Real Time Micro-Organisms PCR in 82 Horseflies in France
Article Information
François Fournier1, Frédéric Durand1, Eric Estramon1, Yannick Lequette2, Christian Perronne3, Michel Franck2, Alexis Lacout*, 4
1Alcide d'Orbigny Natural History Society (SHNAO). 57 rue de Gergovie 63170 Aubiere, France
2ADNucleis 3 route des Pierres Blanches, 69290 Grézieu la Varenne, France
3Infectious and Tropical Diseases. Paris, France
4ELSAN Diagnostic Center, Medical and Surgical Center 83 avenue Charles de Gaulle 15000 Aurillac, France
*Corresponding author: Alexis Lacout, ELSAN Diagnostic Center, Medical and Surgical Center 83 avenue Charles de Gaulle 15000 Aurillac, France.
Received: 13 June 2023; Accepted: 22 June 2023; Published: 04 July 2023
Citation: François Fournier, Frédéric Durand, Eric Estramon, Yannick Lequette, Christian Perronne, Michel Franck, Alexis Lacout. Real Time Micro- Organisms Pcr in 82 Horseflies in France. Archives of Microbiology and Immunology. 7 (2023): 83-95.
View / Download Pdf Share at FacebookAbstract
Introduction: A large number of bacteria other than Borrelia, parasites and viruses are transmitted by tick bites and may be responsible of the persistent polymorphic syndrome possibly due to a tick bite (Syndrome persistant polymorphe après une possible piqûre de tique, SPPT) or the post-treatment Lyme disease syndrome (PTLDS).
Methods: The following micro-organisms were searched for in horseflies, by using real time quantitative polymerase chain reaction (qPCR) : Borrelia burgdorferi sensu lato (sl), Borrelia miyamotoi, Borrelia hermsii, Bartonella spp., Rickettsia spp., Ehrlichia spp., Anaplasma spp., Babesia spp., Theileria spp., Brucella spp., Francisella tularensis, Coxiella burnetii, Chlamydia spp., Mycoplasma spp., Candidatus Neoehrlichia mikurensis, Leishmania spp., Toxoplasma gondii, herperviruses (VZV, EBV, CMV, HHV-6), tick-borne encephalitis virus (TBEV), Bourbon virus, West Nile virus, Chikungunya virus, Dengue virus (1-4), Zika virus, Powassan virus, Eyach virus.
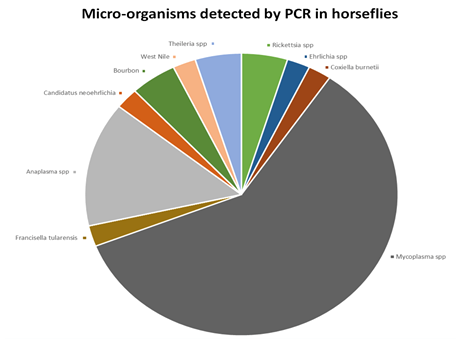
Results: Eighty-two horseflies were analyzed. Thirty-three (40.2 %) horseflies were positive for at least one micro-organism. Among bacteria, Borrelia spp. have not been detected in this study. Mycoplasma spp., Anaplasma spp. and Rickettsia spp. were the most frequent bacteria detected 25 times, 6 times, and twice respectively. Coxiella burnetii, Ehrlichia spp., Francisella tularensis, and Candidatus Neoehrlichia mikurensis were detected once respectively. Among parasites, one Theileria spp. have been detected twice. Among viruses, Bourbon virus and West Nile virus have been detected twice and once respectively.
Conclusion: Our prospective real time qPCR study has shown that horseflies may harbour several micro-organisms which could be pathogenic for animals and humans.
Keywords
Lyme, Borrelia, Babesia, virus, Theileria, PCR, horsefly
Lyme articles, Borrelia articles, Babesia articles, virus articles, Theileria articles, PCR articles, horsefly articles
Article Details
1. Introduction
Lyme disease is a tick-borne infectious disease caused by Borrelia burgdorferi sensu lato (including B. burgdorferi sensu stricto, B. afzelii and B. garinii). The prevalence seems to be increasing in many countries around the world, particularly in France. In addition, some experts make the assumption that Borrelia burgdorferi sensu lato is not the only factor to explain this disease, in particular certain persistent forms as the persistent polymorphic syndrome possibly due to a tick bite (SPPT), officially recognized by the French High Authority for Health (HAS) (a governmental institution https://www.has-sante.fr), and the post-treatment Lyme disease syndrome (PTLDS) (1). A large number of bacteria other than Borrelia i.e., parasites and viruses are transmitted by tick bites and could cause different signs and symptoms in patients, the so-called co-infections (2-5). It might be thus more accurate to change the paradigm, and consider the term “crypto-infections” rather than exclusively “tick-borne infections” (6). The main goal of this study was to look for the different micro-organisms carried and potentially transmitted by horseflies, using real time qPCR, which is a direct diagnostic method amplifying the DNA or RNA of the micro-organisms sought.
2. Methods
This is a prospective observational study associating the natural history society “Alcide d'Orbigny” (Société d'histoire naturelle “Alcide d'Orbigny”, SHNAO), specialist in entomology, and one laboratory performing PCR analyses (AdNucleis).
2.1 Horseflies
Female horseflies (Figure 1) were collected by the SHNAO in the summer of 2020, in various locations in the following French departments: Cantal, Puy de Dôme and Haute-Loire. The location of the sampling was precisely recorded with GPS location. A horsefly trap was set up using a black tarp to attract them. The horseflies were then captured with a net. The female horseflies were then placed in a test tube, numbered and quickly sent to the ADnucleis laboratory for PCR analysis (Figure 2). The identification of horsefly species was previously done by François Fournier from the SHNAO. The precise geographical location of the caught horseflies was determined: department, nearby village, GPS coordinates. The biotope and the presence of domestic and/or wild animals were notified.
2.2 Micro-organisms searched
The following micro-organisms were searched for by using real time PCR (Table 1) : Borrelia burgdorferi sensu lato (s.l.), Borrelia miyamotoi, Borrelia hermsii, Bartonella spp., Rickettsia spp., Ehrlichia spp., Anaplasma spp., Babesia spp., Theileria spp., Brucella spp., Francisella tularensis, Coxiella burnetii, Chlamydia spp., Mycoplasma spp., Candidatus neoehrlichia, Leishmania spp., Toxoplasma gondii, herperviruses (VZV, EBV, CMV, HHV-6), tick-borne encephalitis virus (TBEV), Bourbon virus, West Nile virus, Chikungunya virus, Dengue virus (1-4), Zika virus, Powassan virus, Eyach virus.
Table 1: Real Time Multiplex Polymerase chain reaction (PCR)
|
Selection of Primers |
To allow the detection of bacteria and parasites, primers targeting specific genes of each micro-organism were used to amplify DNA by qPCR. Details of qPCR kits used is listed in Table 2. |
|
Robustness of PCR Mixes |
The porion of target geneswere synthesized and introduced into a plasmid to obtain a control DNA and facilitate its multiplication. This control DNA was used to validate the amplification mixes. Serial dilution of the plasmid was performed and amplified to determine the robustness parameters of eachPCR kit: the limit of detection (LOD), the limit of quantification (LOQ), the repeatability and the reproducibility. |
|
DNA Extraction and Purification |
The DNA was extracted without any prior treatment using 300 μl of whole blood with an equal volume of ADNucleis extraction buffer (5 M guanidium thiocyanate, 500 mM TrisHCL, 50 mM EDTA, 20% Tween 20, 20% Triton X-100, 750 μg proteinase K). After incubation for 20 min at 56°C and 15 min at 80°C, the extracted DNA was purified by means of silica magnetic beads and eluted in 250 μl of elution buffer (10 mM TrisHCl, pH 8.5). |
|
Control of the Extraction |
Human glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) was used as a housekeeping gene as an internal control for PCR extraction and inhibition. The extracted samples were first checked with a PCR targeting the GAPDH gene. If the results of this PCR were consistent (Ct of GAPDH below 32), the samples were then analyzed for the other pathogens. The sequence of interest of GAPDH was insered into a plasmid and this plasmid was used as a positive DNA for the validation of GAPDH primers and PCR mix as well as a positive control for subsequent PCRs. The primers used for GAPDH are described inTable 2. |
|
Real-Time PCR (real time PCR) |
Real-time PCR was carried out in a total volume of 50 μl with a PCR mix containing ADNucleis PCR buffer (20 mM Tris-HCl, 10 mM NH4SO4, 10 mM KCl, 2 mM Mg2+, 0.1% TritonX-100, pH 8.8), 2 mM of each dNTP, 600 nM of each primer, 1 μl of Evagreen and 5 units ofTaqpolymerase ADNucleis. Twelve μl of extracted samples were amplified. An initial denaturation step of 5 min at 95°C was followed by 42 cycles of 15 s at 95°C and 40 s at 60°C (hybridization-elongation). The dissociation curves were generated by a last step of 10 min with temperature increments from 75 to 95°C for qPCR kits using Sybr green technology. |
|
Quantification |
Positive samples were quantified using a standard curve obtained by amplifying known and calibrated concentrations of control DNA of the desired targets. Quantification was obtained using the standard curve equation (Ct = a (Log10 [DNA]) + b) where “a” is the slope and “b” the intercept of the curve. The results were expressed in genome units (UG) per ml of sample. |
LOD: limit of detection
LOQ: limit of quantification
UG: Genome Units
Ct: Cycle threshold.
LOD limit of detection and LOQ limit of quantification
the LOD is calculated by comparing the response of the PCR kit with respect to a reference method, which is most often a method for cultivating the microbial population. Once this microbial population has been cultured, it is stopped when the population is most abundant (eg 10E9); a count is carried out and the microbial population is subjected to successive dilutions in order to be able to have samples from 10E9 to 0, passing through all the intermediates (10E8, 10E7, etc.); these samples constitute the reference and the Borrelia analysis kit is evaluated for each dilution; we look for
the sensitivities of the PCR kits making it possible to detect at least 10E2 DNA copies / PCR reaction volume, at best 10 copies / PCR reaction volume or less. LOD is therefore expressed in DNA copies (or RNA for most viruses) detected per PCR reaction volume; and when we evaluate the regression of the response of the kit (in Ct with respect to each dilution) we must obtain a straight line of which we evaluate the linearity (equation) and the slope (a of the equation y = ax + b, y being the log value of the concentration of the bacterial population, x the value of the response of the kit in Ct); most often, this linearity is not complete, in particular for low concentrations of the microbial population; Then comes the LOQ (limit of quantification) which is the lowest detection value evaluated on the linear part of the regression line. The LOQ is therefore always greater than the LOD, if the latter is of the order of 5 copies / PCR reaction volume, the LOQ can be between 40 and 100 copies / PCR reaction volume; these values are always carefully assessed by the manufacturer before placing the PCR reaction kit on the market.
2.3 Sample collection and nucleic acid sample preparation
Horseflies were kept at -20°C until nucleic acid was extracted. Each individual horsefly was put in a conical tube and grinded using a pestle. Then, 500 µl of PBS were added in the tube and horsefly was grinded again. Tubes with grinded horseflies were centrifuged 3 min at 10000 rpm. 200 µl of supernatant were transferred to a deepwell plate of nucleic acid extraction using a magnetic silica beads system. The kit 96R Ext_ARN PVG300+ Purification BM from ADNucleis was used, according to the manufacturer instructions, to extract both RNA and DNA from horseflies samples. RNA/DNA was eluted in 480 µl of elution buffer and stored at -20°C until used.
2.4 Real time PCR method
Control DNA plasmids containing the amplified fragment were constructed to validate the amplification mixes, to be used as positive control. Serial dilution of the plasmid from 108 copies/PCR to 101 copies/PCR was made and used to determine the limit of detection (LOD), the limit of quantification (LOQ) of each target, Tm for the Sybr mixes (listed in Table 2). The primer and probes are listed in the Table 2. Primers and probes were from the literature or designed for this study. Each target was tested as individual monoplex. The real time PCR was performed using TaqMan or ‘Sybr’ technologies. Each mixt was validated with specific PCR components developed by ADNucleis (including the Taq polymerase). The thermal cycling conditions of rt PCR were as follow: 95°C 5 min, 42 cycles of 95°C 10s and 60°C 40s for the TaqMan rt PCR. The Sybr PCR thermal cycling conditions were the same as for the TaqMan one, except the addition of the melting curve steps (95°C 15s, 75°C 15s, increasing temperature to 95°C and then 95°C 15s). The reaction volume was 40 µl and 12 µl of RNA/DNA samples. A Ct cycle thermic (Ct) higher than 38 was considered as negative for the TaqMan mixes. A Tm within the range TM +/-1 °C was considered positive for the Sybr mixts.
Table 2: List of targets and details of PCR kits
Sybr: Syber Green fluorophore.
The choice of the PCR technique is essentially linked to the sensitivity of each of the techniques; contrary to what is usually said, the two techniques are roughly equivalent, one being better than the other for certain targets and vice versa. And since we are looking for the best sensitivity in all cases, the laboratory has kept both techniques.
FAM: 6-carboxyfluorescéine (fluorophore)
LOD: limit of detection
LOQ: limit of quantification
UG: Genome Units
The sensitivity of each kit, in particular the limit of detection (LOD) and the limit of quantification (LOQ), are the subject of an analytical evaluation calculated according to the recommendations of Regulation (EU) 2017/746. LOD limit of detection and LOQ limit of quantification the LOD is calculated by comparing the response of the PCR kit with respect to a reference method, which is most often a method for cultivating the microbial population. Once this microbial population has been cultured, it is stopped when the population is most abundant (eg 10E9); a count is carried out and the microbial population is subjected to successive dilutions in order to be able to have samples from 10E9 to 0, passing through all the intermediates (10E8, 10E7, etc.); these samples constitute the reference and the analysis kit is evaluated for each dilution; we look for the sensitivities of the PCR kits making it possible to detect at least 10E2 DNA copies or genome unit (UG) / PCR reaction volume, at best 10 copies / PCR reaction volume or less. LOD is therefore expressed in DNA copies (Genome Units UG) detected per PCR reaction volume; and when we evaluate the regression of the response of the kit (in Ct with respect to each dilution) we must obtain a straight line of which we evaluate the linearity (equation) and the slope (a of the equation y = ax + b, y being the log value of the concentration of the bacterial population, x the value of the response of the kit in Ct); most often, this linearity is not complete, in particular for low concentrations of the microbial population; Then comes the LOQ (limit of quantification) which is the lowest detection value evaluated on the linear part of the regression line. The LOQ is therefore always greater than the LOD, if the latter is of the order of 5 copies / PCR reaction volume, the LOQ can be between 40 and 100 copies / PCR reaction volume; these values are always carefully assessed by the manufacturer before placing the PCR reaction kit on the market.
3. Results
3.1 Horseflies
In this study, 82 horseflies were captured and analyzed. Eleven horseflies were caught in Cantal, 11 in Puy de Dôme and 60 in Haute-Loire (Table 3 and 4). Thirty-three (40.2 %) horseflies were positive for at least one micro-organism (Tables 3 and 4, f 3). Four out of 11 (36.4 %) horseflies were positive in Cantal, 4 out of 11 (36.4 %) horseflies were positive in Haute-Loire, 25 out of 60 (41.7 %) were positive in Puy de Dôme. The infected horsefly species were the following: 9 out of 22 (40.9%) Haematopota pluvialis, 9 out of 9 Tabanus spodoreptus, 11 of 37 (29.7 %) Tabanus bromius, 3 out of 11 Tabanus quatuornotatus, 1 out of 2 Tabanus corniger. Another species of horsefly was found but not infected: 1 Tabanus glaucopis.
Table 4
Table 3: Micro-organisms found in the 82 horseflies studied (Cantal (15) Puy de Dôme (63) and Haute-Loire (43), France)
|
Micro-organisms |
Number of positives horseflies (Horsefly numerotation) |
Hosefly species (Number) |
Géographic localisation: village, départment number (Number of positives horseflies) |
Animals in proximity |
|
Borrelia (Burgdorferisl, miyamotoi, hermsii) |
0 |
|||
|
Bartonella spp |
0 |
|||
|
Rickettsia spp. |
2 (24, 64) |
Tabanus spodoreptus (1) Tabanus bromius (1) |
Bansat 63(1) Saint-Jean-en val 63(1) |
Deer, cattle |
|
Ehrlichia spp. |
1 (5) |
Haematopota pluvialis (1) |
Sallèdes 63 |
Wild boar, deer |
|
Anaplasma spp. |
6 (1, 19, 31, 75, 79, 81) |
Haematopota pluvialis (3) Tabanus quatuornotatus (2) Tabanus bromius (1) |
Sallèdes 63 (2) Bansat 63 Saint-Hilaire 43 (3) |
Wild boar, deer, horses |
|
Babesia spp. |
0 |
|||
|
Theileria spp. |
2 (22, 28) |
Tabanus spodoreptus (2) |
Bansat 63 (1) |
Deer, cattle |
|
Dauzat-sur-Vodable 63(1) |
||||
|
Brucella spp. |
0 |
|||
|
Francisella Tularensis |
1 (5) |
Haematopota pluvialis (1) |
Sallèdes 63 (1) |
Wild boar, deer |
|
Coxiella burnetii |
1 (5) |
Haematopota pluvialis (1) |
Sallèdes 63 |
Wild boar, deer |
|
Chlamydia spp. |
0 |
|||
|
Mycoplasma spp. |
25 (5, 6, 15,18-29, 31, 33, 35, 38, 45, 46, 55, 56, 62, 80) |
Haematopota pluvialis (9) Tabanus bromius (6) Tabanus spodoreptus (8) Tabanus corniger (1) Tabanus quatuornotatus (1) |
Sallèdes 63 (5) Sansac-de-Marmiesse 15 (1) Bansat 63 (10) Dauzat-sur-Vodable 63 (1) Saint-Babel 63 (1) Saint-Jean-en val 63 (6) Saint-Hilaire 43 (1) |
Wild boar, deer, horses |
|
Candidatus neoehrlichia |
1 (1) |
Haematopota pluvialis (1) |
Sallèdes 63 |
Wild boar, deer |
|
Leishmania spp. |
0 |
|||
|
Toxoplasma gondii |
0 |
|||
|
EBV |
0 |
|||
|
HHV6 |
0 |
|||
|
CMV |
0 |
|||
|
VZV |
0 |
|||
|
TBEV |
0 |
|||
|
Bourbon virus |
2 (2, 14) |
Haematopota pluvialis (2) |
Sallèdes 63 (1) Sansac-de-Marmiesse 15 (1) |
Wild boar, deer |
|
West Nile virus |
1 (12) |
Tabanus bromius (1) |
Sansac-de-Marmiesse 15 |
|
|
Chikungunya virus |
0 |
|||
|
Dengue virus 1-4 |
0 |
|||
|
Zika virus |
0 |
|||
|
Powassan virus |
0 |
|||
|
Eyach virus |
0 |
Spp.: species plurimae (all species in the genus).
TBEV: Tick-borne encephalitis virus
3.2 Real time qPCR
Bacteria
Twenty-five (30.5%) horseflies were positive for Mycoplasma spp.; 6 (7.3%) horseflies were positive for Anaplasma spp.; 2 horseflies were positive for Rickettsia spp.; 1 (1.2%) horsefly was positive for Coxiella burnetii; 1 (1.2%) horsefly was positive for Ehrlichia spp.; 1 (1.2%) horsefly was positive for Francisella tularensis; 1 (1.2%) horsefly was positive for Candidatus Neoehrlichia mikurensis. Borrelia burgdorferi sl., Borrelia miyamotoi, Bartonella spp., Brucella spp., Chlamydia spp. have not been detected in this study.
Parasites
2 (2.4%) horseflies were positive for Theileria spp. Babesia spp., Leishmania spp. and Toxoplasma gondii have not been detected in this study.
Viruses
Two horseflies (2.4%) were positive for Bourbon virus (Sallèdes, Puy de Dôme) and (Sansac-de-Marmiesse, Cantal); 1(1.2%) horsefly was positive for West Nile virus (Sansac-de-Marmiesse, Cantal). EBV, HHV6, CMV, VZV, TBEV, Chikungunya virus, Dengue virus, Zika virus, Powassan virus, Eyach virus have not been detected in this study.
Polyinfection
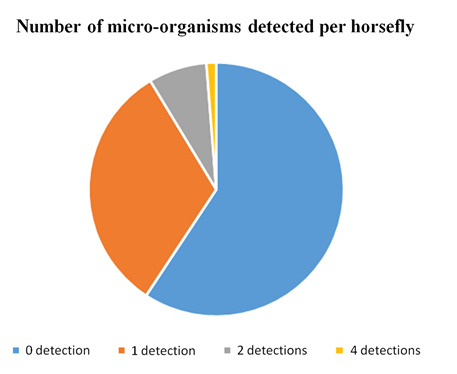
Twenty-six horseflies (31.7 %) were positive for one micro-organism, 6 (7.3 %) for two micro-organisms, none for three micro-organisms, and 1 (1.2 %) for 4 micro-organisms (Table 3, Figure 4). The latter carried Ehrlichia spp., Coxiella burnetii, Francisella tularensis and Mycoplasma spp (Horsefly Tabanus bromius caught in Sallèdes, Puy de Dôme. Exluding Mycoplasma spp., twelve horseflies (14,6 %) were positive, 10 for one micro-organism, 1 (1.2%) for two micro-organisms, 1 (1.2%) for three micro-organisms.
4. Discussion
4.1 Lyme disease, SPPT, PTLDS and fibromyalgia
Lyme disease is known to be transmitted primarily by ticks. Other transmission routes are known or suspected: blood (transfusions for example), maternal-fetal, or by other hematophagous insects (7, 8). In addition, we remind that ticks can be vectors of many micro-organisms and that patients who have been diagnosed with Lyme disease can be poly-infected, by other micro-organisms than Borrelia burgdorferi sl.
4.2 Transmission by hematophagous insects
Borrelia burgdorferi has already been detected in insects, deerflies and some species of horseflies (Hybomitra lasiophtalma and Hybomitra epistates) and mosquitoes (Aedes canadensis and Aedes stimulans) (8, 9). It is possible that these insects can transmit Lyme disease because a hamster exposed to infected mosquitoes has developed specific antibodies (8). Šikutov et al, found spirochetes (different from Borrelia burgdorferi) in black flies and mosquitoes, but did not find them in horseflies (10). Horseflies could also transmit other micro-organisms such as trypanosomes and Francisella tularensis (11, 12).
4.3 Horseflies
The Tabanidae family, belongs to the order Diptera and has about 4000 species in the world. Among other criteria, the wings have a specific venation, and the females are hematophagous while the males are floricultural. The female does not bite but has mandibles and maxillaries, otherwise known as stylets, adapted to pierce the skin and then suck blood. When taking a blood meal, the stylets are pushed into the skin and act like scissors, creating microhematoma. Saliva is introduced into the wound via the salivary duct. The flexible labellum folds up and allows the insect to suck blood from the wound. It needs blood to ensure its vitality and to reproduce. The adult horsefly lives two to three weeks. The females lay 100 to 800 eggs per clutch at the sites. These eggs take one to three weeks to develop into larvae, which develop in a variety of relatively moist environments: mud, decaying plants, humus, wet soil, and around water sources. It takes a few months to 3 years to produce an adult. The females have a sensitivity to the degree of polarization of the reflected light. Thus, the more a surface reflects a highly polarized light, the more the female will be attracted. Therefore, a dark and uniform animal (highly polarized surface) is more attractive than a white or with spots or stripes (low polarized surface). Thus, black plastic tarps attract these insects because they are polarizing and constitute attractive traps. In our study, sight hunting was conducted at several different sites.
4.4 Mirco-organisms found
This study showed that many horseflies harbour micro-organisms: 33 (40.2 %) horseflies positive for at least one micro-organism, 12 (14.6 %) excluding Mycoplasma spp., that are well detected using real time qPCR. It is surprising that our study did not show the presence of Borrelia while this bacterium, responsible for Lyme disease, has already been observed in horseflies (8). The explanation could be that horseflies are not the primary vector, or that the sample studied (82 horseflies) was insufficient.
Babesia spp. infections have already been described in immunocompetent patients, sometimes recurrent, with a torpid, chronic presentation (13-15), and are most likely underestimated (16). It is interesting to observe that parasites of the Theileria species, a parasite close to Babesia, have been detected in our study. There is a very high genetic proximity between Babesia microti and Theileria microti and our PCR primers are designed to specifically discriminate the genus Babesia from the genus Theileria. Theileria are piroplasms, well known in veterinary medicine as they usually infest horses (17). Theileria have been recently detected for the first time by PCR in patients with polymorphic signs and symptoms that may be related to a tick bite (17, 18).
Candidatus Neoehrlichia mikurensis, a recently described tick-borne agent has been detected once in our study. This bacterium, close to Rickettsia spp., can cause an influenza-like syndrome with high fever (resembling erhrlichiosis) in immunocompromised patients, sometimes associated with thrombosis or lymphoma.
Interestingly, we did not find any tick-borne encephalitis virus, but two cases of Bourbon virus and one case of West Nile virus. Bourbon virus, transmitted by ticks, described mainly in America, causes a viral syndrome with leukopenia and thrombocytopenia resembling ehrlichiosis. West Nile virus, is known to be transmitted by mosquitoes and seems to be increasing in Europe, and can give various clinical signs, from flu-like syndrome to encephalitis (19, 20). Some mycoplasmas such as Mycoplasma fermentans can be transmitted by ticks and be pathogenic (21). Mycoplasma could however be transmitted by other way than the tick bite (for example by sexual contamination) or be commensal microbial agents. Our study shows that horseflies can also carry Mycoplasma spp. and possibly transmit it. In future studies it would be interesting to sequence the DNA for a precise characterization of the Mycoplasma species.
Although horseflies can carry many micro-organisms, it is not formally demonstrated that these insects can necessarily transmit infectious diseases. On the one hand, it is known that a tick must often remain attached to its host for some time (several hours) to transmit Borrelia burgdorferi sl. On the other hand, mosquitoes transmit viruses (malaria, dengue for example), during a much shorter bite. It is therefore possible that horseflies can transmit diseases associated with the observed micro-organisms. As horseflies introduce saliva into the bitten animal, it could favour transmission. This should be confirmed in further studies. However, the transmission of some micro-organisms by horsefly bites is probably more difficult than by tick bites (22).
In conclusion, our prospective study has shown that horseflies, like ticks, harbour and could transmit multiple pathogenic micro-organisms observed in patients presenting with SPPT/PTLDS. Further studies are needed to determine if the micro-organisms isolated from horseflies can be transmitted to humans and can be pathogenic.
Conflict of interest disclosure
Michel Franck is CEO of ADNucleis
The other authors have nothing to disclose.
Authors' contributions
François Fournier: identification of horsefly species, horsefly collection, review and editing.
Frédéric Durand: Review and editing, writing, horsefly collection.
Eric Estramon: Review and editing, writing, horsefly collection.
Michel Franck: Investigation, writing, review and editing, resources, validation
Yannick Lequette, Writing, review and editing
Christian Perronne: Writing, review and editing, supervision.
Alexis Lacout: Conceptualization, writing original draft.
Declaration
Ethics approval and consent to Participate: not applicable
Consent for Publication: All authors have seen and approved the manuscript, contributed significantly to the work. The manuscript has neither been previously published nor is being considered for publication elsewhere.
Competing Interests: Michel Franck is CEO of ADNucleis, Yannick Lequette, employee of ADNucleis. The others authors do not declare any conflict of interest.
Funding: This work was supported by “FRANCE BOIS FORET”. The authors declare that this study received funding from Association BonSens.org to cover the publication fees.
Authors’ Contributions: All authors have seen and approved the manuscript, contributed significantly to the work.
Availability of data and material: Data and material are available and included in the manuscript.
References
- https://www.hhs.gov/ash/advisory-committees/tickbornedisease/meetings/2018-07-24/meeting-summary/index.html?language=es
- Cisak E, Wójcik-Fatla A, Zajac V, Dutkiewicz J. Prevalence of tick-borne pathogens at various workplaces in forest exploitation environment. Med Pr 65 (2014): 575-81.
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R. Polymicrobial Nature of Tick-Borne Diseases. mBio 10 (2019): e02055-19.
- Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, et al. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol 29 (2014): 103.
- Nelder MP, Russell CB, Sheehan NJ, Sander B, Moore S, Li Y, et al. Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasit Vectors 9 (2016): 265.
- Christian Perronne. Crypto-Infections: Denial, Censorship and Suppression the Truth About What Lies Behind. ed. Hammersmith Health Books (2021).
- Middelveen MJ, Burke J, Sapi E, Bandoski C, Filush KR, Wang Y, et al. Culture and identification of Borrelia spirochetes in human vaginal and seminal secretions 3 (2014): 309.
- Magnarelli LA, Anderson JF. Ticks and biting insects infected with the etiologic agent of Lyme disease, Borrelia burgdorferi. J Clin Microbiol 26 (1988): 1482-6.
- Stanek G, Flamm H, Groh V, Hirschl A, Kristoferitsch W, Neumann R, et al. Epidemiology of borrelia infections in Austria. Zentralbl Bakteriol Mikrobiol Hyg A 263 (1987): 442-9.
- Sikutová S, Halouzka J, Mendel J, Knoz J, Rudolf I. Novel spirochetes isolated from mosquitoes and black flies in the Czech Republic. J Vector Ecol 35 (2010): 50-5.
- Rogers L. Note on the Rôle of the Horse Fly in the Transmission of Trypanosoma Infection, with a Reply to Colonel Bruce's Criticisms. Br Med J 26 (1904): 1454-5.
- Eliasson H, Bäck E. Myositis and septicaemia caused by Francisella tularensis biovar holarctica. Scand J Infect Dis 35 (2003): 510-1.
- Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 22 (2008): 469-88.
- Krause PJ. Human babesiosis. Int J Parasitol 49 (2019): 165-174.
- Krause PJ, Spielman A, Telford SR 3rd, Sikand VK, McKay K, Christianson D, et al. Persistent parasitemia after acute babesiosis. N Engl J Med 16 (1998): 160-5.
- Martinot M, Paleau A, Greigert V, Brunet J, Hansmann Y, Jouglin M, et al. Babésiose en France et en Europe : une pathologie à redéfinir. Med mal inf Volume 48, Issue 4, Supplement (2018): S112.
- Onyiche TE, Suganuma K, Igarashi I, Yokoyama N, Xuan X, Thekisoe O. A Review on Equine Piroplasmosis: Epidemiology, Vector Ecology, Risk Factors, Host Immunity, Diagnosis and Control. Int J Environ Res Public Health 16 (2019): E1736.
- Lacout A, Mas M, Pajaud J, Perronne V, Lequette Y, Franck M, et al. Real time micro-organisms PCR in 104 patients with polymorphic signs and symptoms that may be related to a tick bite. Eur J Microbiol Immunol (Bp) 3 (2021).
- Clark MB, Schaefer TJ. West Nile Virus. 2021 Aug 11. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2021).
- Komar N, Hamby N, Palamar MB, Staples JE, Williams C. Indirect Evidence of Bourbon Virus (Thogotovirus, Orthomyxoviridae) Infection in North Carolina. N C Med J 81 (2020): 214-215.
- Eskow E, Adelson ME, Rao RV, Mordechai E. Evidence for disseminated Mycoplasma fermentans in New Jersey residents with antecedent tick attachment and subsequent musculoskeletal symptoms. J Clin Rheumatol 9 (2003): 77-87.
- Scoles GA, Miller JA, Foil LD. Comparison of the efficiency of biological transmission of Anaplasma marginale (Rickettsiales: Anaplasmataceae) by Dermacentor andersoni Stiles (Acari: Ixodidae) with mechanical transmission by the horse fly, Tabanus fuscicostatus Hine (Diptera: Muscidae). J Med Entomol 45 (2008): 109-14.