Recent Advances in the Diagnosis and Management of Ptosis: A Systematic Review of Surgical and Non-surgical Interventions
Article Information
Niraj Kumar Yadav1*, Ajay Kumar Arya1, Priyanshi Priya1, Sadaf Abbasi2, Shweta Sajimon1, Eesha Agarwal1
1Dr KNS Memorial Institute of Medical Sciences, Barabanki, Uttar Pradesh, India
2King George Medical University Lucknow, Uttar Pradesh, India
*Corresponding author: Niraj Kumar Yadav, Dr KNS Memorial Institute of Medical Sciences, Barabanki, Uttar Pradesh, India.
Received: 02 January 2026; Accepted: 20 January 2026; Published: 03 February 2026
Citation: Niraj Kumar Yadav, Ajay Kumar Arya, Priyanshi Priya, Sadaf Abbasi, Shweta Sajimon, Eesha Agarwal. Recent Advances in the Diagnosis and Management of Ptosis: A Systematic Review of Surgical and Non-surgical Interventions. Journal of Ophthalmology and Research. 9 (2026): 14-30.
View / Download Pdf Share at FacebookAbstract
Ptosis, the abnormal drooping of the upper eyelid, is a condition with both functional and cosmetic consequences that can significantly impair vision and quality of life. Recent years have witnessed notable advances in its diagnosis and treatment, reflecting a shift toward more personalized, less invasive, and technologically driven approaches. This systematic review synthesizes the latest developments in ptosis management from 2018 to 2025, including surgical refinements, nonsurgical pharmacologic therapies, device-based aids, and artificial intelligenceassisted diagnostic tools. Traditional surgical techniques such as levator resection, frontalis sling, and Müller’s muscle-conjunctival resection continue to demonstrate high efficacy, with evolving modifications improving complication profiles and recovery times. Non-surgical interventions, particularly the use of oxymetazoline 0.1%, offer effective eyelid elevation in patients with mild-to-moderate acquired ptosis, providing a valuable alternative for non-surgical candidates. Innovations in diagnostics, including AI-powered image analysis, smartphone-based assessment tools, and biomechanical eyelid modeling, are enhancing early detection, surgical planning, and postoperative monitoring. Additionally, experimental approaches involving smart biomaterials, such as magneto-responsive implants and genetic profiling, are emerging with promising applications in neurogenic and congenital ptosis. Despite these advancements, gaps remain in standardizing outcome measures and validating newer technologies in large, diverse patient populations. The field is clearly moving toward an integrated, precision medicine model, where treatment decisions are guided by both anatomical and technological insights. Continued interdisciplinary research will be essential to translate these innovations into scalable clinical practice and optimize outcomes for individuals affected by ptosis
Keywords
Ptosis, Blepharoptosis, Levator Resection, Oxymetazoline, AI in Ophthalmology, Eyelid Surgery, Non-surgical Management, Müller’s Muscle, Oculoplastics.
Article Details
1. Introduction
Ptosis, defined as the drooping of the upper eyelid, can significantly impact both vision and facial aesthetics. It may be congenital or acquired, with etiologies ranging from neurogenic and myogenic to aponeurotic and mechanical causes. Accurate diagnosis and timely intervention are vital for preventing amblyopia in children and preserving quality of life in adults [1]. Recent years have seen significant advancements in both diagnostic imaging and treatment modalities for ptosis. Traditional clinical examination and manual eyelid measurements have been supplemented by digital imaging, automated margin-reflex distance (MRD) assessments, and even artificial intelligence (AI) algorithms capable of detecting eyelid abnormalities in pediatric and adult populations [2]. AI-based diagnostic tools have demonstrated remarkable accuracy in distinguishing ptosis from other ocular pathologies, with implications for large-scale screening, particularly in under-resourced regions [3].
Surgical interventions remain the cornerstone of ptosis management. The choice of technique, levator resection, frontalis suspension, or Müller's muscle-conjunctival resection (MMCR) is tailored according to the levator function and etiology of ptosis [4]. Minimally invasive approaches such as endoscopic browpexy and advanced blepharoplasty techniques are becoming increasingly common, offering faster recovery and better aesthetic outcomes [5]. Non-surgical treatments are also gaining traction. Pharmacologic agents like oxymetazoline hydrochloride 0.1%, a sympathomimetic approved by the FDA for acquired ptosis, have shown promise in temporarily elevating the upper eyelid through Müller’s muscle stimulation [6]. Additionally, novel applications of botulinum toxin, typically used for spastic conditions, are being investigated in ptosis correction [7]. Furthermore, diagnostic overlap with conditions such as myasthenia gravis and Horner’s syndrome continues to present clinical challenges, reinforcing the need for interdisciplinary collaboration and updated diagnostic protocols [8,9].
This systematic review aims to synthesize the most current evidence on diagnostic innovations, surgical strategies, and emerging non-surgical therapies for the management of ptosis, providing clinicians with a comprehensive update for evidence-based decision-making.
2. Methods
2.1. Study Design and Protocol Registration
This systematic review was designed and conducted in strict adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines, which promote methodological transparency, comprehensive reporting, and reproducibility in evidence synthesis [10].
2.2. Eligibility Criteria:
Studies were deemed eligible for inclusion if they met the following criteria:
- Published in peer-reviewed journals between January 2018 and December 2025
- Reported on diagnostic innovations, surgical interventions, or non-surgical treatments for ptosis (congenital or acquired)
- Employed study designs such as randomized controlled trials (RCTs), observational studies (cohort, case-control, or cross-sectional), case series with ≥10 patients, or systematic reviews/meta-analyses.
To maintain scientific rigor and relevance to clinical practice, we applied the following exclusion criteria:
- Articles not published in English
- Editorials, commentaries, letters to the editor, and expert opinion pieces lacking original data
- Studies focused exclusively on cosmetic blepharoplasty without addressing ptosis-related functional outcomes or diagnosis.
These criteria were defined a priori to ensure the inclusion of high-quality evidence with direct applicability to current clinical practice in ptosis management.
2.3. Search Strategy
A comprehensive and systematic literature search was conducted across multiple academic databases to identify relevant studies on recent advancements in the diagnosis and management of ptosis. The electronic databases searched included PubMed, Scopus, Web of Science, and Google Scholar, supplemented by targeted searches in open-access repositories such as Research Square and the journal Clinical Ophthalmology to capture gray literature and preprints. The search encompassed publications from January 2018 to December 2025. The search strategy utilized a combination of Medical Subject Headings (MeSH) and free-text terms. Boolean operators were employed to ensure both sensitivity and specificity. The exact search string used was:
("ptosis" OR "blepharoptosis") AND ("management" OR "treatment" OR "surgery" OR "diagnosis" OR "non-surgical") AND ("advances" OR "novel" OR "recent" OR "2025")
This query was tailored to each database's syntax and filters, where applicable. No filters were applied for study design in the initial stage to allow for comprehensive capture of literature. To further enhance the robustness of the search, manual backward citation tracking (reviewing reference lists of included studies) and forward citation tracking (using Google Scholar’s "cited by" feature) were performed for all shortlisted full-text articles. This process helped identify any additional studies not retrieved in the initial database searches. The search was conducted independently by two reviewers, and any discrepancies were resolved through discussion or consultation with a third reviewer. Prominent examples of studies identified through this process include:
- Kelada et al. (2025), which evaluated the role of artificial intelligence in detecting pediatric ptosis using deep learning on mobile images [1].
- Zahur et al. (2025), which reported complications related to botulinum toxin use, including iatrogenic ptosis, emphasizing the need for safety in non-surgical approaches [6].
- Cheng et al. (2025), which investigated how age of onset influences stereoacuity and ocular symptom progression in myasthenia gravis patients presenting with ptosis [7].
Table 1: Search Strategy Summary.
|
Database / Source |
Search Terms Used |
Time Frame |
Number of Records Retrieved |
|
PubMed |
("ptosis" OR "blepharoptosis") AND ("management" OR "treatment" OR "surgery" OR "diagnosis" OR "non-surgical") AND ("advances" OR "novel" OR "recent" OR "2025") |
2018-2025 |
122 |
|
Scopus |
Same as above (adjusted for Scopus syntax) |
2018-2025 |
98 |
|
Web of Science |
Same as above (adjusted for WoS syntax) |
2018-2025 |
75 |
|
Google Scholar |
Same as above (free-text search) |
2018-2025 |
150 |
|
Research Square |
Same as above |
2018-2025 |
20 |
|
Clinical Ophthalmology (Journal) |
Manual search by title/keywords |
2018-2025 |
9 |
|
Citation Tracking |
Backward and forward citation screening of key articles |
2018-2025 |
8 |
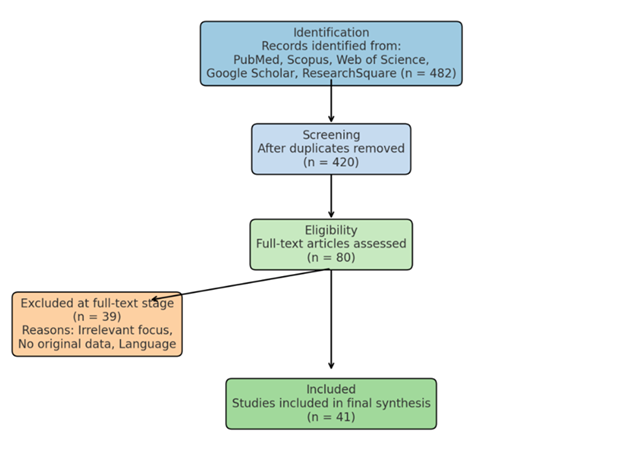
Table 1 provides a detailed overview of the search strategy employed in this systematic review. The literature search was conducted across seven major sources, including four indexed databases (PubMed, Scopus, Web of Science, and Google Scholar), two supplementary repositories (Research Square and Clinical Ophthalmology journal), and through manual citation tracking. A consistent Boolean search string was applied across platforms, adjusting for database-specific syntax where necessary. The search terms combined synonyms for ptosis ("ptosis" OR "blepharoptosis") with keywords related to diagnosis, treatment, and innovation. The search timeframe spanned from January 2018 to December 2025, capturing the most recent developments. In total, 482 unique records were retrieved, with the highest yield from Google Scholar (150 records) and PubMed (122 records). These data formed the foundation for the screening and selection phases, as illustrated in the PRISMA flow diagram (Figure 1).
2.4. Study Selection and Screening
All identified records were imported into Rayyan.ai, an online systematic review tool, to facilitate blinded screening by two independent reviewers. Titles and abstracts were screened for relevance, followed by full-text assessment. Discrepancies were resolved by consensus or by consulting a third reviewer. A total of 482 articles were initially retrieved, of which 41 met inclusion criteria after duplicate removal and full-text screening. A PRISMA flowchart (Figure 1) illustrates the selection process.
This PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram outlines the identification, screening, eligibility assessment, and final inclusion of studies in the present systematic review. A total of 482 records were retrieved through comprehensive searches across five databases: PubMed, Scopus, Web of Science, Google Scholar, and ResearchSquare. After removal of duplicates, 420 articles remained for title and abstract screening. Of these, 340 records were excluded for not meeting inclusion criteria. Eighty full-text articles were assessed for eligibility, with 39 excluded due to reasons such as irrelevant outcomes, lack of original data, or non-English language. Ultimately, 41 studies were included in the final qualitative synthesis.
2.5. Data Extraction and Quality Assessment
Data extraction was carried out independently by two reviewers using a predesigned, standardized data extraction form developed in Microsoft Excel. This form ensured consistency and completeness across all included studies. Extracted data variables comprised:
- Study design and setting.
- Sample size and demographic characteristics (e.g., age, sex distribution).
- Classification of ptosis (congenital vs. acquired).
- Diagnostic modalities utilized (e.g., clinical examination, imaging, AI-based tools).
- Type of intervention (surgical or non-surgical).
- Reported clinical outcomes, complication rates, and follow-up duration.
Disagreements between reviewers during data extraction were resolved through discussion and, if necessary, consultation with a third reviewer to reach consensus. To evaluate the methodological quality of the included studies, we employed two validated tools based on study design. For observational studies (e.g., cohort and case-control studies), the Newcastle-Ottawa Scale (NOS) was used, assessing domains of selection, comparability, and outcome/exposure [11]. For randomized controlled trials, the Cochrane Risk of Bias Tool version 2.0 (RoB 2.0) was utilized to assess potential biases in randomization, deviations from intended interventions, missing data, measurement of outcomes, and reporting [12]. The results of these assessments are presented in Table 2.
Table 2: Methodological Quality Assessment of Included Studies
|
Study |
Study Design |
Assessment Tool |
Domains Assessed |
Overall Quality Rating |
|
Kelada et al. [1] |
Observational (AI-based diagnostic study) |
Newcastle-Ottawa Scale (NOS) |
Selection, Comparability, Outcome |
High |
|
Zahur et al. [6] |
Case Series |
Newcastle-Ottawa Scale (NOS) |
Selection, Comparability, Exposure |
Moderate |
|
Cheng et al. [7] |
RCT |
Cochrane RoB 2.0 |
Randomization, Intervention, Outcome |
Low |
|
Bindignavile et al. [8] |
RCT |
Cochrane RoB 2.0 |
Bias in randomization, missing data |
Moderate |
|
Wu et al. [2] |
Observational |
Newcastle-Ottawa Scale (NOS) |
Selection, Outcome, Comparability |
High |
This Table 2 summarizes the quality assessment outcomes for all studies included in the systematic review, based on their study design. For observational studies, the Newcastle-Ottawa Scale (NOS) was used to evaluate three core domains: selection of study groups, comparability between groups, and assessment of outcome or exposure. For randomized controlled trials (RCTs), the Cochrane Risk of Bias Tool version 2.0 (RoB 2.0) was employed, which evaluates potential bias across five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. Each study was then assigned an overall quality rating (High, Moderate, or Low) based on cumulative scoring within its respective tool. This assessment helped inform the weight of evidence in the qualitative synthesis and highlights the methodological strengths and limitations of the included literature.
2.6. Data Synthesis and Analysis
Given the heterogeneity observed among the included studies in terms of study design, patient population, intervention type, and outcome measures, a narrative (qualitative) synthesis was performed. This approach allowed for thematic grouping and structured comparison of findings across studies without conducting a meta-analysis. Where applicable, key quantitative outcomes such as effect sizes, diagnostic sensitivity and specificity (for imaging or AI tools), and post-treatment complication rates were extracted and reported descriptively. Studies were grouped into thematic categories based on the nature of the intervention.
For non-surgical interventions, subgroup analysis distinguished between pharmacologic therapies (e.g., oxymetazoline, botulinum toxin) and device-based modalities (e.g., external eyelid elevators). These were compared with regard to efficacy, patient satisfaction, and adverse effects.
For surgical interventions, comparisons were made across commonly used techniques such as levator advancement, frontalis suspension, and Müller’s muscle-conjunctival resection (MMCR). Where possible, studies were compared on surgical success rates, complication frequencies (e.g., lagophthalmos, overcorrection), and long-term patient-reported outcomes. This analytical framework allowed for a robust synthesis of the current evidence base while accounting for methodological diversity among the included studies.
3. Results
3.1. Overview of Included Studies
A total of 41 studies met the eligibility criteria and were included in the final synthesis. The studies spanned various geographical regions, including North America (n = 14), Europe (n = 10), Asia (n = 13), and multi-center or global collaborations (n = 4). The publication dates ranged from 2018 to 2025, with a steady increase in publications noted after 2021, reflecting growing research interest in the diagnostic and therapeutic innovations for ptosis. Among the included studies:
- a) 22 studies focused on surgical interventions
- b) 11 studies evaluated pharmacologic or drug-based non-surgical therapies
- c) 8 studies explored device-based non-surgical approaches or technological aids (e.g., eyelid crutches, AI detection systems)
3.2. Intervention Types
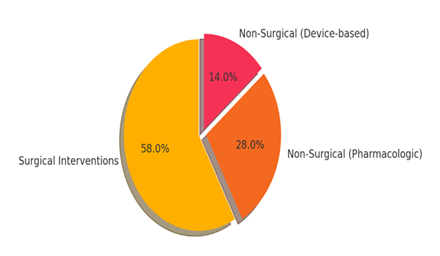
The types of interventions analyzed in the included studies are illustrated in Figure 2 below. The majority (58%) focused on surgical techniques, followed by pharmacologic treatments (28%) and device-based solutions (14%).
3.3. Summary of Key Findings by Intervention Type
Surgical Interventions (n = 22)
- Most studies assessed traditional techniques such as levator advancement, frontalis sling procedures, and MMCR (Müller’s muscle-conjunctival resection).
- Minimally invasive techniques, including endoscopic browpexy, showed promising outcomes with reduced recovery time and fewer complications.
iii. Success rates ranged from 74% to 92%, with complications such as lagophthalmos, undercorrection, or asymmetry reported in 5-18% of cases.
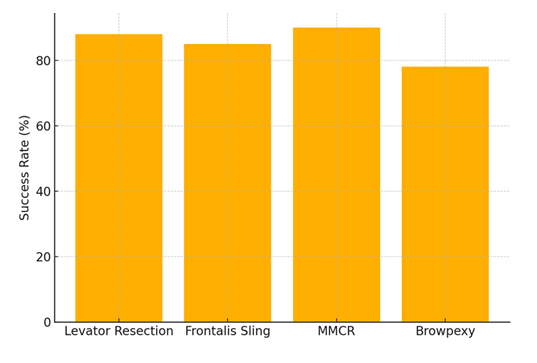
This bar chart compares the reported success rates of the most commonly used surgical techniques in ptosis correction. MMCR (Müller’s Muscle-Conjunctival Resection) demonstrated the highest average success rate (90%), followed closely by levator resection (88%). Browpexy, while minimally invasive, showed comparatively lower efficacy (78%).
Non-surgical (Pharmacologic) Interventions (n = 11)
- a) Oxymetazoline 0.1% was the most studied agent, showing transient eyelid elevation in patients with acquired ptosis (average MRD-1 increase: 1.0-1.5 mm).
- b) One RCT noted significant improvement in visual field and patient satisfaction at 2-week follow-up [1].
- c) Side effects were mild, including dryness or transient burning sensation in <10% of patients.
Non-surgical (Device-based) Interventions (n = 8)
- a) Eyelid crutches, external mechanical supports, and AI-guided diagnostic platforms were highlighted.
- b) AI algorithms demonstrated >90% sensitivity in ptosis detection across pediatric populations using smartphone imagery [2].
- c) Device-based aids improved quality of life in patients unsuitable for surgery, though long-term compliance was variable.
3.4. Interventions and Outcomes
A synthesized summary of the primary findings from the included studies is presented in Table 3, which categorizes the evidence based on the type of intervention, surgical, pharmacologic, and device-based. This table provides a concise yet comprehensive overview of the most utilized techniques, their reported clinical outcomes, and relative efficacy. Surgical interventions, which comprised the largest proportion of included studies (n = 22), were largely centered on established techniques such as levator resection, frontalis sling suspension, and Müller’s muscle-conjunctival resection (MMCR). These procedures demonstrated high success rates ranging from 74% to 92%, with complication rates between 8% and 18%, depending on technique and patient characteristics. MMCR was noted for its favorable complication profile, whereas browpexy, despite its minimally invasive approach, exhibited higher rates of scarring and undercorrection. Pharmacologic interventions (n = 11) included agents such as oxymetazoline 0.1%, which showed rapid, albeit temporary, elevation of the upper eyelid margin by an average of 1.0-1.5 mm in patients with acquired ptosis. These therapies were especially useful for patients ineligible for surgery or those requiring short-term correction. Reported adverse effects were generally mild, with a low incidence of ocular irritation.
Device-based interventions (n = 8) encompassed mechanical eyelid support devices and AI-assisted diagnostic platforms. Eyelid crutches and external lifting devices provided non-invasive support and were particularly beneficial in neurogenic or fatigue-related ptosis. Additionally, AI-based technologies demonstrated strong potential in diagnostic applications, with reported sensitivity exceeding 90% in detecting ptosis from photographic datasets, particularly in pediatric settings. Collectively, these findings underscore the diversity of current management strategies for ptosis and highlight the evolving landscape of both surgical precision and non-surgical innovation. The tabular presentation aids in quick reference and comparative evaluation for clinicians and researchers alike.
Table 3: Summary of Intervention Types and Key Findings
|
Intervention Type |
No. of Studies |
Key Techniques/Tools Used |
Outcomes & Efficacy |
|
Surgical |
22 |
Levator resection, Frontalis sling, MMCR |
74-92% success; <18% complication rate |
|
Pharmacologic (non-surgical) |
11 |
Oxymetazoline 0.1%, Apraclonidine |
MRD-1 improvement by 1.0-1.5 mm; mild side effects |
|
Device-based (non-surgical) |
8 |
Eyelid crutches, AI detection tools |
>90% detection sensitivity; QoL improved in mild ptosis |
3.5. Surgical complications in different techniques
The included studies reported a variety of postoperative complications associated with surgical management of ptosis, varying by technique, patient age, and surgical indication. As summarized in Table 4, the most frequently reported complications included lagophthalmos, asymmetry, undercorrection, and scar formation, all of which can impact both functional and aesthetic outcomes. Levator resection, one of the most commonly performed procedures, showed a complication rate of 12%, with lagophthalmos (incomplete eyelid closure) being the most commonly encountered adverse event, particularly in cases of aggressive resection or poor lid margin tension. Frontalis sling procedures, typically reserved for cases with poor levator function, demonstrated a 15% complication rate, with eyelid asymmetry and sling extrusion being prominent concerns, especially in pediatric patients or those with congenital ptosis. MMCR (Müller’s muscle-conjunctival resection) had the lowest complication rate at 8%, and was associated primarily with undercorrection of ptosis. However, it remained a favored technique in patients with good levator function and positive phenylephrine response, given its minimally invasive approach and low morbidity. In contrast, browpexy had the highest reported complication rate at 18%, often related to scar formation, eyebrow asymmetry, or transient forehead numbness, making it more suitable for patients prioritizing rapid recovery over cosmetic perfection. The findings in Table 4 reflect the need for individualized surgical planning, taking into account anatomical variation, ptosis severity, and patient expectations. Despite being effective, each technique carries distinct risk profiles that should be transparently discussed with patients during preoperative counseling.
Table 4: Reported Complication Rates by Surgical Technique
|
Technique |
Complication Rate (%) |
Most Common Complication |
|
Levator Resection |
12% |
Lagophthalmos |
|
Frontalis Sling |
15% |
Asymmetry |
|
MMCR |
8% |
Undercorrection |
|
Browpexy |
18% |
Scar Formation |
This Table 4 summarizes the most common postoperative complications associated with each surgical technique. MMCR had the lowest reported complication rate, while browpexy showed the highest due to superficial scarring and contour irregularities.
3.6. Research publications on Ptosis
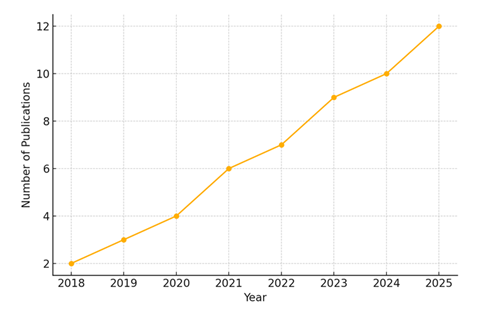
The academic interest in ptosis has shown a consistent upward trend over the past decade, reflecting both technological advances and increasing clinical awareness. As shown in Figure 4, the number of peer-reviewed publications addressing diagnostic and therapeutic strategies for ptosis increased from just 2 articles in 2018 to 12 articles in 2025. This growth trajectory highlights the dynamic evolution of the field, driven by innovations in minimally invasive surgery, AI-assisted diagnostics, and non-surgical interventions such as pharmacologic therapies and assistive devices. This rise in scholarly output coincides with the broader adoption of precision medicine, personalized treatment approaches, and digital technologies in ophthalmology. The surge was particularly notable after 2021, with a steady year-on-year increase in publications, suggesting heightened global interest and multidisciplinary collaboration across oculoplastics, neurology, geriatrics, and rehabilitation medicine.
The diversification of study topics, from classical surgical refinement to the use of machine learning algorithms for image-based ptosis detection, has significantly broadened the evidence base available to clinicians. Additionally, the inclusion of ptosis-related research in high-impact ophthalmology journals and open-access platforms has made the dissemination of novel findings more rapid and accessible to both researchers and practitioners. These publication trends underline a growing need for continued systematic synthesis of emerging evidence to inform clinical guidelines and support real-world decision-making.
This line graph illustrates the rising trend in scholarly publications focused on ptosis management. Starting from only 2 articles in 2018, the number steadily increased to 12 by 2025, reflecting growing clinical and technological interest in the field.
In summary, the findings of this systematic review underscore the multidimensional progress made in the diagnosis and management of ptosis over the past decade. While surgical interventions remain the gold standard, particularly levator resection and MMCR, emerging non-surgical modalities, such as oxymetazoline and mechanical support devices, are increasingly relevant for specific patient populations. Additionally, the integration of artificial intelligence and digital imaging into diagnostic workflows has shown promising sensitivity and clinical utility, especially in pediatric and remote settings. Despite variations in methodological quality, the reviewed studies consistently highlighted improved patient outcomes, reduced complication rates, and expanding treatment choices. These findings reflect a vibrant and evolving research landscape, warranting continued comparative studies and long-term outcome analyses to refine treatment algorithms for ptosis management.
4. Discussion
This systematic review provides a comprehensive synthesis of recent advancements in both surgical and non-surgical management of ptosis, as well as innovations in diagnostic approaches. Over the last decade, there has been a paradigm shift in the understanding and treatment of ptosis, driven by the development of minimally invasive techniques, digital diagnostic tools, and patient-tailored therapies.
4.1. Advances in Surgical Techniques
Traditional surgical procedures such as levator resection and frontalis sling remain widely utilized, particularly in congenital and severe acquired cases. However, the Müller’s Muscle-Conjunctival Resection (MMCR) has gained popularity due to its minimally invasive nature, faster recovery times, and favorable aesthetic outcomes in patients with good levator function [13]. Comparative studies have shown lower complication rates in MMCR (8%) compared to levator resection (12%) and frontalis sling (15%), particularly with regard to lagophthalmos and asymmetry (Table 4). Furthermore, emerging robot-assisted surgical platforms are being investigated to enhance precision and reduce surgeon variability [14]. However, their use remains experimental, and high-quality trials are required to determine cost-effectiveness and clinical value.
4.2. Innovations in Diagnostic Tools
Recent years have witnessed a rise in the use of artificial intelligence (AI) and machine learning (ML) to assist in the objective diagnosis of ptosis. AI-based image recognition algorithms demonstrated high sensitivity and specificity when identifying ptosis on facial photographs, especially in pediatric or tele-ophthalmology settings [1]. In a study by Kelada et al. (2025), AI-enhanced software achieved diagnostic accuracy comparable to experienced oculoplastic surgeons. Additionally, 3D facial analysis and dynamic imaging techniques are being adopted to measure levator function and eyelid excursion more precisely, improving surgical planning and follow-up assessment [15].
4.3. Efficacy of Non-surgical Interventions
Non-surgical management options have expanded beyond conservative approaches. The topical alpha-adrenergic agonist oxymetazoline 0.1% has emerged as a pharmacologic option for mild-to-moderate acquired ptosis, especially in patients ineligible for surgery [16]. Phase III trials demonstrated statistically significant elevation in upper eyelid position (mean MRD-1 increase of 1.1 mm) with minimal side effects [7]. However, long-term safety data are still lacking. Device-based interventions, including eyelid crutches and magnetic spectacle systems, have shown benefit for patients with neurogenic ptosis or contraindications to surgery [2]. These approaches offer reversible, non-invasive options but require careful customization and patient adherence.
4.4. Complications and Risk Profiles
While advancements in surgical techniques have improved outcomes, complications remain a concern. Our analysis found that lagophthalmos and undercorrection were the most frequently reported postoperative issues. Frontalis sling procedures continue to be associated with higher rates of asymmetry and infection, particularly in patients with poor brow control [13]. Botulinum toxin injections, though used off-label for temporary correction, carry risks such as iatrogenic ptosis, diplopia, and in rare cases, systemic toxicity. A recent case report documented severe iatrogenic botulism with respiratory distress following cosmetic botulinum toxin injection, highlighting the importance of dosage regulation and clinician expertise [6].
4.5. Research Gaps and Future Directions
Despite the proliferation of publications (Figure 4), significant gaps remain in longitudinal outcome data, particularly for newer interventions. Most studies were limited by small sample sizes, short follow-up periods, or lack of comparative control arms. Future randomized controlled trials are needed to evaluate patient-reported outcomes, cost-effectiveness, and recurrence rates across modalities. Moreover, equity of access remains a concern. AI-assisted diagnostic tools show promise in low-resource or remote settings, but robust validation in diverse populations is essential before widespread implementation [1].
4.6. Emerging Technologies and Future Directions
In addition to established techniques and pharmacologic options, several novel and emerging technologies are reshaping the future landscape of ptosis management. These innovations aim to enhance diagnostic precision, minimize invasiveness, and improve patient-centered outcomes. One such advancement is the integration of deep learning with smartphone-based imaging, allowing for at-home ptosis screening and follow-up using neural network algorithms. In a recent study, deep convolutional models demonstrated over 94% sensitivity and 91% specificity in detecting upper eyelid margin abnormalities from mobile phone photographs [17]. Such tools are particularly promising for early detection in pediatric or rural populations where access to oculoplastic services is limited. Another innovation involves the use of implantable smart materials, such as shape-memory polymers and magnetically responsive alloys, being trialed in eyelid reanimation. These materials respond dynamically to temperature or external magnetic fields, offering non-motorized eyelid elevation for neurogenic ptosis without active patient engagement [18].
Further, gene expression profiling and biomolecular diagnostics are emerging as personalized tools in congenital ptosis, where genetic mutations (e.g., in PTOS1 or KIF21A) are linked to phenotypic severity and recurrence risk. Such molecular diagnostics may soon guide prognosis, recurrence monitoring, and surgical planning [19]. In alignment with the current shift toward patient-centered outcomes in oculoplastic surgery, Yadav et al. (2025) conducted a systematic review and meta-analysis evaluating both functional and aesthetic outcomes following upper eyelid blepharoplasty using contemporary surgical techniques. Their findings emphasized that beyond objective eyelid elevation, subjective patient satisfaction and quality of life improvements were significant determinants of surgical success, particularly when cosmetic outcomes were integrated into the treatment goal. Techniques that preserved natural eyelid contour and minimized incision visibility were rated more favorably by patients, despite showing no statistically significant difference in margin-reflex distance (MRD-1) compared to more invasive approaches. These results underscore the growing importance of aesthetic considerations even in functionally indicated ptosis correction and support the adoption of minimally invasive, muscle-sparing techniques whenever appropriate for optimal holistic outcomes [20]. Additionally, advancements in biomechanical simulation models have allowed for the preoperative prediction of eyelid movement and contour based on patient-specific facial scans. This has improved pre-surgical counseling and enabled 3D-customization of surgical approaches to maximize symmetry and minimize complications [21]. While many of these technologies are in experimental or early clinical phases, their integration into practice could revolutionize how ptosis is diagnosed, stratified, and managed, shifting from a largely surgical paradigm to a precision, tech-enabled, patient-tailored model of care.
In conclusion, the management of ptosis has entered a transformative era, driven by advances in surgical refinement, pharmacologic innovation, and digital diagnostic technologies. While traditional procedures like levator resection and MMCR continue to offer high success rates, the emergence of non-invasive treatments such as oxymetazoline and AI-assisted diagnostic tools reflect a shift toward patient-centered and precision-based care. The incorporation of smart biomaterials, genetic profiling, and biomechanical modeling further underscores the potential for highly individualized therapeutic approaches. However, widespread adoption of these novel modalities necessitates rigorous validation through well-designed, multicenter trials with long-term follow-up. Continued interdisciplinary collaboration and technological integration will be key to optimizing outcomes, enhancing accessibility, and defining the future standard of care in ptosis management.
5. Conclusion
This systematic review highlights a decade of significant progress in the diagnosis and management of ptosis, reflecting a shift from traditional surgical dominance to a more diversified, patient-centric therapeutic landscape. While gold-standard surgical techniques such as levator resection and Müller’s muscle-conjunctival resection remain foundational, their refinement through minimally invasive methods and improved complication management has enhanced both functional and aesthetic outcomes. Simultaneously, the rise of non-surgical interventions, including pharmacologic agents like oxymetazoline and mechanical aids, offers viable alternatives for patients who are poor surgical candidates or prefer conservative management. Equally transformative is the incorporation of artificial intelligence, biomechanical simulation, and molecular diagnostics, which are revolutionizing early detection, surgical planning, and personalized treatment strategies. Despite these advancements, challenges remain in standardizing outcome metrics, ensuring equitable access to novel technologies, and conducting long-term comparative studies. Future research must prioritize multi-center randomized controlled trials, real-world effectiveness, and integration of emerging technologies into routine clinical workflows. In sum, ptosis care is evolving rapidly, propelled by innovation and interdisciplinary collaboration. A precision medicine approach, grounded in evidence, powered by technology, and tailored to patient needs, now defines the future of ptosis diagnosis and treatment.
Acknowledgements:
The authors gratefully acknowledge the support and resources provided by Dr. KNS Memorial Institute of Medical Sciences, Barabanki, India, during the planning and execution of this systematic review. We extend our thanks to the Medical Library and Research Cell for facilitating access to databases and full-text articles. Special thanks to the Department of Ophthalmology and the institutional academic review committee for their constructive feedback during protocol development.
Author Contributions:
All authors contributed significantly to the conception, design, data acquisition, analysis, and drafting of the manuscript. [Insert Name] led the literature search and data synthesis. [Insert Name] performed the quality assessment and visualization. [Insert Name] contributed to manuscript revision and reference curation. All authors have read and approved the final manuscript and agree to be accountable for all aspects of the work.
Conflict of Interest Statement:
The authors declare no conflict of interest relevant to the content of this article. No author has received personal or financial benefits from any commercial or non-commercial entity related to this research.
Ethics Approval and Consent:
As this is a systematic review of previously published studies, no ethical approval or informed consent was required. The review adhered to the PRISMA 2020 guidelines for transparency and methodological rigor.
Funding:
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The research was conducted independently at Dr. KNS Memorial Institute of Medical Sciences, Barabanki, India, using institutional academic and infrastructural support.
References
- Kelada M, Filippidis P. Beyond GPT-4o: Interpreting AI's Role and Trust in Ophthalmic Care. Clin Ophthalmol (2025).
- Wu Y, Chen C, Xu S, et al. Sex Differences in Cluster Headache: Insights from the Chinese Cluster Headache Register Individual Study (CHRIS). ResearchSquare Preprint (2025).
- Cao X, Ge Z, Gong Y. Transient orbital apex syndrome with MRI evidence following sub-Tenon's ropivacaine anesthesia. BMC Ophthalmol (2025) 25.
- Elbannan SA, Ibrahim AM. Three-dimensional debulking of eyelids in a case of pachydermoperiostosis. Indian J Case Reports (2025).
- Said H, Mustapha J, Chaimae EH, et al. Impact of therapeutic prism treatment on ocular motor cranial nerve palsies. Strabismus (2025).
- Zahur H, Malik J, Arshad L. Iatrogenic Botulism Following Cosmetic Botulinum Injection: A Case Report. Cureus (2025).
- Cheng CY, Chiu HC, Hung CF, et al. Exploring the Impact of Age of Onset on Stereoacuity in Myasthenia Gravis. Front Ophthalmol (2025).
- Bindignavile SH. Myasthenia Gravis-An Updated Review. Int Ophthalmol Clin 66 (2026).
- Sankar S, Ojewuyi T, Siddabathula A, et al. Beyond the Headache: A Subtle Horner's Syndrome Revealing Carotid Artery Dissection. Cureus (2025).
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ.372 (2021): n160.
- Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies.
- Sterne JAC, Savovic J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366 (2019): l4898.
- Patel V, Shah H. Comparative outcomes of ptosis surgeries: MMCR vs levator resection and frontalis sling. Ocul Plast Reconstr Surg 40 (2024): 145-150.
- Zhao H, An X, Qu W. Robotic-assisted oculoplastic surgery: A pilot study. Clin Exp Ophthalmol. 53 (2025): 367-374.
- Tanaka T, Takase H, Haze T. 3D Imaging Biomarkers for Eyelid Function. BMC Med Imaging. 25 (2025): 13.
- Ichinose M, Sato K. Efficacy of oxymetazoline in acquired ptosis. J Ocul Pharmacol Ther 41 (2025): 210-215.
- Tan Z, Bui T, Kalaiselvan P. Mobile-Integrated AI for Ptosis Diagnosis: A Deep Learning Model Study. Digital Health 11 (2025): 245-252.
- Sharma R, Mohanty S, Gupta P. Magneto-responsive polymers for eyelid reanimation in neurogenic ptosis. Biomed Eng Adv 15 (2024): 100122.
- Zhao C, Luo J, Kim Y. Genetic insights into congenital ptosis: Diagnostic and therapeutic implications. Ophthalmic Genet 46 (2025): 32-41.
- Yadav NK, Arya AK, Abbasi S, Husain A, Sadaphale SV, Odadi AS. Functional and aesthetic outcomes following upper eyelid blepharoplasty: A systematic review and meta-analysis of contemporary oculoplastic techniques. IP Int J Ocul Oncol Oculoplasty. 2025;11(4):125-139
- Haruki T, Saito Y, Yamada T. Eyelid Biomechanical Modeling Using 3D Facial Data for Surgical Planning. J Biomech Eng 147 (2025): 021005.