VS for all patients are presented in Table 2. Thus, ipsilateral Ra was significantly reduced during VS related to pre VS values (0.79 ± 0.42 vs 1.17 ± 0.52 mmHg*sec/cm, p = 0.04), whereas both pre-VS and VS values were significantly reduced ipsilateral compared to contralateral side (1.17 ± 0.52 vs 1.41 ± 0.9, p = 0.02 and 0.79 ± 0.42 vs 1.28 ± 0.61 mmHg*sec/cm, p = 0.03, respectively). Ipsilateral tau during VS was significantly shortened in relation with pre VS period of measurements (0.17 ± 0.08 vs 0.25 ± 0.17 sec, p = 0.04), as well as compared to contralateral side (0.17 ± 0.08 vs 0.19 ± 0.09 sec, p = 0.01, Table 2). Similar differences were found for CrCPAaslid, with ipsilateral CrCP exhibiting significantly reduced values during VS (9.69 ± 23.28 vs 27.23 ± 23.31 mmHg, p = 0.01). Ipsilateral Mxa was increased during VS compared to pre VS values but differences were not statistically significant (0.31 ± 0.21 vs 0.24 ± 0.20, p = 0.16). Similarly, both CrCPVarsos and Ca were decreased (36.45 ± 10.76 vs 41.31 ± 15.67, mmHg, p = 0.15 and 0.21 ± 0.09 vs 0.23 ± 0.13 cm/mmHg, p = 0.8, respectively).
4.2 Temporal and spatial comparisons in cohorts with and without DCI
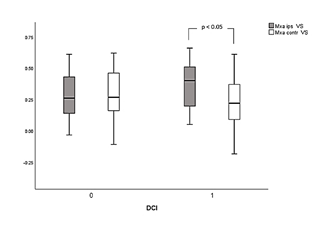
Statistically significant comparisons before and during VS, as well between ipsilateral and contralateral side to VS in dichotomized categories of patients with DCI are presented in Table 3. Ipsilateral Mxa was significantly increased during VS in DCI vs non-DCI groups of patients (0.36 ± 0.18 vs 0.26 ± 0.23, p = 0.04). Furthermore, and during the same period of measurements, Mxa was significantly higher at ipsilateral related to contralateral side of VS in the DCI cohort (0.36 ± 0.18 vs 0.21 ± 0.23, p = 0.03), indicating an interhemispheric asymmetry of autoregulation. Finally, ipsilateral Mxa was significantly increased during VS compared to pre VS values only in patients who developed DCI (0.36 ± 0.18 vs 0.23 ± 0.23, p = 0.04, Table 3), probably due to more disturbed autoregulation. CrCPAaslid during VS was found significantly reduced ipsilateral compared to contralateral side (6.61 ± 24.5 vs 17.24 ± 19.4 mmHg, p = 0.04) in the DCI group. Similar results but without reaching statistical significance were found for non-DCI patients (Table 3). Furthermore, ipsilateral CrCPAaslid was non-significantly reduced during VS in DCI vs non-DCI patients (6.61 ± 24.5 vs 12.87 ± 21.4 mmHg, p = 0.06). Finally, ipsilateral values during VS were significantly reduced in DCI cohort related to pre VS measurements (6.61 ± 24.5 vs 30.58 ± 26.55 mmHg, p < 0.001).
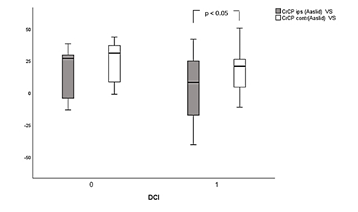
CrCP Varsos did not exhibit significant differences neither between nor within groups. Thus, a slight decrease of ipsilateral CrCP in both DCI and non-DCI patients during VS compared to pre VS values was found, but without any temporal or spatial difference between groups.
|
Variables |
Site of measurement |
Pre VS |
During VS |
p value |
|
Ra |
ipsilateral |
1.17 ± 0.52 |
0.79 ± 0.42 |
0.04 |
|
[mmHg*sec/cm] |
contralateral |
1.41 ± 0.9 |
1.28 ± 0.61 |
0.72 |
|
Tau |
p value |
0.02 |
0.03 |
|
|
[sec] |
ipsilateral |
0.25 ± 0.17 |
0.17 ± 0.08 |
0.04 |
|
contralateral |
0.28 ± 0.16 |
0.19 ± 0.09 |
0.5 |
|
|
CrCPAaslid |
p value |
0.32 |
0.01 |
|
|
[mmHg] |
ipsilateral |
27.23 ± 23.31 |
9.69 ± 23.28 |
0.01 |
|
contralateral |
29.12 ± 23.4 |
19.31 ± 18.52 |
0.39 |
|
|
p value |
0.62 |
< 0.001 |
||
|
VS: vasospasm, Ra: cerebrovascular resistance, Tau: time constant, CrCP: critical closing pressure, SWs: slow waves. |
||||
Table 2: Statistically significant temporal (pre VS vs VS) and spatial (ipsilateral vs contralateral) differences of mean values of different parameters across the overall study population (N = 32, paired samples t-test).
|
Spatial comparisons during VS (ipsilateral vs contralateral) |
|||||
|
Side of measurement |
DCI |
Non-DCI |
p value |
||
|
Variables |
(N=19) |
(N=13) |
|||
|
Mxa |
ipsilateral |
0.36 ± 0.18 |
0.26 ± 0.23 |
0.04 |
|
|
contralateral |
0.21 ± 0.23 |
0.23 ± 0.32 |
0.52 |
||
|
p value |
0.03 |
0.62 |
|||
|
CrCPAaslid |
ipsilateral |
6.61 ± 24.5 |
12.87 ± 21.4 |
0.06 |
|
|
[mmHg] |
contralateral |
17.24 ± 19.4 |
22.75 ± 17.4 |
0.5 |
|
|
p value |
0.04 |
0.08 |
|||
|
Temporal comparisons (ipsilateral pre VS vs during VS) |
|||||
|
Time of measurement |
DCI |
Non-DCI |
p value |
||
|
Variables |
(N=19) |
(N=13) |
|||
|
Mxa |
pre VS |
0.23 ± 0.23 |
0.26 ± 0.16 |
0.52 |
|
|
during VS |
0.36 ± 0.18 |
0.26 ± 0.23 |
0.04 |
||
|
p value |
0.04 |
0.32 |
|||
|
CrCPAaslid |
pre VS |
30.58 ± 26.55 |
22.19 ± 17.2 |
0.4 |
|
|
[mmHg] |
during VS |
6.61 ± 24.56 |
12.87 ± 21.14 |
0.06 |
|
|
p value |
< 0.001 |
0.72 |
|||
|
VS: vasospasm, DCI: delayed cerebral ischemia, CrCP: critical closing pressure, SWs: slow waves. |
|||||
Table 3: Statistically significant temporal (pre VS vs VS) and spatial (ipsilateral vs contralateral) differences of mean values of different parameters between DCI and non-DCI groups of patients (independent samples t-test).
Figures 1a and 1b illustrate differences of Mxa across different groups, between time and side of measurements, respectively. Similarly, figures 2a and b illustrate differences of CrCPAaslid across different groups, between time and site of measurements.
Figure 1a: Box plot of mean values and standard deviation (SD) of Mxa distribution between and within groups 1 (DCI) and 0 (non-DCI), respectively. Statistically significant differences of ipsilateral Mxa values (Mxa ips) during vasospasm (VS) are illustrated between DCI and non-DCI groups, as well as between pre VS and during VS measurements within group 1.
Ipsilateral tau was non significantly reduced during VS related to pre VS values in both groups (0.18 ± 0.09 vs 0.31 ± 0.2 sec, p = 0.06 for DCI group and 0.17 ± 0.1 vs 0.2 ± 0.09 sec, p = 0.4 for non-DCI group, respectively), whereas no differences were found between cohorts during both pre VS and VS periods of recordings.
4.3 Bivariate correlations
The ipsilateral Mxa during VS was found to be negatively correlated with CrCP Aaslid with a Pearson coefficient r = - 0.435, p = 0.015. Thus, the more disturbed auto-regulation (higher Mxa) seems to be related with reduced critical closing pressure. Ipsilateral CrCPVarsos during VS exhibited a positive correlation with tau (r = 0.74, p < 0.001), reflecting a probable association between a shortened time of filling of the arterial bed distal to the level of insonated vessel with reduced vasomotor tone.
5. Discussion
In the present study, we measured different TCD-derived hemodynamic indices of cerebrovascular dynamics, in order to investigate their behavior during VS in patients suffering from SAH, dichotomized by the presence of DCI.
For the whole group of patients, we observed that VS was associated with significantly reduced ipsilateral Ra, tau and CrCP estimated with Aaslid’s method. Similar differences were found between ipsilateral vs contralateral side during VS (paired spatial comparisons). These findings suggest that VS can cause a shortening of tau at the site of aneurysm, mostly due to reduced Ra, since Ca was not found to change significantly. Our results are in keeping with a similar previous study by Kasprowicz and colleagues [17], who also found that tau is significantly reduced during VS even before formal TCD signs of VS are observed, suggesting a potentially reducing time to escalation of treatment. It has been postulated that both cerebral Ca and Ra might decrease during VS, since tension of arterial smooth muscles increases, whereas there is a decrease in Ra due to dilatation of small resistance vessels in the territory distal to the affected artery [17]. Since assessment of Ca and Ra requires that the unknown diameter of insonated vessel remains constant, their values cannot be calibrated during states of narrowing of cerebral arteries, excluding comparisons between patients. As a consequence, their product tau has been suggested to overcome such limitations, since it is independent of the vessel’s radius and is measured in seconds. Tau describes how fast the arterial bed distal to the point of insonation is filled with blood and reflects the mutual relationship between Ca and Ra.
In this respect, Ca has been found to be affected mostly by changes in ABP, since arterial hypertension, as well as old age can cause an increase in arterial wall stiffness and a concomitant decrease in arterial distensibility, something that can be represented through a decrease in Ca [13]. In our study, patient did not differ in terms of ABP or age. Furthermore, ABP was not significantly different between pre VS and during VS periods of recordings. Contrary to Ca, Ra seems to be affected mainly by changes in pCO2 levels, since hypercapnia through dilatation of small cerebral vessels can decrease Ra and shorten tau [13]. In this study, although pCO2 levels were not monitored, all patients were awake and calm, breathing spontaneously during all periods of TCD recordings. Thus, we assume that pCO2 changes during measurements were not that significant to affect Ra and subsequently, tau. Reduced CrCP during VS was verified by 2 different setting (both temporal and spatial effect), as well as by 2 different methods (Aaslid and Varsos), although only CrCP Aaslid exhibited statistically significant changes. According to Burton’s model [14], CrCP reflects a lower critical threshold for ABP, below which the small brain vessels collapse and CBF stops. Dewy and colleagues [15] showed in an experimental study that CrCP is determined by vasomotor tone and ICP. In this respect, any observed changes in CrCP can reflect alterations either in wall tension (WT) or ICP. Normal CrCP values for humans are estimated at around 40 mmHg, whereas alterations in vasomotor tone may induce changes in CrCP between 10 and 95 mmHg. This model applies only to the resistance vessels and particularly the precapillary arterioles [15].
Our results are in agreement with a similar study by Varsos and colleagues [19] who found a significant decrease in CrCP during VS, in both temporal and spatial assessments, in patients suffering from SAH. In addition, unfavorable outcome estimated with GOS upon discharge and 3 months after ictus, was associated with significantly reduced CrCP after the onset of VS. Although ICP was not measured in our study, it was assumed that changes in CrCP corresponded to changes in WT. In this case and according to Dewy’ s theory [15], VS in large conductive vessels might decrease blood flow to the resistance vessels in the periphery and induce dilatation, a decrease in wall tension and finally, decrease in CrCP. In our investigation we assume that CrCP changes might reflect mainly a dilatatory effect of proximal VS on distal microcirculation, since patients were stable during periods of measurements without any change in their neurological status. In any case, and despite the fact that ICP monitoring in patients suffering from SAH is not routinely performed, lack of ICP measurements limits accuracy and reproducibility of our results.
In another similar study, Soehle and coworkers [18] also showed that CrCP decreased significantly during VS as compared with baseline, whereas ICP remained unaffected by VS. In addition, ipsilateral values were significantly lower in relation with measurements at the contralateral side. According to the authors, such findings indicate that either autoregulation-related distal vasodilatation outweighs proximal VS, or CrCP is underestimated since the turbulence associated with VS may have impaired the linear relationship between flow and pressure. Since a significantly negative correlation was found between CrCPAaslid and Mxa ipsilateral to aneurysm rupture during VS, we suggest that disturbed autoregulation in our patients was associated with maximal vasodilatation and reduced WT. Similar correlations were found in another study by Varsos and colleagues in patients suffering from TBI [32]. As they have suggested, impaired autoregulation can be associated with maximal vasodilatation or even vasoparalysis, reflected in reduced CrCP values. In our study, Mxa was increased during VS, as well as at the ipsilateral vs contralateral side but without reaching statistical significance. Moreover, the strong positive correlation between CrCPVarsos and tau that was found during VS, might reflect a potential association between reduced WT and rapid arterial filling distal to the point of insonation.
Cerebral autoregulation estimated with Mxa was found to be significantly impaired during VS in patients who developed DCI related to pre VS measurements. Moreover, ipsilateral Mxa values during VS were significantly increased in DCI compared to non-DCI groups (temporal comparisons). Finally, patients with DCI exhibited a significant interhemispheric difference in autoregulation, since Mxa during VS was found to increase in the ipsilateral related to contralateral side of aneurysm rupture (spatial comparisons). Such findings are in keeping with previous studies of Budohoski and colleagues [4,5] who showed that development of DCI was associated with a significant interhemispheric difference of autoregulation in the first 5 days after ictus. Measurement of ABP rather than CPP for calculation of Mx might limit accuracy of our results. However, both Mx and Mxa have been shown to exhibit good correlation, particularly in cases of impaired autoregulation in TBI patients, although Mxa was not found to differ between patients with different outcome [33]. In this respect, our findings agree with those of Calviere and coworkers [3], who showed that the combination of both large artery VS and impaired autoregulation estimated with Mxa during the first 7 days from ictus, was correlated to subsequent DCI. Another potential confounder in our study might be the arterial tension of pCO2, since thresholds of disturbed autoregulation strongly depend on its values, limiting accuracy of comparisons between patients [34]. Never-the-less, none from our patients had a history of Chronic Obstructive Pulmonary Disease (COPD) or any other pulmonary disease that might affect pCO2 levels, assuming that its potential impact upon our measurements might be insignificant. In any case, our results agree with those from other studies where no arterial tension pCO2 measurements were performed [3-5]. The DCI group exhibited inverse changes in CrCPAaslid compared to Mxa. Moreover, CrCP was significantly decreased in both temporal and spatial assessments, whereas no significant differences were found between groups. Such findings imply that in patients who developed DCI, WT estimated with CrCP was reduced during VS due to potential vasoparalysis, since autoregulation was also significantly disturbed.
Some of the major limitations of this study is its retrospective nature, as well as the small sample size that might account for some of the negative associations found. Never-the-less, we included only conscious patients, in order to increase homogeneity of studying population, as well as to have clinically proven diagnosis of DCI, since its diagnosis in sedated subjects is more difficult and depends on different imaging techniques. Our findings are in keeping with results from other investigators who evaluated relationships between different TCD-derived cerebrovascular parameters with outcome, in similar patients’ groups. Non-invasive ABP measurement for Mx calculation through Finapres system could constitute another limitation in terms of accuracy of results. However, agreement between invasive and non-invasive assessment of Mx has been tested and a good correlation between the two methods was found [35]. Since TCD has limited spatial resolution, it is conceivable that regional disturbance of autoregulation, particularly affecting the posterior circulation might have been missed. Another limitation associated with current TCD technology is the fact that due to short periods of recordings, CrCP measurements corresponded only to these particular time points. CrCP could also have been a poor estimate of wall tension due to lack of invasive ICP measurements. However, patients with SAH do not usually receive ICP monitoring, whereas similar findings with our study have been presented before, confirming our results. Regarding different associations found between the 2 methods applied for CrCP measurement and various hemodynamic parameters, we can only speculate that using Varsos technique, expected values of WT may fluctuate from 16% to 85% of CPP, due to the non-linear, monotonically increasing function of the denominator in equation 6, as has been suggested by Varsos [11]. In this case, potential correlations with measured variables in this study might be false-negative. Never-the-less, the Aaslid method still produced a lot of negative values with very large standard deviations.
In conclusion, we suggest that more sophisticated signal processing techniques, such as wavelet transformation of CBFV signals, could shed more light into different patho-physiological phenomena during VS and DCI. Moreover, simultaneous analysis of ICP and CBFV could enhance accuracy of measurements through estimation of CPP instead for ABP, whereas parallel monitoring of arterial tension of pCO2 that affects thresholds of disturbed autoregulation might improve accuracy of comparisons between patients. Finally, a future analysis including also patients without VS could further assess the complexity of interrelations between different variables measured in this study, as well as their potential association with outcome.
6. Conclusions
VS was associated with reduced tau and CrCP in both temporal and spatial assessments, in all patients with SAH. Impaired autoregulation and shortened arterial filling time distal to insonation vessel correlated with decreased CrCP, possibly due to low wall tension. VS in patients who developed DCI reduced CrCP (temporal and spatial effect) signifying vasodilatation, and increased Mxa, reflecting disturbed autoregulation. Consequently, we suggest that following SAH, daily monitoring of different cerebrovascular parameters through TCD measurements at the bedside, could improve understanding of complex physiology and eventually, optimize treatment.
References
- Budohoski K, Guilfoyle M, Helmy A, et al. The pathophysiology and treatment of delayed cerebral ischemia following subarachnoid hemorrhage. J Neurol Neurosurg Psychiatry 85 (2014): 1343-1353.
- Rowland MJ, Hadjipavlou G, Kelly M, et al. Delayed cerebral ischemia after subarachnoid hemorrhage: looking beyond vasospasm. BJA 109 (2012): 315-329.
- Calviere L, Nasr N, Arnaud C, et al. Prediction of delayed cerebral ischemia after subarachnoid hemorrhage using cerebral blood flow velocities and cerebral auto-regulation assessment. Neurocrit Care 32 (2015): 253-258.
- Budohoski K, Czosnyka M, Kirkpatrick PJ, et al. Bilateral failure of cerebral autoregulation is related to unfavorable outcome after subarachnoid hemorrhage. Neurocrit Care 22 (2015): 65-73.
- Budohoski K, Czosnyka M, Smielewski P, et al. Impairment of cerebral auto-regulation predicts delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 43 (2002): 3230-3237.
- Cahill J, Calvert JW, Zhang JH. Mechanism of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26 (2006): 1341-1353.
- Budohoski K, Czosnyka M, Kirkpatrick P, et al. Clinical relevance of cerebral autoregulation following subarachnoid hemorrhage. Nat Rev Neurol 9 (2013): 152-163.
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler recordings of flow velocity in basal cerebral arteries. J Neurosurg 57 (1982): 769-774.
- Czosnyka M, Smielewski P, Kirkpatrick P, et al. Monitoring of cerebral autoregulation in head-injured patients. Stroke 27 (1996): 1829-1834.
- Czosnyka M, Richards H, Pickard JD, et al. Frequency-dependent properties of cerebral blood transport-an experimental study in anaesthetized rabbits. Ultrasound Med Biol 20 (1994): 391-399.
- Varsos GV, Kasprowicz M, Smielewski P, et al. Model-based indices describing cerebrovascular dynamics. Neurocrit Care 20 (2014): 142-157.
- Kim DJ, Kasprowicz M, Carrera E, et al. The monitoring of relative changes in compartmental compliances of brain. Physiol Meas 30 (2009): 647-659.
- Kasprowicz M, Diedler J, Reinhard M, et al. Time constant of the cerebral arterial bed in normal subjects. Ultrasound Med Biol 38 (2012): 1129-1137.
- Nichol J, Girling F, Jerrard W, et al. Fundamental instability of the small blood vessels and critical closing pressure in vascular beds. Am J Physiol 164 (1951): 330-344.
- Dewey RC, Pierer HP, Hunt WE. Experimental cerebral hemodynamics. Vaso-motor tone, critical closing pressure and vascular bed resistance. J Neurosurg 41 (1974): 597-606.
- Czosnyka M, Smielewski P, Piechnik S, et al. Critical closing pressure in cerebro-vascular circulation. J Neurol Neurosurg Psychiatry 66 (1999): 606-611.
- Kasprowicz M, Czosnyka M, Soehle M, et al. Vasospasm shortens cerebral arterial time constant. Neurocrit Care 16 (2011): 213-218.
- Soehle M, Czosnyka M, Pickard JD, et al. Critical closing pressure in subarachnoid hemorrhage: effect of cerebral vasospasm and limitations of a transcranial Doppler-derived estimation. Stroke 35 (2004): 1393-1398.
- Varsos GV, Budohoski KP, Czosnyka M, et al. Cerebral vasospasm affects arterial critical closing pressure. J Cereb Blood Flow Metab 35 (2015): 285-291.
- Papaioannou V, Budohoski K, Placek M, et al. Association of transcranial doppler blood flow velocity slow waves with delayed cerebral ischemia in patients suffering from subarachnoid hemorrhage: a retrospective study. Intensive Care Med Exp 9 (2021): 11.
- Suarez JI, Qureshi AI, Yahia AB, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med 30 (2002): 1348-1355.
- Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir (Wien) 100 (1989): 12-24.
- Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41 (2010): 2391-295.
- Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for health care professionals from the American Heart Association/American Stroke Association. Stroke 43 (2012): 1711-1737.
- Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 32 (2001): 2012-2020.
- Avezaat CJJ, van Eijndhoven JHM. Cerebrospinal fluid pulse pressure and craniospinal dynamics. A theoretical and experimental study. Thesis. The Hague: A Jongbloed (1984).
- Sorrentino E, Budohoski KP, Kasprowicz M, et al. Critical thresholds for transcranial Doppler indices of cerebral autoregulation in traumatic brain injury. Neurocrit Care 14 (2011): 188-193.
- Soehle M, Czosnyka M, Pickard JD, et al. Continuous assessment of cerebral autoregulation in subarachnoid hemorrhage. Anesth Analg 98 (2004): 1133-1139.
- Aaslid R, Lash SR, Bardy GH, et al. Dynamic pressure-flow velocity relationships in the human cerebral circulation. Stroke 34 (2003): 1645-1649.
- Mitchel E, Hillebrand S, von Twickel J, et al. Frequency dependence of cerebro-vascular impedance in preterm neonates: a different view on critical closing pressure. J Cereb Blood Flow Metab 17 (1997): 1127-1131.
- Varsos GV, Richards H, Kasprowicz M, et al. Critical closing pressure determined with a model of cerebrovascular impedance. J Cereb Blood Flow Metab 33 (2012): 235-243.
- Varsos GV, Budohoski KP, Kolias AG, et al. Relationship of vascular wall tension and autoregulation following traumatic brain injury. Neurocrit Care 21 (2014): 266-274.
- Lewis PM, Smielewski P, Pickard JD, et al. Dynamic cerebral autoregulation: should intracranial pressure be taken into account? Acta Neuroschir (Wien) 149 (2007): 549-555.
- Czosnyka M, Brady K, Reinhard M, et al. Monitoring of cerebral autoregulation: Facts, myths, amd missing links. Neurocrit Care 10 (2009): 373-386.
- Lavinio A, Schmidt EA, Haubrich C, et al. Noninvasive evaluation of dynamic cerebrovascular autoregulation using Finapres plethysmograph and transcranial Doppler. Stroke 38 (2007): 402-404.