Rare Ovarian Steroid Cell Tumor, Not Otherwise Specified (NOS) With Massive Ascites and Elevated CA125: A Case Report
Article Information
Yiqun Tang1*, Dandan Chu1, Yanli Li1, Bin Cai1, Shuo Shi2*
1Department of Gynecology and Obstetrics, Shanghai Jiaotong University, Shanghai General Hospital, Shanghai, China
2Department of Gynecology, Obstetrics and Gynecology Hospital of Fudan University, No.128 Shenyang Road, Shanghai, China
*Corresponding Author’s: Yiqun Tang, Department of Gynecology and Obstetrics, Shanghai Jiaotong University, Shanghai General Hospital, Shanghai, China.
Shuo Shi, Department of Gynecology, Obstetrics and Gynecology Hospital of Fudan University, No.128 Shenyang Road, Shanghai, China.
Received: 05 May 2023; Accepted: 11 May 2023; Published: xx May 2023
Citation: Yiqun Tang, Dandan Chu, Yanli Li, Bin Cai, Shuo Shi. Rare ovarian steroid cell tumor, not otherwise specified (NOS) with massive ascites and elevated CA125: A case report. Obstetrics and Gynecology Research. 6 (2023): 156-159
View / Download Pdf Share at FacebookAbstract
Ovarian steroid cell tumor, not otherwise specified (SCT-NOS) is an extremely rare ovarian tumor. We reported a unique case with massive ascites and elevated CA125 in a 45-year-old woman who presented with persistent abdominal distention for one month. Preoperative diagnosis was suspected as a malignant ovarian tumor initially due to aggressive signs including markedly elevated CA125 and massive ascites. Therefore, an exploratory laparotomy was performed to prepare for a complete staging surgery once get the pathological evidence of malignancy. However, taking the result of the intraoperative frozen section examination into account, a conservative uniliteral adnexectomy was conducted. Eventually, the pathological diagnosis revealed a benign ovarian steroid cell tumor, not otherwise specified. During the stringent postoperative surveillance, the serum level of testosterone and CA125 decreased to the normal range within one month. At the one-year and two-year follow-up visits, the patient did not have any complaints, and the testosterone serum level remained within the normal range. This rare and unique SCT-NOS case may help gynecologists better understand the clinical behavior of SCTNOS, and a comprehensive assessment including clinical presentation, radiology, serum testosterone level, and intraoperative frozen section examination may provide guidance in making an operative proposal and choosing the optimal individualized treatment strategies.
Keywords
Ovarian Steroid Cell Tumor; Not Otherwise Specified; Magnetic Resonance Imaging; Massive Ascites; CA125; Testosterone
Ovarian Steroid Cell Tumor articles Ovarian Steroid Cell Tumor Research articles Ovarian Steroid Cell Tumor review articles Ovarian Steroid Cell Tumor PubMed articles Ovarian Steroid Cell Tumor PubMed Central articles Ovarian Steroid Cell Tumor 2023 articles Ovarian Steroid Cell Tumor 2024 articles Ovarian Steroid Cell Tumor Scopus articles Ovarian Steroid Cell Tumor impact factor journals Ovarian Steroid Cell Tumor Scopus journals Ovarian Steroid Cell Tumor PubMed journals Ovarian Steroid Cell Tumor medical journals Ovarian Steroid Cell Tumor free journals Ovarian Steroid Cell Tumor best journals Ovarian Steroid Cell Tumor top journals Ovarian Steroid Cell Tumor free medical journals Ovarian Steroid Cell Tumor famous journals Ovarian Steroid Cell Tumor Google Scholar indexed journals Not Otherwise Specified articles Not Otherwise Specified Research articles Not Otherwise Specified review articles Not Otherwise Specified PubMed articles Not Otherwise Specified PubMed Central articles Not Otherwise Specified 2023 articles Not Otherwise Specified 2024 articles Not Otherwise Specified Scopus articles Not Otherwise Specified impact factor journals Not Otherwise Specified Scopus journals Not Otherwise Specified PubMed journals Not Otherwise Specified medical journals Not Otherwise Specified free journals Not Otherwise Specified best journals Not Otherwise Specified top journals Not Otherwise Specified free medical journals Not Otherwise Specified famous journals Not Otherwise Specified Google Scholar indexed journals Magnetic Resonance Imaging articles Magnetic Resonance Imaging Research articles Magnetic Resonance Imaging review articles Magnetic Resonance Imaging PubMed articles Magnetic Resonance Imaging PubMed Central articles Magnetic Resonance Imaging 2023 articles Magnetic Resonance Imaging 2024 articles Magnetic Resonance Imaging Scopus articles Magnetic Resonance Imaging impact factor journals Magnetic Resonance Imaging Scopus journals Magnetic Resonance Imaging PubMed journals Magnetic Resonance Imaging medical journals Magnetic Resonance Imaging free journals Magnetic Resonance Imaging best journals Magnetic Resonance Imaging top journals Magnetic Resonance Imaging free medical journals Magnetic Resonance Imaging famous journals Magnetic Resonance Imaging Google Scholar indexed journals Massive Ascites articles Massive Ascites Research articles Massive Ascites review articles Massive Ascites PubMed articles Massive Ascites PubMed Central articles Massive Ascites 2023 articles Massive Ascites 2024 articles Massive Ascites Scopus articles Massive Ascites impact factor journals Massive Ascites Scopus journals Massive Ascites PubMed journals Massive Ascites medical journals Massive Ascites free journals Massive Ascites best journals Massive Ascites top journals Massive Ascites free medical journals Massive Ascites famous journals Massive Ascites Google Scholar indexed journals CA125 articles CA125 Research articles CA125 review articles CA125 PubMed articles CA125 PubMed Central articles CA125 2023 articles CA125 2024 articles CA125 Scopus articles CA125 impact factor journals CA125 Scopus journals CA125 PubMed journals CA125 medical journals CA125 free journals CA125 best journals CA125 top journals CA125 free medical journals CA125 famous journals CA125 Google Scholar indexed journals Testosterone articles Testosterone Research articles Testosterone review articles Testosterone PubMed articles Testosterone PubMed Central articles Testosterone 2023 articles Testosterone 2024 articles Testosterone Scopus articles Testosterone impact factor journals Testosterone Scopus journals Testosterone PubMed journals Testosterone medical journals Testosterone free journals Testosterone best journals Testosterone top journals Testosterone free medical journals Testosterone famous journals Testosterone Google Scholar indexed journals ovarian neoplasms articles ovarian neoplasms Research articles ovarian neoplasms review articles ovarian neoplasms PubMed articles ovarian neoplasms PubMed Central articles ovarian neoplasms 2023 articles ovarian neoplasms 2024 articles ovarian neoplasms Scopus articles ovarian neoplasms impact factor journals ovarian neoplasms Scopus journals ovarian neoplasms PubMed journals ovarian neoplasms medical journals ovarian neoplasms free journals ovarian neoplasms best journals ovarian neoplasms top journals ovarian neoplasms free medical journals ovarian neoplasms famous journals ovarian neoplasms Google Scholar indexed journals endocrine potentials articles endocrine potentials Research articles endocrine potentials review articles endocrine potentials PubMed articles endocrine potentials PubMed Central articles endocrine potentials 2023 articles endocrine potentials 2024 articles endocrine potentials Scopus articles endocrine potentials impact factor journals endocrine potentials Scopus journals endocrine potentials PubMed journals endocrine potentials medical journals endocrine potentials free journals endocrine potentials best journals endocrine potentials top journals endocrine potentials free medical journals endocrine potentials famous journals endocrine potentials Google Scholar indexed journals unilateral ovaries articles unilateral ovaries Research articles unilateral ovaries review articles unilateral ovaries PubMed articles unilateral ovaries PubMed Central articles unilateral ovaries 2023 articles unilateral ovaries 2024 articles unilateral ovaries Scopus articles unilateral ovaries impact factor journals unilateral ovaries Scopus journals unilateral ovaries PubMed journals unilateral ovaries medical journals unilateral ovaries free journals unilateral ovaries best journals unilateral ovaries top journals unilateral ovaries free medical journals unilateral ovaries famous journals unilateral ovaries Google Scholar indexed journals premenopausal articles premenopausal Research articles premenopausal review articles premenopausal PubMed articles premenopausal PubMed Central articles premenopausal 2023 articles premenopausal 2024 articles premenopausal Scopus articles premenopausal impact factor journals premenopausal Scopus journals premenopausal PubMed journals premenopausal medical journals premenopausal free journals premenopausal best journals premenopausal top journals premenopausal free medical journals premenopausal famous journals premenopausal Google Scholar indexed journals
Article Details
INTRODUCTION
Ovarian steroid cell tumors (SCT) are rare ovarian neoplasms, accounting for less than 0.1% of all ovarian tumors and 5% of ovarian sex-cord stromal tumors [1]. The classification of ovarian SCT was initially proposed by Hayes and Scully in 1987, who divided the type of the tumor into three subtypes based on the cell of origin [2]. Stromal luteomas and Leydig cell tumors arise from ovarian stromal cells and Leydig cells, respectively. The most common subtype is ovarian steroid cell tumor, not otherwise specified (SCT-NOS), which comprises approximately 60% of all ovarian steroid cell tumors. These tumors usually affect unilateral ovaries and have endocrine potentials, with only 25% being nonfunctioning. Therefore, patients often present with virilization due to overexpression of testosterone [2]. Ovarian SCT-NOS typically occurs in premenopausal women with a mean age of 43 years old, and the proportion of malignancy ranges from 25% to 43% [3]. Currently, accurate preoperative diagnosis and management strategy of SCT-NOS can be challenging for clinicians. Benign SCT-NOS behaving in a clinically malignant manner has rarely been reported so far. Herein, we reported an atypical case of benign SCT-NOS with malignant signs and a good prognosis.
CASE PRESENTATION
A 45-year-old Chinese female, premenopausal, gravida 3, para 1, who presented with persistent abdominal distention for one month was admitted to our hospital in December 2018. The patients had a normal menstrual cycle, length, and bleeding, and did not complain of any pain or changes in micturition or bowel movements. She was healthy in general previously and denied any history of taking herbal medication, steroids, supplemental drugs, etc. Physical examination revealed remarkable abdominal distension.
On laboratory analysis, the serum carcinoma antigen 125 (CA125) level was elevated at 355.2 U/ml (normal, < 35 U/ml), and the serum testosterone level also remained high at 358.9 ng/dl (normal, < 100 ng/dl). Other tumor markers were within normal limits as well as serum dehydroepiandrosterone and dehydroepiandrosterone sulfate level.
Sonography reported a large amount of ascites in the abdominal cavity and an endometrial-like ovarian cystic mass measuring 3 - 4 cm was detected on the right adnexal (Figure 1A). Magnetic resonance image (MRI) manifested as a 35 mm well-circumscribed nodule with hyperintensity on Diffusion Weighted Imaging (DWI) along with copious ascites and thickened peritoneum (Figure 1B). Positron emission tomography-computed tomography (PET-CT) showed a solid mass in the right ovary with higher glucose metabolic rate suspicious of malignancy. The peritoneal cytology showed non-malignant ascites. Cervical and endometrial cytology was normal. Gastrointestinal endoscopy and biopsy showed chronic inflammation.
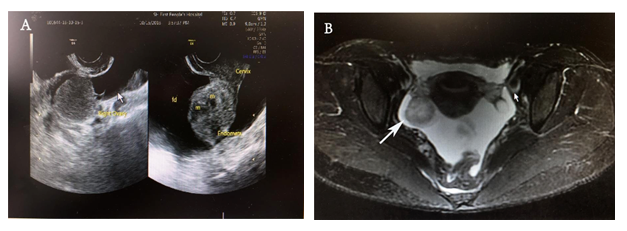
Figure 1: Preoperative pelvic imaging findings. (A) Sonography examination shows a cystic-like mass in the right ovary. (B) The magnetic resonance image displays a mixed cystic and solid mass (indicated by the white arrow) with clear boundaries located in the right adnexal.
Subsequently, an open surgery was performed to achieve complete resection of the tumor. Nearly two-liter straw-colored ascites were collected. A yellowish solid tumor was identified at the right ovary with no hemorrhage and necrosis on the surface. Frozen section analysis showed the tumor cell is densely packed with vacuolated cytoplasm and some cells resembled signet-ring cells. After careful inspection of abdomen intraoperatively, we performed a unilateral adnexectomy. The final pathologic examination confirmed an ovarian steroid cell tumor, not otherwise specified.
Histological image (Hematoxylin and eosin staining on paraffin-embedded sections) showed cytoplasmic vacuoles that displaced the nuclei simulating a signet-ring cell-like appearance (Figure 2A). Immunohistochemically, the tumor is composed of cells positive for estrogen receptor (ER), progesterone receptor (PR), CD117, CD99, CD56, vimentin, Melan A, OCT3/4 and negative for cytokeratin, Epithelial membrane antigen (EMA), AR, CD10, RCC, CyclinD-1, Syn, Placental alkaline phosphatase(PLAP), PAX-8, β-catenin, a-inhibin, CgA, WT1 (Table 1). The Ki67 labeling index was less than 1%.
|
Expression |
Immunohistochemical markers |
|
Positive |
Ki67(1%+), ER, PR, Vimentin, CD117(Part), CD56, CD99, OCT3/4, MelanA |
|
Negative |
Pan-cytokeratin, EMA, α-inhibin, AR, CD10, Cyclin D1, RCC, Syn, PLAP, PAX-8, β-catenin, CgA, WT-1 |
ER = estrogen receptor, PR = progesterone receptor, EMA = epithelial membrane antigen, AR = androgen receptor, RCC
Table 1: Immunohistochemistry staining results
After two days of operation, the patient’s serum testosterone level experienced a significant drop from 358.9 ng/dl to 9.6 ng/dl. The serum level of CA125 decreased relatively slowly and reached to normal level 1 month later. In a one-year follow-up and two-year follow-up, the patient has been asymptomatic with normal serum testosterone and without any evidence of recurrences.
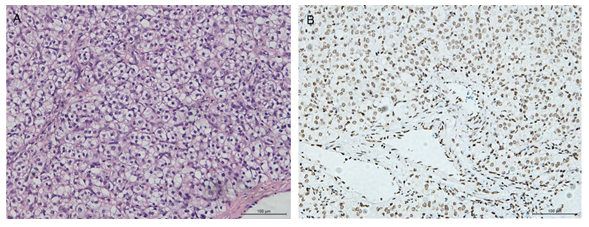
Figure 2: Histological images. (A) Hematoxylin and Eosin staining image shows round-like cells which are densely arranged with many vacuoles in the cytoplasm resembling the signet-ring-like cells. (B) Immunohistochemistry staining of OCT3/4 shows the cell nuclei were stained and highlighted by OCT3/4 staining. Bar:100um
Discussion
Ovarian steroid cell tumor, not otherwise specified (SCT-NOS) is an extremely rare ovarian sex cord-stromal tumor with the incidence estimated to be less than 0.1% of all types of ovarian tumors [4]. Due to a variety of steroid hormones, particularly testosterone released by most of these tumors, patients often present with virilization symptoms including hirsutism, temporal baldness, amenorrhea, and so on. Although estrogenic symptoms may also occur, they are less common, affecting only 6%-23% of patients [4]. Massive ascites, on the other hand, are a rare symptom of SCT-NOS. The etiology and pathogenesis remain largely unknown, which contributes to delayed diagnosis and a lack of standard treatment strategies. One study suggested that the dysregulation of the wnt/β-catenin pathway, indicated by the D32Y mutation in exon 3 of CTNNB1, may be involved in the occurrence and development of this subtype of SCT [5]. Other reported cases have suggested a link between Von Hippel-Lindau syndrome and ovarian steroid cell tumors [5-7]. In the present case, we demonstrate that SCT-NOS with malignant signs preoperatively does not necessarily indicate ovarian cancer. A conservative fertility preservation surgical strategy may be applied in such cases, with careful follow-up surveillance of serum testosterone and CA125.
Accurate diagnosis of SCT-NOS is challenging, and there is no gold standard diagnostic approach, even with the indicator of immunohistochemistry staining. Diagnosis relies on a comprehensive evaluation of symptoms, hormone levels, radiologic findings, histology, and immunohistochemical examinations. Most of these tumors secrete testosterone, leading to virilization symptoms such as hirsutism, acne, amenorrhea, abnormal facial hair growth, and a times alopecia [8-10]. Additionally, estrogen-secreting tumors can cause endometrial hyperplasia and bleeding in postmenopausal women. Rarely, pregestational effects or Cushing’s syndrome may occur [10]. In the present case, the patient did not show any symptoms relating to hormone abnormalities but presented with abdominal distention mainly due to a large amount of ascites. The elevated serum level of CA125 and testosterone within a short time, along with imaging findings and massive ascites, led us to suspect a primary ovarian malignant tumor. Unlike previously reported cases, this case was characterized by copious amounts of yellow ascites and an elevated serum level of CA125. Kim et al [11] inferred that the tumor and its ascites mechanically irritated the mesothelium causing overexpression of CA125. During the surgery, a well-circumscribed mass measuring 3 cm in diameter with clear tumor boundaries, no bleeding, and no necrosis was observed in the right ovary. Microscopically, the frozen section showed signet-ring like cell with abundant cytoplasm. After ruling out a gastric signet -like cell tumor and no metastasis was detected in the abdominal cavity, we opted for unilateral adnexectomy and awaited the paraffin sections immunohistochemistry staining results for further treatment decisions.
According to a study, a significant proportion of SCT-NOS, ranging from 25 – 43 % are malignant ovarian tumors [3]. Hayes and Scully analyzed several criteria for predicting the malignancy of these tumors, such as the presence of two or more mitotic figures per 10 high-power fields (92% malignant), exhibiting necrosis (86% malignant) or hemorrhage (77% malignant) and a tumor diameter greater than 7 cm (78% malignant) [2]. However, such criteria do not apply to every single case. It was reported that a Japanese woman diagnosed with ovarian SCT displayed peritoneal dissemination [12]. Although the tumor was morphologically and histologically considered benign on account of Hayes and Scully's criteria, eventually, the clinical diagnosis was malignant and the cytologic result also indicates malignancy. It is important to note that some SCT-NOS can exhibit benign histological features but behave in a malignant way. In our case, the gross and pathological features confirmed a benign ovarian SCT-NOS. The histological staining on paraffin section revealed cytoplasmic vacuoles that displaced the nuclei simulating a signet-ring cell-like appearance.
Although there are no specific diagnostic immunohistochemical markers of SCT-NOS, some of our immunohistochemical outcomes were consistent with the results described in other cases [8]. The Ki67 index was less than 1%, suggesting a degenerative nature rather than a high-grade malignancy. Pan-cytokeratin and EMA staining were negative, ruling out the diagnosis of epithelial carcinoma. Vimentin positive shows the tumor originated from mesothelium tissue. Melan-A is 100% positive in the adrenal gland and related tumors, and could also be found positive in some SCT-NOS cases[13], which probably manifests their derivation from the adrenal gland in SCT-NOS.
Surgery is a common treatment no matter for benign or malignant tumors. An appropriate surgical method requires a customized analysis of the patient's age, demand for preserving fertility, clinical characteristics, histology, and the extent of malignancy of the tumor. To prevent recurrences of malignant tumors, some patients usually received postsurgical adjuvant chemotherapy or radiotherapy, however, the effectiveness has not been verified. For those patients who are not suitable for these therapies, GnRHa may be a good choice to control hyperandrogenism, Zhang reported a patient who had persistent testosterone elevation after surgery, when the patient received GnRHa administration, the testosterone level normalized with no obvious recurrence for 32 months [14]. During the postoperative follow-up, monitoring the hormone level is necessary, and strict long-term follow-up should be adopted in cases with worse prognostic histological features.
In conclusion, SCT-NOS is a rare ovarian tumor and difficult to diagnose preoperatively. The biological behavior of this kind of tumor is not clearly understood. In clinical practice, when confronted with ovarian mass, we should raise awareness of ovarian steroid cell tumors and pay more attention to sex hormone abnormalities and improve the knowledge of clinicopathological features and immunohistochemical markers.
Conflict of Interest Statement
The authors declare no potential conflicts of interest.
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analyzed.
REFERENCES
- Outwater E K, Wagner B J, Mannion C, McLarney J K, Kim B. Sex cord-stromal and steroid cell tumors of the ovary. RadioGraphics 1998; 18: 1523-1546.
- Hayes M, Scully RE. Ovarian Steroid Cell Tumors (Not Otherwise Specified): A Cli...: The American Journal of Surgical Pathology. Am J Surg Pathol 1987; 11: 835-845.
- Varras M, Vasilakaki T, Skafida E, Akrivis C. Clinical, ultrasonographic, computed tomography and histopathological manifestations of ovarian steroid cell tumour, not otherwise specified: Our experience of a rare case with female virilisation and review of the literature. Gynecological Endocrinology 2011; 27: 412-418.
- Lin M, Bao K, Lu L, Xu S, Liang Y, Cheng X, et al. Ovarian steroid cell tumors, not otherwise specified: analysis of nine cases with a literature review. BMC Endocr Disord 2022; 22.
- Wagner M, Browne H N, Marston Linehan W, Merino M, Babar N, Stratton P. Lipid cell tumors in two women with von Hippel-Lindau syndrome. Obstetrics and Gynecology 2010;116 Suppl 2: 535-539.
- Marques A, Portugal R. Ovarian Steroid Cell Tumor in an Adolescent With Von Hippel-Lindau Syndrome: A Case Report and Review of the Literature. Int J Gynecol Pathol 2020; 39: 473-477.
- Morani A C, Mubarak A I, Bhosale H R, Ramani N S, Waguespack S G, Ying A. Steroid Cell Ovarian Tumor in a Case of von Hippel-Lindau Disease: Demonstrating Lipid Content of the Mass with MR Imaging. Magn Reson Med Sci 2019; 18: 251-252.
- Matsuda S, Yamaguchi Y, Kaseki H, Watanabe K, Ono S, Yamamoto A, et al. Case of ovarian steroid cell tumor diagnosed after presenting acute heart failure. J Obstet Gynaecol Res 2020; 46: 1211-1215.
- Lobaton-Ginsberg M, Malanco-Hernández LM, Ferreira-Hermosillo A. Rare virilizing tumor: ovarian steroid cell tumor, not otherwise specified: a case report. J Med Case Rep 2022; 16.
- Kaelin W G. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2002;2:673-682.
- Kim Y T, Kim S W, Yoon B S, Kim S H, Kim J H, Kim J W, et al. An ovarian steroid cell tumor causing virilization and massive ascites. Yonsei Med J 2007; 48: 142-146.
- Kosaka N, Hasegawa K, Kiuchi K, Ochiai S, Nagai T, Machida H, et al. Cytological Findings of Ascitic Fluid with a Malignant Ovarian Steroid Cell Tumor: A Case Report and Literature Review. Acta Cytol 2017; 61: 165-171.
- Haroon S, Idrees R, Fatima S, Memon A, Kayani N. Ovarian steroid cell tumor, not otherwise specified: A clinicopathological and immunohistochemical experience of 12 cases. Journal of Obstetrics and Gynaecology Research 2015; 41: 424-431.
- Wang P H, Chao H T, Lee W L. Use of a long-acting gonadotropin-releasing hormone agonist for treatment of steroid cell tumors of the ovary. Fertil Steril 1998; 69: 353-355.
