Region-Specific and Pregnancy-Enhanced Vasodilator Effects of Hydrogen Sulfide
Article Information
Pankaj Yadav1, Dong-Bao Chen2, Sathish Kumar1,3*
1Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin, Madison, Wisconsin, United States of America
2Department of Obstetrics & Gynecology, University of California, Irvine, California, United States of America
3Department of Obstetrics and Gynecology, School of Medicine and Public Health, University of Wisconsin, Madison, Wisconsin, United States of America
*Corresponding Author: Sathish Kumar, Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin, Madison, Wisconsin, United States of America.
Received: 07 December 2023; Accepted: 14 December 2023; Published: 22 December 2023
Citation:
Pankaj Yadav, Dong-Bao Chen, Sathish Kumar. Region-Specific and Pregnancy-Enhanced Vasodilator Effects of Hydrogen Sulfide. Obstetrics and Gynecology Research. 6 (2023): 309-316.
View / Download Pdf Share at FacebookAbstract
Hydrogen sulfide (H2S) is a cardiovascular signaling molecule that causes vasodilation in vascular smooth muscle cells, but its mechanism is unclear. We examined how H2S affects mesenteric and uterine arteries without endothelium in nonpregnant and pregnant rats and the underlying mechanisms. H2S donors GYY4137 and NaHS relaxed uterine arteries more than mesenteric arteries in both pregnant and nonpregnant rats. GYY4137 and NaHS caused greater relaxation in the uterine artery of pregnant versus nonpregnant rats. High extracellular K+ abolished NaHS relaxation in pregnant uterine arteries, indicating potassium channel involvement. NaHS relaxation was unaffected by voltage-gated potassium channel blockers, reduced by ATP-sensitive potassium channel blockers, and abolished by calcium-activated potassium (BKCa) channel blockers. Thiol-reductant dithiothreitol also prevented NaHS relaxation. Thus, H2S has region-specific and pregnancy-enhanced vasodilator effects in the uterine arteries, mainly mediated by BKCa channels and sulfhydration.
Keywords
H2S; artery; vasodilation; pregnant; potassium channels
H2S articles H2S Research articles H2S review articles H2S PubMed articles H2S PubMed Central articles H2S 2023 articles H2S 2024 articles H2S Scopus articles H2S impact factor journals H2S Scopus journals H2S PubMed journals H2S medical journals H2S free journals H2S best journals H2S top journals H2S free medical journals H2S famous journals H2S Google Scholar indexed journals artery articles artery Research articles artery review articles artery PubMed articles artery PubMed Central articles artery 2023 articles artery 2024 articles artery Scopus articles artery impact factor journals artery Scopus journals artery PubMed journals artery medical journals artery free journals artery best journals artery top journals artery free medical journals artery famous journals artery Google Scholar indexed journals vasodilation articles vasodilation Research articles vasodilation review articles vasodilation PubMed articles vasodilation PubMed Central articles vasodilation 2023 articles vasodilation 2024 articles vasodilation Scopus articles vasodilation impact factor journals vasodilation Scopus journals vasodilation PubMed journals vasodilation medical journals vasodilation free journals vasodilation best journals vasodilation top journals vasodilation free medical journals vasodilation famous journals vasodilation Google Scholar indexed journals pregnant articles pregnant Research articles pregnant review articles pregnant PubMed articles pregnant PubMed Central articles pregnant 2023 articles pregnant 2024 articles pregnant Scopus articles pregnant impact factor journals pregnant Scopus journals pregnant PubMed journals pregnant medical journals pregnant free journals pregnant best journals pregnant top journals pregnant free medical journals pregnant famous journals pregnant Google Scholar indexed journals potassium channels articles potassium channels Research articles potassium channels review articles potassium channels PubMed articles potassium channels PubMed Central articles potassium channels 2023 articles potassium channels 2024 articles potassium channels Scopus articles potassium channels impact factor journals potassium channels Scopus journals potassium channels PubMed journals potassium channels medical journals potassium channels free journals potassium channels best journals potassium channels top journals potassium channels free medical journals potassium channels famous journals potassium channels Google Scholar indexed journals normal pregnancy articles normal pregnancy Research articles normal pregnancy review articles normal pregnancy PubMed articles normal pregnancy PubMed Central articles normal pregnancy 2023 articles normal pregnancy 2024 articles normal pregnancy Scopus articles normal pregnancy impact factor journals normal pregnancy Scopus journals normal pregnancy PubMed journals normal pregnancy medical journals normal pregnancy free journals normal pregnancy best journals normal pregnancy top journals normal pregnancy free medical journals normal pregnancy famous journals normal pregnancy Google Scholar indexed journals uterus articles uterus Research articles uterus review articles uterus PubMed articles uterus PubMed Central articles uterus 2023 articles uterus 2024 articles uterus Scopus articles uterus impact factor journals uterus Scopus journals uterus PubMed journals uterus medical journals uterus free journals uterus best journals uterus top journals uterus free medical journals uterus famous journals uterus Google Scholar indexed journals uterine blood flow articles uterine blood flow Research articles uterine blood flow review articles uterine blood flow PubMed articles uterine blood flow PubMed Central articles uterine blood flow 2023 articles uterine blood flow 2024 articles uterine blood flow Scopus articles uterine blood flow impact factor journals uterine blood flow Scopus journals uterine blood flow PubMed journals uterine blood flow medical journals uterine blood flow free journals uterine blood flow best journals uterine blood flow top journals uterine blood flow free medical journals uterine blood flow famous journals uterine blood flow Google Scholar indexed journals third trimester articles third trimester Research articles third trimester review articles third trimester PubMed articles third trimester PubMed Central articles third trimester 2023 articles third trimester 2024 articles third trimester Scopus articles third trimester impact factor journals third trimester Scopus journals third trimester PubMed journals third trimester medical journals third trimester free journals third trimester best journals third trimester top journals third trimester free medical journals third trimester famous journals third trimester Google Scholar indexed journals developing fetus articles developing fetus Research articles developing fetus review articles developing fetus PubMed articles developing fetus PubMed Central articles developing fetus 2023 articles developing fetus 2024 articles developing fetus Scopus articles developing fetus impact factor journals developing fetus Scopus journals developing fetus PubMed journals developing fetus medical journals developing fetus free journals developing fetus best journals developing fetus top journals developing fetus free medical journals developing fetus famous journals developing fetus Google Scholar indexed journals
Article Details
Introduction
During a normal pregnancy, there is a significant increase in blood flow to the uterus, with a 20–80-fold rise in uterine blood flow observed in the third trimester of a singleton pregnancy [1]. This enhanced uterine perfusion is crucial for maintaining the health of both the mother and the developing fetus as it facilitates the transfer of nutrients and oxygen from the mother to the fetus while also removing carbon dioxide and metabolic waste products from the fetus. Proper uterine blood flow is essential for optimal fetal development and survival, and compromised blood flow has been implicated in various pregnancy-related conditions such as preeclampsia and intrauterine growth restriction [2-5].
The mechanisms governing the dilation of uterine arteries during pregnancy are intricate and not fully understood. However, evidence suggests a pivotal role for locally produced vasodilators in relaxing the smooth muscle of uterine arteries. Among these vasodilators, prostacyclin and nitric oxide (NO) have been extensively studied [6-8]. However, the inhibition of prostaglandin and NO synthesis does not entirely abolish the pregnancy-induced increase in uterine artery blood flow [9-11], suggesting the participation of additional mechanisms. Recent studies indicate an increase in the production of hydrogen sulfide (H2S) in uterine arteries during pregnancy, adding it to the family of gaseous signaling molecules that includes NO and carbon monoxide [12, 13].
H2S is synthesized from L-cysteine by specific enzymes, and its production in uterine arteries during pregnancy is primarily associated with the upregulation of cystathionine-β-synthase (CBS) [14]. Unlike systemic vasculature, where endothelial cell cystathionine-γ lyase (CSE) is the primary source of H2S, uterine artery H2S production is linked to both smooth muscle and endothelial CBS upregulation, and this unique H2S production is implicated in uterine vasodilation during pregnancy [12, 13]. Studies using a slow-releasing H2S donor further support the role of H2S as a novel uterine vasodilator [13].
The mechanism by which H2S induces uterine artery dilation is not fully understood. Previous research in mesenteric vascular beds has implicated the activation of ATP-sensitive potassium (KATP) channels and large conductance Ca2+-activated voltage-dependent potassium (BKCa) channels in mediating H2S-induced vasodilation [15-17]. However, the contribution of KATP channels to uterine vasodilation associated with pregnancy seems limited [15]. In contrast, BKCa channels have been shown to play a critical role in mediating H2S-induced vasodilation in rat mesenteric arteries [18]. This H2S-induced mesenteric vasodilation was lost in endothelium-denuded arteries [23], suggesting the presence of endothelium is essential for H2S-induced BKCa-mediated mesenteric vasodilation. In contrast, relaxation of the rat aorta and human uterine artery induced by H2S appears less dependent on the endothelium [17,19,20], indicating H2S directly acts on vascular smooth muscle cells to induce vasodilation. Although BKCa channels in vascular smooth muscle cells are implicated in pregnancy-induced uterine artery dilation [19,21-23], their involvement in H2S-induced vasodilation in rat arteries remains uncertain, and if their sensitivity differs between pregnancy and the nonpregnant state is unknown. Given the recognized heterogeneity in structure, functions, and pharmacological sensitivities of vascular beds, we sought to examine the impact of H2S on the vascular relaxation of peripheral resistance arteries, specifically the rat mesenteric and uterine arteries. Since H2S is proposed as a therapeutic vasodilator for preeclampsia, wherein the endothelial function is compromised [24-26], it is essential to investigate whether H2S directly influences vascular smooth muscle to induce vasodilation. Thus, in this study, we examined the vascular relaxation responses of H2S in endothelium-denuded mesenteric and uterine arteries isolated from both pregnant and nonpregnant (virgin) rats and the underlying mechanisms.
Methods
All the experiments were conducted as per National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996) with approval by the Institutional Animal Care and Use Committee at the University of Wisconsin at Madison (IACUC protocol V005847). Nonpregnant and timed pregnant Sprague-Dawley rats, purchased from Envigo Laboratories (Indianapolis, IN) on gestation day (GD) 16, were maintained under controlled conditions with a 12L:12D photoperiod in a temperature-regulated room (23°C). These rats were provided with ad libitum food and water. On GD20, pregnant and age-matched nonpregnant rats were euthanized using CO2 asphyxiation. Uterine and mesenteric arteries were isolated and immersed in the ice-cold Krebs physiological solution (KPS) (composition-118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 11 mM glucose, 0.026mM EDTA, 1.25 mM CaCl2; pH 7.4) for study of vascular response.
Ex-Vivo Vascular Reactivity
Arteries were cleaned from surrounding adventitial tissues, and 1-1.5 mm rings were made, and mounted in wire myograph (Danish Myo Technology, Aarhus, Denmark) using tungsten wires. The bath was filled with 6 ml of KPS continuously aired with carbogen gas (95% O2 and 5% CO2) and maintained at 37°C. The endothelium was denuded by gently rubbing the interior of the rings with tungsten wire. Rings were equilibrated for 1 hour in KPS and normalized using a specialized software package (Myodata V8.1.13; Danish Myo Technology). Arterial viability and strength were confirmed by depolarizing the rings with KCl 80 mM (two times). The denudation of the endothelial layer was established by the absence of relaxation response to acetylcholine in rings precontracted with a submaximal concentration of phenylephrine (PE). The concentration of PE that induced 80% of the maximal response was utilized for precontraction. Data acquisition was carried out using LabChart software (ADInstruments, Australia).
Assessment of Vascular Relaxation Responses
The arterial rings were subjected to an 80 mM KCl solution until reproducible contractions were observed. Following a subsequent round of washing and equilibration with KPS, the vascular contractile responses were done by exposing the rings to increasing concentrations of PE (10-9 to 10-5 M).
The direct effect of H2S on vascular smooth muscle relaxation was compared in endothelium-denuded mesenteric and uterine arterial rings of nonpregnant and pregnant rats, using two different H2S donors: GYY4137 (a slow-releasing compound; 10-10 – 10-5 M) and NaHS (a fast-releasing compound; 10-9 – 10-5 M) in PE precontracted arteries.
Assessment of H2S-induced Vascular Relaxation Pathways
The contribution of potassium channels in the H2S-induced vasorelaxation response was assessed by incubating the rings with specific potassium channel blockers, such as 4-aminopyridine (4-AP, voltage-gated potassium channel, 1 mM), glibenclamide (ATP-sensitive potassium channel, 10 µM), iberiotoxin plus apamin (large and intermediate conductance BKCa, potassium channels, 100 nM each), KCl (membrane depolarizer, 80 mM) for 20 min and then examining H2S-induced relaxation using NaHS (10-9 – 10-5 M) on PE precontracted arteries.
To assess the involvement of S-sulfhydration in H2S-induced relaxation, the arterial tissues were preindubated with dithiothreitol (DTT, 2 mM) for 20 min, and then relaxation response to NaHS (10-9 – 10-5 M) was carried out on PE pre-constricted arteries.
Statistical analysis
Analyses were performed using Prism (GraphPad 9, San Diego, CA, USA). Cumulative relaxation-response curves were fitted to a 4-parameter sigmoid curve for vascular reactivity. Relaxant responses were expressed as a percentage of relaxation from the PE-induced contraction. The data were presented as the mean ± standard error of the mean (SEM). Statistical significance was considered at a p-value of less than 0.05.
Results
H2S-induced vascular smooth muscle relaxation response in mesenteric artery and uterine artery from virgin and pregnant rat
In endothelium-denuded arterial rings isolated from nonpregnant rats and precontracted with PE, maximum relaxation to GYY4137 and NaHS was greater in the uterine artery versus the mesenteric artery (Figure 1A and Table 1). Similarly, in the rings of pregnant rats, GYY4137 and NaHS were more potent in causing relaxation in the uterine artery than in the mesenteric artery (Figure 1B and Table 1). The relaxation to GYY4137 and NaHS was not different in the mesenteric artery of pregnant versus virgin rats but was greater in the uterine artery of pregnant versus nonpregnant rats, indicating an enhanced sensitivity of uterine artery to H2S-induced relaxation during pregnancy (Figure 1 and Table 1).
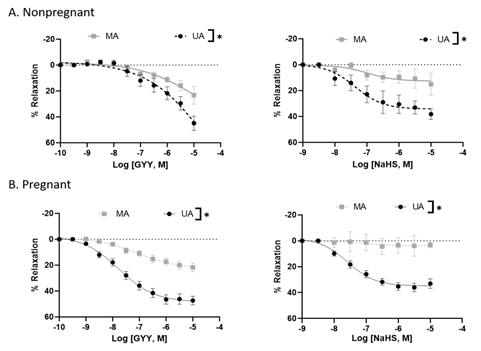
Figure 1: GYY4137 and NaHS-induced concentration-dependent relaxation responses in nonpregnant and pregnant rat mesenteric and uterine arterial rings pre-constricted with PE. The vertical bar represents mean ± S.E.M. (n= 6, *p < 0.05 UA vs. MA). MA – Mesenteric artery; UA – Uterine artery.
Table 1: GYY4137 and NaHS-induced relaxation of the mesenteric and uterine arteries in nonpregnant and pregnant rats.
|
Nonpregnant |
Pregnant |
|||
|
MA |
UA |
MA |
UA |
|
|
GYY4137-induced relaxation |
||||
|
Maximal relaxation |
23.28 ± 6.81 |
44.88 ± 5.35* |
21.70 ± 3.27 |
47.30 ± 3.28* |
|
NaHS-induced relaxation |
||||
|
Maximal relaxation |
15.11 ± 8.65 |
38.14 ± 4.07* |
3.19 ± 1.48 |
36.03 ± 3.22* |
*p < 0.05 UA vs MA in respective groups (n=6 in each group). MA – Mesenteric artery; UA – Uterine artery
Role of high K concentration on H2S-induced relaxation of pregnant uterine arteries
Because the uterine arteries of pregnant rats showed greater vascular relaxation than those of nonpregnant rats, we investigated the mechanisms of H2S relaxation in these arterial rings. A high K+ concentration (80 mM), which inhibits the opening of K channels involved in smooth muscle relaxation [27], completely blocked the relaxation response to NaHS and shifted the H2S response to slight vasoconstriction (Figure 2).
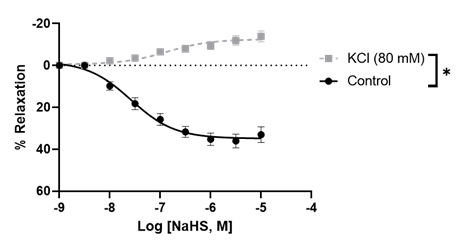
Figure 2: NaHS-induced concentration-dependent relaxation in pregnant rat uterine arterial rings in the presence and absence of high extracellular potassium concentration (80 mM). The vertical bar represents mean ± S.E.M. (n= 6, *p < 0.05 compared to control).
Role of specific potassium channels on H2S-induced relaxation of pregnant uterine arteries
The involvement of different potassium channels in the H2S-mediated relaxation of uterine arteries of pregnant rats was examined by using specific blockers. Blockade of the voltage-gated potassium channel with 4-AP (1 mM) did not affect the H2S-induced relaxation response (Figure 3A). Glibenclamide (10 µM) partially reduced the relaxation response of NaHS, suggesting that ATP-sensitive potassium channels have minimal contribution to H2S relaxation (Figure 3B). Iberiotoxin (100 nM) plus apamin (100 nM) completely abolished the relaxation response to NaHS (10-9 - 10-5 M) (Figure 3C), indicating that BKca channels were essential for H2S relaxation. These results demonstrate that H2S-induced relaxation of uterine arteries of pregnant rats is mediated by different types of potassium channels, especially BKCa channels (Figure 3 and Table 2).
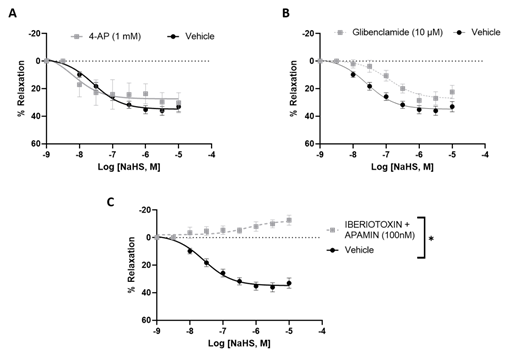
Figure 3: NaHS-induced concentration-dependent relaxation in pregnant rat uterine arterial rings pre-incubated with (A) 4-aminopyridine (1 mM), (B) Glibenclamide (10 µM) and (C) iberiotoxin (100 nM) plus apamin (100 nM) for 20 min and then pre-constricted with PE. The vertical bar represents mean ± S.E.M. (n= 6, *p < 0.05 compared to control).
Table 2: NaHS-induced response in the presence and absence of K+ channel blockers and KCl
|
Pregnant Uterine arteries |
Control |
4-AP |
Glibenclamide |
Iberiotoxin and apamin |
|
Maximal relaxation |
36.03 ± 3.22 |
30.22 ± 7.21 |
28.55 ± 4.94* |
-8.03 ± 3.77* |
*p < 0.05 compared to control (n = 6 in each group).
Effect of DTT on H2S-induced relaxation of pregnant uterine arteries
H2S exerts its biological effects by S-sulfhydrating cysteine residues on target proteins. Dithiothreitol (DTT) is a reducing agent that can inhibit S-sulfhydration. To test whether S-sulfhydration is involved in the relaxation response of uterine arteries to H2S, the arteries were preincubated with DTT (2 mM). DTT significantly decreased the relaxation response of NaHS from 43.38 ± 5.39% to 11.22 ± 2.88% (Figure 4), suggesting that blockade of S-sulfhydration activity and reduces H2S-induced vasodilation.
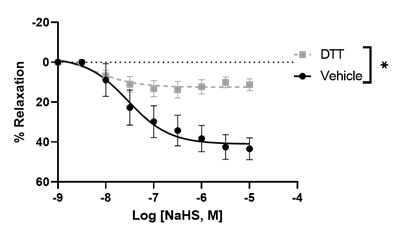
Figure 4: NaHS-induced concentration-dependent relaxation in pregnant rat uterine arterial rings was pre-incubated with dithiothreitol (DTT, 2 mM) for 20 min and then pre-constricted with PE. The vertical bar represents mean ± S.E.M. (n= 6, *p < 0.05 compared to control).
Discussion
The major findings of the study are, First, GYY4137 and NaHS evoked a concentration-dependent endothelium-independent vasorelaxation that was more pronounced in the uterine than mesenteric arteries. Second, high extracellular K+ concentrations abolished the NaHS-induced vasodilation of pregnant rat uterine arteries. Third, NaHS-induced relaxation in pregnant uterine arteries involved activation of KATP and BKCa channels. Fourth, suppression of S-sulfhydration reduced the relaxation of pregnant rat uterine arteries to NaHS. Our results demonstrated a vascular bed and pregnancy-specific relaxant response of arteries to H2S, with uterine arteries being more sensitive to H2S-induced relaxation during pregnancy. Stimulating KATP and BKCa channels and S-sulfhydration in vascular SMCs by H2S represent important cellular mechanisms for the H2S effect in the uterine artery during pregnancy. These data, together with previous studies in which blockade of H2S synthesis decreased pup weight and increased pup mortality in rats [28], suggest that the H2S may play an important role in maintaining relaxation of the uterine vascular bed and, therefore, in uterine blood flow in rats during pregnancy.
In this study, GYY4137 and NaHS induced only minimal relaxation of the vascular smooth muscle of mesenteric arteries. This is in contrast to prior studies, which demonstrated significant relaxation in mesenteric arteries when exposed to H2S [29-31]. The disparity in these findings may be attributed to differences in the mesenteric artery preparations utilized; our study employed endothelium-denuded rings, while earlier studies utilized endothelium-intact rings. The presence of endothelium appears to play a crucial role in H2S-induced relaxation in the mesenteric artery, as indicated by previous studies showing reduced H2S relaxations in preparations lacking endothelium in small mesenteric arteries and in the presence of an inhibitor of NO synthase [32]. This implies that endothelium-derived NO is involved in H2S relaxations in mesenteric arteries. Our study also provides evidence that H2S-induced relaxation in mesenteric arteries was not different between arteries from nonpregnant and pregnant rats, indicating that pregnancy does not influence H2S-induced mesenteric vasodilation during pregnancy. Thus, these findings suggest that H2S exerts only minimal direct vascular smooth muscle relaxation effects, and this effect remains unaltered by pregnancy. It would be interesting to examine whether the sensitivity of H2S vasodilation is affected by pregnancy in endothelium-intact mesenteric arteries.
On the other hand, H2S induced greater relaxation in the uterine than mesenteric arteries. Studies show that the uterine artery relaxes more than the mesenteric artery during pregnancy. This is because the uterine artery needs to accommodate the increased blood flow to the placenta and the fetus, while the mesenteric artery does not have such a demand. One study found that the uterine artery resistance index, which is a measure of vascular resistance, decreased significantly from early to late gestation, while the mesenteric artery resistance index did not change much [33]. Another study reported that the uterine artery pulsatility index, which is another measure of vascular resistance, decreased over gestation, while the mesenteric artery pulsatility index increased slightly [34]. These findings suggest that the uterine artery relaxes more than the mesenteric artery during pregnancy to facilitate placental perfusion and fetal growth. However, the mechanisms contributing to the enhanced vasodilation of the pregnant uterine arteries were unclear. Our finding that H2S produces greater relaxation in the uterine than mesenteric arteries and that the relaxation effect in the uterine arteries is further enhanced during pregnancy suggests that H2S could be an important factor in supporting gestational uterine vascular adaptations.
Various mechanisms have been proposed to elucidate the vasodilatory effects of H2S, depending on the specific vascular preparations under investigation. Previous studies focusing on resistance arteries have identified the involvement of KATP channels [16], BKCa channels [18, 35], and KV channels [29, 36] in H2S-induced vasodilation. Patch-clamp studies have demonstrated that H2S activates KATP channels in vascular smooth muscle from mesenteric arteries [16] and hyperpolarizes mesenteric arteries through an iberiotoxin-sensitive mechanism, indicating the participation of BKCa channels [18, 35]. In separate experiments, H2S was found to hyperpolarize rat aorta and directly activate KV channels in CHO cells [37]. Recent investigations have revealed that H2S donors directly activate KV channels, providing protection against neuropathic pain [38]. Additionally, direct persulfidation of KV channels by H2S has been implicated in skeletal muscle hypercontractility in human malignant hyperthermia syndrome [39]. In alignment with findings in pregnant uterine arteries, our study indicates that H2S-induced relaxations are sensitive to high extracellular potassium and are blocked by inhibitors of both ATP-sensitive and BKCa channels. This suggests the involvement of KATP and BKCa channels in H2S-mediated relaxation. Intriguingly, the presence of a KATP channel blocker has a lesser impact on the magnitude of H2S-induced relaxation compared to BKCa channel blockers, implying a predominant role of BKCa channels in mediating H2S-induced relaxation in pregnant rat uterine arteries. This aligns with prior research demonstrating that H2S enhances BKCa currents in a dose-dependent manner, leading to the relaxation of PE pre-constricted human uterine arterial rings, which was inhibited by the BKCa channel blocker [19].
Although our findings do not elucidate the mechanism by which H2S modulates BKCa channel activity, at least 2 possibilities exist. Firstly, BK channels are known to be regulated by zinc (Zn) through coordination with histidine and aspartic acid residues in the RCK1 (regulator of conductance for K+) domain of the channel [40]. Removal of Zn reduces channel activation, suggesting a potential structural arrangement for Zn binding by these residues [40]. Concurrently, H2S has been identified as a binder of Zn, capable of altering the activity of Zn-dependent proteins [41]. A second potential mechanism through which H2S may regulate BKCa channel activity is sulfhydration. Recent mass spectrometric analyses have unveiled the addition of extra sulfur to thiol (-SH) groups of cysteines, forming a hydropersulfide (-SSH) moiety [42]. This posttranslational modification affects a subset of proteins, including but not limited to GAPDH, β-tubulin, and actin, altering their functions [43]. Notably, our observation that inhibition of sulfhydration with DTT diminishes H2S-induced relaxation supports the hypothesis that H2S modifies critical cysteine residues regulating BK channel activity. This aligns with previous bioinformatics studies identifying pregnancy-dependent H2S-responsive human uterine artery SSH peptides/proteins, particularly those involved in vascular smooth muscle contraction/relaxation [44]. However, further verification is necessary to confirm whether H2S regulates the sulfhydration of BKCa channels and if this effect is heightened to induce more substantial vasodilation in pregnant rat uterine arteries.
In conclusion, H2S exhibit regional changes in their vasodilator activity in the vascular smooth muscle of the mesenteric artery and uterine artery. The H2S-induced vasodilator effect is enhanced in the uterine artery during gestation. The data also support the premise that H2S-mediated uterine vasodilatation may be mediated more by BKCa channels and sulfhydration. The study supports the proposition that pregnancy-associated changes in vascular H2S activity in the uterine versus systemic circulation could regulate uterine blood flow and distribution of blood in the fetoplacental unit by promoting vasodilation during normal pregnancy. Future studies should investigate the potential usefulness of H2S donors in promoting vasodilation and improving maternal hemodynamics and uterine artery blood flow in preeclamptic pregnancies.
Funding
The study was in part supported by the National Institutes of Health (NIH) grants R01ES033345 (to S.K.) and R01HL70562 (to D.B.C.).
Conflicts of interest:
None
References
- Browne, V.A., et al., Uterine artery blood flow, fetal hypoxia and fetal growth. Philos Trans R Soc Lond B Biol Sci 370 (2015): p. 20140068.
- Romero, R., S.K. Dey, S.J. Fisher, Preterm labor: one syndrome, many causes. Science 345 (2014): p.760-765.
- Osol, G. M. Mandala, Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24 (2009): p.58-71.
- Thornburg, K.L., et al., Hemodynamic changes in pregnancy. Semin Perinatol 24 (2004): p.11-14.
- Barker, D.J., Intrauterine programming of adult disease. Mol Med Today 1 (1995): p.418-423.
- Gokina, N.I., O.Y. Kuzina, A.M. Vance, Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol 299 (2010): p.H1642-1652.
- Magness, R.R., et al., Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol 272 (1997): p.H1730-1740.
- Kimura, T., Y. Yoshida, N. Toda, Mechanisms of relaxation induced by prostaglandins in isolated canine uterine arteries. Am J Obstet Gynecol 167 (1992): p.1409-1416.
- Naden, R.P., et al., Hemodynamic effects of indomethacin in chronically instrumented pregnant sheep. Am J Obstet Gynecol 151 (1985): p.484-494.
- Miller, S.L., G. Jenkin, D.W. Walker, Effect of nitric oxide synthase inhibition on the uterine vasculature of the late-pregnant ewe. Am J Obstet Gynecol 180 (1999): p.1138-1145.
- Rosenfeld, C.R., et al., Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest 98 (1996): p.2158-2166.
- Lechuga, T.J., et al., Ovine uterine artery hydrogen sulfide biosynthesis in vivo: effects of ovarian cycle and pregnancydagger. Biol Reprod 100 (2019): p.1630-1636.
- Sheibani, L., et al., Augmented H2S production via cystathionine-beta-synthase upregulation plays a role in pregnancy-associated uterine vasodilation. Biol Reprod 96 (2017): p.664-672.
- Kimura, H., Hydrogen sulfide and polysulfides as biological mediators. Molecules 19 (2014): p.16146-16157.
- Xiao, D., L.D. Longo, L. Zhang, Role of KATP and L-type Ca2+ channel activities in regulation of ovine uterine vascular contractility: effect of pregnancy and chronic hypoxia. Am J Obstet Gynecol 203 (2010): p.596 e6-12.
- Tang, G., et al., Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol 68 (2005): p.1757-1764.
- Zhao, W., et al., The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20 (2001): p.6008-6016.
- Jackson-Weaver, O., et al., Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca(2)(+)-activated K(+) channels and smooth muscle Ca(2)(+) sparks. Am J Physiol Heart Circ Physiol 304 (2013): p.H1446-1454.
- Li, Y., et al., Hydrogen Sulfide Relaxes Human Uterine Artery via Activating Smooth Muscle BK(Ca) Channels. Antioxidants (Basel) 9 (2020): 1127.
- Tang, G., et al., H(2)S is an endothelium-derived hyperpolarizing factor. Antioxid Redox Signal 19 (2013): p.1634-1646.
- Hu, X.Q., et al., Pregnancy Reprograms Large-Conductance Ca(2+)-Activated K(+) Channel in Uterine Arteries: Roles of Ten-Eleven Translocation Methylcytosine Dioxygenase 1-Mediated Active Demethylation. Hypertension 69 (2017): p.1181-1191.
- Rosenfeld, C.R., et al., Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J Soc Gynecol Investig 12 (2005): p.402-408.
- Rosenfeld, C.R., D.N. Cornfield, T. Roy, Ca(2+)-activated K(+) channels modulate basal and E(2)beta-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol 281 (2001): p.H422-431.
- Saif, J., et al., Hydrogen sulfide releasing molecule MZe786 inhibits soluble Flt-1 and prevents preeclampsia in a refined RUPP mouse model. Redox Biol 38 (2021): p.101814.
- Rezai, H., et al., MZe786, a hydrogen sulfide-releasing aspirin prevents preeclampsia in heme oxygenase-1 haplodeficient pregnancy under high soluble flt-1 environment. Redox Biol 38 (2021): p.101768.
- Wang, K., et al., Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 127(2013): p.2514-2522.
- Dogan, M.F., et al., Potassium channels in vascular smooth muscle: a pathophysiological and pharmacological perspective. Fundam Clin Pharmacol 33 (2019): p.504-523.
- Yakovleva, O.V., et al., Hydrogen Sulfide Ameliorates Developmental Impairments of Rat Offspring with Prenatal Hyperhomocysteinemia. Oxid Med Cell Longev (2018): p.2746873.
- Hedegaard, E.R., et al., Involvement of Potassium Channels and Calcium-Independent Mechanisms in Hydrogen Sulfide-Induced Relaxation of Rat Mesenteric Small Arteries. J Pharmacol Exp Ther 356 (2016): p.53-63.
- d'Emmanuele di Villa Bianca, R., et al., Hydrogen sulfide-induced dual vascular effect involves arachidonic acid cascade in rat mesenteric arterial bed. J Pharmacol Exp Ther 337 (2011): p.59-64.
- Ali, M.Y., et al., Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol 149 (2006): p.625-634.
- Li, L., et al., Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117 (2008): p.2351-2360.
- Marshall, S.A., et al., Relaxin Deficiency Leads to Uterine Artery Dysfunction During Pregnancy in Mice. Front Physiol 9 (2018): p.255.
- Tian, Y, X, Yang. A Review of Roles of Uterine Artery Doppler in Pregnancy Complications. Front Med (Lausanne) 9 (2022): p.813343.
- Jackson-Weaver, O., et al., Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ Res 108 (2011): p.1439-1447.
- Schleifenbaum, J., et al., Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens 28 (2010): p.1875-1882.
- Martelli, A., et al., Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol Res 70 (2013): p.27-34.
- Di Cesare Mannelli, L., et al., Effects of natural and synthetic isothiocyanate-based H(2)S-releasers against chemotherapy-induced neuropathic pain: Role of Kv7 potassium channels. Neuropharmacology 121 (2017): p.49-59.
- Vellecco, V., et al., Anomalous K(v) 7 channel activity in human malignant hyperthermia syndrome unmasks a key role for H(2) S and persulfidation in skeletal muscle. Br J Pharmacol 177 (2020): p.810-823.
- Hou, S., et al., Zn2+ activates large conductance Ca2+-activated K+ channel via an intracellular domain. J Biol Chem, 2010. 285(9): p.6434-6442.
- Szabo, C., Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6 (2007): p.917-935.
- Mustafa, A.K., et al., H2S signals through protein S-sulfhydration. Sci Signal 2(2009): p.ra72.
- Miyamoto, R., et al., Polysulfides (H(2)S(n)) produced from the interaction of hydrogen sulfide (H(2)S) and nitric oxide (NO) activate TRPA1 channels. Sci Rep 7 (2017): p.45995.
- Bai, J., et al., Mapping Pregnancy-dependent Sulfhydrome Unfolds Diverse Functions of Protein Sulfhydration in Human Uterine Artery. Endocrinology 164 (2023): bqad107.
