Ranolazine in Preventing Ventricular Fibrillation During Aortic Valve Replacement in Patients with Low Ejection Fraction: A Single-Centre Retrospective Study
Article Information
Simopoulos Vasilios1, Papadopoulos Eleftherios1, Mitilis Vasilios1, Tasoudis Vasilios2, Dipla Konstantina3, Hatziefthimiou Apostolia4, Tsilimingas Nikolaos1, Aidonidis Isaac4*
1University of Thessaly, Department of Thoracic and Cardiovascular Surgery, University Hospital of Larissa, Larissa, Greece
2University General Hospital of Larissa, Anesthesiology, Larissa, Greece
3Department of Physical Education and Sports Science at Serres, Aristotle University of Thessaloniki, Thessaloniki, Greece
4Department of Physiology, Medical School of Larissa, University of Thessaly, Larissa, Greece
*Corresponding Author: Isaac Aidonidis, Department of Physiology (Exp. Surgery Laboratory, Cardiovascular Section), University of Thessaly, Medical School of Larissa, Biopolis, Panepistimiou Street 3, LARISSA 41500, Greece
Received: 20 March 2020; Accepted: 28 March 2020; Published: 08 April 2020
Citation: Simopoulos Vasilios, Papadopoulos Eleftherios, Mitilis Vasilios, Tasoudis Vasilios, Dipla Konstantina, Hatziefthimiou Apostolia, Tsilimingas Nikolaos, Aidonidis Isaac. Ranolazine in Preventing Ventricular Fibrillation During Aortic Valve Replacement in Patients with Low Ejection Fraction: A Single-Centre Retrospective Study. Journal of Surgery and Research 3 (2020): 058-065.
View / Download Pdf Share at FacebookAbstract
Objectives: Aortic valve replacement (AVR) is often associated with intraoperative ventricular fibrillation (VF) complicating early postoperative outcomes. Ca2+ triggering mechanisms induced by ischemia-reperfusion injury have been implicated as possible causes of this arrhythmia, however, the exact mechanisms remain unclear. The aim of this study was to investigate the role of late sodium current inhibition by ranolazine (RAN) in preventing VF in these patients.
Methods: We retrospectively examined 53 patients with aortic stenosis or insufficiency, receiving either mechanical or bioprosthetic AVR in the last two years. Of these, 29 (19 M/10 F, age 66.4 ± 11.3 y) were under treatment with RAN 500 mg × 2 daily (RAN group) and 24 (17 M/7 F, age 62.8 ± 17 y) had not been treated with RAN (control group). Internal defibrillation was usually applied at consecutively escalating energy levels to reset sinus rhythm in patients manifesting VF.
Results: At the time of aortic unclamping, 18 out of 29 patients (62%) in the control group and 6 out of 24 patients (25%) in the RAN group developed VF (P=0.007, χ2 test). Left ventricular ejection fraction (EF) was reduced in both groups (≤40%) with no significant between group differences (P=0.51). Cross-clamp time was 48.3 ± 9.6 min in the control group and 51.7 ± 8.8 min in the RAN group (P=0.09). QTc intervals measured postoperatively in the ICU after ECG stabilization, were within upper normal limits (430.3 ± 23.6 vs. 413.5 ± 20.2 ms, in control and RAN group, respectively; P= 0.008). The Tpeak-Tend interval was significantly reduced in RAN vs controls (p<0.001).
Conclusion: Patients with low EF receiving RAN before AVR surgery, showed a 37% lower incidence of VF compared to the control group. This is possibly associated to the RAN&r
Keywords
Cardiac surgery, Aortic valve replacement, Reduced ejection fraction, Intraoperative ventricular fibrillation, Ranolazine
Cardiac surgery articles, Aortic valve replacement articles, Reduced ejection fraction articles, Intraoperative ventricular fibrillation articles, Ranolazine articles
Cardiac surgery articles Cardiac surgery Research articles Cardiac surgery review articles Cardiac surgery PubMed articles Cardiac surgery PubMed Central articles Cardiac surgery 2023 articles Cardiac surgery 2024 articles Cardiac surgery Scopus articles Cardiac surgery impact factor journals Cardiac surgery Scopus journals Cardiac surgery PubMed journals Cardiac surgery medical journals Cardiac surgery free journals Cardiac surgery best journals Cardiac surgery top journals Cardiac surgery free medical journals Cardiac surgery famous journals Cardiac surgery Google Scholar indexed journals Aortic valve replacement articles Aortic valve replacement Research articles Aortic valve replacement review articles Aortic valve replacement PubMed articles Aortic valve replacement PubMed Central articles Aortic valve replacement 2023 articles Aortic valve replacement 2024 articles Aortic valve replacement Scopus articles Aortic valve replacement impact factor journals Aortic valve replacement Scopus journals Aortic valve replacement PubMed journals Aortic valve replacement medical journals Aortic valve replacement free journals Aortic valve replacement best journals Aortic valve replacement top journals Aortic valve replacement free medical journals Aortic valve replacement famous journals Aortic valve replacement Google Scholar indexed journals Reduced ejection fraction articles Reduced ejection fraction Research articles Reduced ejection fraction review articles Reduced ejection fraction PubMed articles Reduced ejection fraction PubMed Central articles Reduced ejection fraction 2023 articles Reduced ejection fraction 2024 articles Reduced ejection fraction Scopus articles Reduced ejection fraction impact factor journals Reduced ejection fraction Scopus journals Reduced ejection fraction PubMed journals Reduced ejection fraction medical journals Reduced ejection fraction free journals Reduced ejection fraction best journals Reduced ejection fraction top journals Reduced ejection fraction free medical journals Reduced ejection fraction famous journals Reduced ejection fraction Google Scholar indexed journals Intraoperative ventricular fibrillation articles Intraoperative ventricular fibrillation Research articles Intraoperative ventricular fibrillation review articles Intraoperative ventricular fibrillation PubMed articles Intraoperative ventricular fibrillation PubMed Central articles Intraoperative ventricular fibrillation 2023 articles Intraoperative ventricular fibrillation 2024 articles Intraoperative ventricular fibrillation Scopus articles Intraoperative ventricular fibrillation impact factor journals Intraoperative ventricular fibrillation Scopus journals Intraoperative ventricular fibrillation PubMed journals Intraoperative ventricular fibrillation medical journals Intraoperative ventricular fibrillation free journals Intraoperative ventricular fibrillation best journals Intraoperative ventricular fibrillation top journals Intraoperative ventricular fibrillation free medical journals Intraoperative ventricular fibrillation famous journals Intraoperative ventricular fibrillation Google Scholar indexed journals Ranolazine articles Ranolazine Research articles Ranolazine review articles Ranolazine PubMed articles Ranolazine PubMed Central articles Ranolazine 2023 articles Ranolazine 2024 articles Ranolazine Scopus articles Ranolazine impact factor journals Ranolazine Scopus journals Ranolazine PubMed journals Ranolazine medical journals Ranolazine free journals Ranolazine best journals Ranolazine top journals Ranolazine free medical journals Ranolazine famous journals Ranolazine Google Scholar indexed journals ischemia-reperfusion injury articles ischemia-reperfusion injury Research articles ischemia-reperfusion injury review articles ischemia-reperfusion injury PubMed articles ischemia-reperfusion injury PubMed Central articles ischemia-reperfusion injury 2023 articles ischemia-reperfusion injury 2024 articles ischemia-reperfusion injury Scopus articles ischemia-reperfusion injury impact factor journals ischemia-reperfusion injury Scopus journals ischemia-reperfusion injury PubMed journals ischemia-reperfusion injury medical journals ischemia-reperfusion injury free journals ischemia-reperfusion injury best journals ischemia-reperfusion injury top journals ischemia-reperfusion injury free medical journals ischemia-reperfusion injury famous journals ischemia-reperfusion injury Google Scholar indexed journals Traditional Aortic Valve Surgery: articles Traditional Aortic Valve Surgery: Research articles Traditional Aortic Valve Surgery: review articles Traditional Aortic Valve Surgery: PubMed articles Traditional Aortic Valve Surgery: PubMed Central articles Traditional Aortic Valve Surgery: 2023 articles Traditional Aortic Valve Surgery: 2024 articles Traditional Aortic Valve Surgery: Scopus articles Traditional Aortic Valve Surgery: impact factor journals Traditional Aortic Valve Surgery: Scopus journals Traditional Aortic Valve Surgery: PubMed journals Traditional Aortic Valve Surgery: medical journals Traditional Aortic Valve Surgery: free journals Traditional Aortic Valve Surgery: best journals Traditional Aortic Valve Surgery: top journals Traditional Aortic Valve Surgery: free medical journals Traditional Aortic Valve Surgery: famous journals Traditional Aortic Valve Surgery: Google Scholar indexed journals cardiopulmonary bypass articles cardiopulmonary bypass Research articles cardiopulmonary bypass review articles cardiopulmonary bypass PubMed articles cardiopulmonary bypass PubMed Central articles cardiopulmonary bypass 2023 articles cardiopulmonary bypass 2024 articles cardiopulmonary bypass Scopus articles cardiopulmonary bypass impact factor journals cardiopulmonary bypass Scopus journals cardiopulmonary bypass PubMed journals cardiopulmonary bypass medical journals cardiopulmonary bypass free journals cardiopulmonary bypass best journals cardiopulmonary bypass top journals cardiopulmonary bypass free medical journals cardiopulmonary bypass famous journals cardiopulmonary bypass Google Scholar indexed journals cardioplegia articles cardioplegia Research articles cardioplegia review articles cardioplegia PubMed articles cardioplegia PubMed Central articles cardioplegia 2023 articles cardioplegia 2024 articles cardioplegia Scopus articles cardioplegia impact factor journals cardioplegia Scopus journals cardioplegia PubMed journals cardioplegia medical journals cardioplegia free journals cardioplegia best journals cardioplegia top journals cardioplegia free medical journals cardioplegia famous journals cardioplegia Google Scholar indexed journals
Article Details
1. Introduction
Ventricular fibrillation (VF) is relatively common during cardiac surgery and most frequently occurs due to interventions inducing ischemia-reperfusion injury. This arrhythmia may potentially aggravate the electrical and hemodynamic stability after surgery, increasing the risk of cardiac death especially in patients with compromised left ventricular function [1]. Lidocaine is usually administered shortly before aortic declamping to prevent the occurrence of VF during the bypass operation. However, there exist contradictory reports regarding its effectiveness [2-5]. In addition, lidocaine can critically block peak Na+ channels in the ischemically-depolarized myocardium, thereby blocking slowly conducted areas in the myocardium. This may favor reentrant excitation and recurrence of VF. However, when lidocaine incompletely blocks conduction, it may produce proarrhythmic effects.
Furthermore, lidocaine abrogates sympathetic overactivity to the heart after VF, and thus, could reduce the effectiveness of defibrillation and promote cardiac arrest [6, 7]. Ranolazine (RAN) is a selective inhibitor of the late Na+ current in cardiac myocytes, primarily indicated for patients with stable angina. In the last decade, RAN has experimentally and clinically showed potent antiarrhythmic properties against atrial and ventricular tachyarrhythmias. Its antiarrhythmic mechanism seems to be mediated by suppressing Ca2+ triggered ventricular arrhythmias following reperfusion [8, 9]. However, the RAN’s role in preventing VF during AVR surgery is not completely clear. Therefore, this study aimed to investigate the efficacy of RAN as add-on therapy in preventing VF during AVR surgery.
2. Materials and Methods
Patients undergoing aortic valve stenosis or insufficiency surgery were examined to estimate whether RAN prevents VF during AVR surgery. Diagnostic evaluation of valvular disease and left ventricular systolic function was carried out by M-mode and 2D-echocardiography in the setting of essential preoperative measures. All patients were analyzed for the incidence of intraoperative VF under RAN treatment (RAN group) or without RAN (control group).
2.1 Surgical procedure
2.1.1 Traditional Aortic Valve Surgery: Operative interventions were performed using an identical protocol executed by the same surgical, anesthetic, and perfusionist team. Aortic valve replacement was performed through a median sternotomy. Briefly, once the pericardium was opened, the patient was cannulated. The aortic cannulation was performed by a cannula placed in the aorta and the venous cannulation by a single atrial venous cannula inserted through the right atrium. Next, the patient was connected to the cardiopulmonary bypass apparatus, (heart-lung machine, Stoeckert Instrumente GmbH, Munchen, Germany). This apparatus is a mechanical pump that maintains a patient’s blood circulation and oxygenation, while the surgeon replaces the heart valve. The patient’s heart was then stopped, using cardioplegia.
In more details, a catheter was placed in the left ventricle through the right superior pulmonary vein to prevent left ventricular distention before and after cardiac arrest. When the set-up was completed, the aorta was clamped shut with a cross-clamp to cease blood pumping through the heart and cardioplegia was infused. The surgeon then performed an aortotomy (i.e. an incision of the aorta a few millimeters above the sinotubular junction, just above the coronary ostia, where the coronary arteries join to the aorta). Cardioplegia was then delivered directly through the ostia. With the application of cardioplegia, the heart was still and the surgeon removed the patient's diseased aortic valve. The cusps of the aortic valve were excised, and calcium was removed (debrided) from the aortic annulus. After valve replacement, the aorta was closed, and the patient was placed in a Trendelenburg position. The heart was then de-aired and restarted and the patient was disconnected from the cardiopulmonary bypass apparatus. During this crossclamp removal, VF frequently occurred, abruptly requiring low energy internal defibrillation (10-30 J). Transesophageal echocardiogram was used to verify that the new valve was functioning properly. Pacing wires were applied, for manually controlling the heart, in case of complications after surgery. Drainage tubes were also inserted, to drain fluids from the chest. These were usually removed within 36 hours, while the pacing wires were left in place, until the patient’s discharge from the hospital. After extubation and until discharge all patients received a standard drug regimen that included acetylsalicylic acid (100 mg daily), atorvastatin (20-40 mg daily), the b-blocker metoprolol (50-100 mg daily), and the angiotensin-converting enzyme inhibitor perindopril (5-10 mg daily), in addition to their standard preoperative treatment. Postoperative monitoring in the coronary care unit included daily blood cell count, basic biochemical tests, X-rays, and continuous electrocardiogram.
2.2 Protocol endpoints
Primary endpoint of this study was the incidence of VF after AVR surgery in the control and in the chronically RAN-treated patients (500 mg × 2 daily). Secondary endpoints included comparisons of electrocardiographic findings associated with inducing VF, duration of surgical procedure, and crossclamp times, as well as plasma K+ levels prior to aortic declamping.
2.3 Statistics
Statistical analyses were performed with GraphPad Prism 5.01 (GraphPad Software, San Diego, CA, USA). Demographic data and baseline characteristics of the patients were expressed as mean ± standard deviation (in case of normally distributed data), or median and interquartile range (in case non-normally distributed data) or percentiles, as appropriate. Differences between the control and the RAN group in continuous variables were compared using independent t-tests or Mann-Whitney tests, as appropriate and in categorical variables using the χ2 test. A p value <0.05 was considered as statistically significant.
3. Results
From June 2018 to August 2019, a total of 53 patients undergoing AVR surgery were studied for incidence of operative VF with or without RAN treatment. Most of demographic and clinical characteristics were well balanced within the two groups, with no significant differences between groups (Table 1). In a few variables, there were differences between the control and RAN group, but without a pathological impact, as the absolute values ranged within normal to upper normal limits. The proportion of patients who developed intraoperative VF was significantly lower in the RAN group (25%) compared with 62% in the control group (Table 1, Figure 1). Values of left ventricular ejection fraction (LVEF%) were typical for reduced EF heart failure (HFrEF ≤ 40%) in both groups. Patients in the control group required in average 1.80 ± 0.2 countershocks, whereas, patients in the RAN group required in average 1.16 ± 0.1 countershocks to terminate VF (p<0.001). Previous anti-HF standard treatment was similar in both groups, albeit β-blockers were markedly down titrated in patients with AVI, in order to prevent bradycardia induced by increased backflow during diastole. RAN did not pathologically change the QRS duration. Although it prolonged the P-R intervals near a 1st degree AV block, and shortened the QTc intervals, these alterations were not associated with a hemodynamic deterioration of the patients. Tpeak-Tend (Tp-Te) interval, measured in ECG precordial leads, approximately reflects the real dispersion of transmural ventricular repolarization, which is a marker of arrhythmogenesis. RAN significantly reduced (p<0.001) this dispersion compared to control values (Table 2). Postoperative atrial fibrillation (POAF) was rarely manifested in the RAN group 2-3 days after surgery.
|
Variable |
Control group |
RAN group |
P value |
|
N (Male/Female) |
29 (19/10) |
24 (17/7) |
____ |
|
Age (y) |
66.4 ± 11.3 |
62.8 ± 17 |
0.71 |
|
Medical history |
|||
|
HTN, N (%) |
5 (17.2) |
7 (29.2) |
0.3 |
|
T2DM, N (%) |
5 (17.2) |
8 (33.3) |
0.17 |
|
AVS/AVI |
16/13 |
24/0 |
____ |
|
Echocardiography |
|||
|
LVEF (%) |
35.5 ± 5.2 |
36.5 ± 5.3 |
0.51 |
|
LAd (cm) |
4.83 ± 0.2 |
4.86 ± 0.3 |
0.99 |
|
Previous medications |
|||
|
Diuretics, N (%) |
16 (55.2) |
23 (96) |
<0.001 |
|
β-Blockers, N (%) |
8 (27.6) |
11 (45.8) |
0.16 |
|
ACE inhibitors, N (%) |
7 (24) |
10 (41.7) |
0.17 |
|
Antidiabetics, N (%) |
5 (17.2) |
8 (33.3) |
0.17 |
|
Statins, N (%) |
13 (44.8) |
12 (50) |
0.70 |
Abbreviations: N-number of patients; HTN-arterial hypertension; T2DM-type II diabetes mellitus; AVS/AVI-aortic valve stenosis/insufficiency; LVEF-left ventricular ejection fraction; Lad-left atrial diameter; ACE-angiotensin converting enzyme
Table 1: Demographic and preoperative data of patients undergoing AVR surgery.
|
Variable |
Control group |
RAN group |
P value |
|
VF, N (%) |
18 (62) |
6 (25) |
0.007 |
|
Valve type Mech/Bio |
12/17 |
10/14 |
____ |
|
Total CPB (min) |
79.3 ± 8.2 |
82.5 ± 6.8 |
0.13 |
|
CC time (min) |
48.3 ± 9.6 |
51.7 ± 8.8 |
0.09 |
|
Biochemical data |
|||
|
K (mEq/L) |
4.6 ± 0.21 |
4.7 ± 0.22 |
0.87 |
|
Urea (mg/dL) |
43.5 ± 6.9 |
43.9 ± 4.8 |
0.54 |
|
Creatinine (mg/dL) |
1.12 ± 0.07 |
1.10 ± 0.09 |
0.63 |
|
ECG evaluation |
|||
|
P-R (ms) |
197.6 ± 8.9 |
215.4 ± 13.6 |
<0.001 |
|
QRS (ms) |
81.9 ± 8.1 |
86.8 ± 9.5 |
0.04 |
|
QTc (ms) |
430.3 ± 23.6 |
413.5 ± 20.2 |
0.008 |
|
Tp-Te (ms) |
34.6 ± 5.5 |
25.3 ± 5.3 |
<0.001 |
|
POAF, N (%) |
6 (20.7) |
3 (12.5) |
0.43 |
Abbreviations: N-number of patients; Mech/Bio-mechanical/bioprosthetic valves; CPB-cardiopulmonary bypass; CC time-crossclamp time; QTc-corrected QT interval; Tp-Te-Tpeak-Tend interval; POAF-postoperative atrial fibrillation
Table 2: Operative and postoperative measurements.
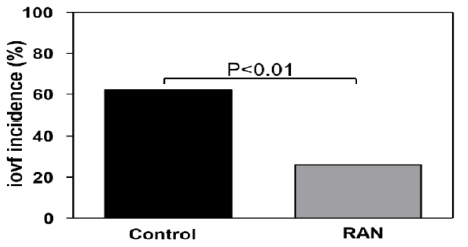
Figure 1: RAN significantly decreased the incidence of intraoperative ventricular fibrillation (iovf) compared to the control group.
4. Discussion
This is the first study comparing the incidence of VF during AVR surgery in reduced EF patients with or without previous RAN treatment. Patients in the RAN group experienced significantly lower VF rates (25%) than the control group (62%). No adverse electrophysiologic effects were observed in patients which were operated under RAN treatment, e.g., sinus bradycardia, atrioventricular node blockade, or QTc prolongation, regardless of the effectiveness of the drug. The anti-VF effect achieved by RAN was largely similar to that reported by 200 mg iv lidocaine when administered 3 min prior to aortic declamping [3]. In contrast to these findings, another study failed to show an effect of lidocaine or amiodarone in reducing the VF episodes compared to controls [4].
Although RAN and lidocaine have structural similarities, RAN is more atrial-selective than lidocaine regarding the peak and late Na+ channel blockade and does not shorten ventricular repolarization and refractoriness due to a concomitant blockade of K+ channels. However, in the partially depolarized ischemic ventricular myocardium, both agents might stronger affect local conduction and eventually exert pro-arrhythmic activity. Lidocaine has been found to abrogate the increase in the post-fibrillatory sympathetic drive to the heart, producing dose-dependently ineffective defibrillation and cardiac arrest [6, 7]. Moreover, a bolus of lidocaine has a short half-life time, thus, leaving unprotected the heart from a later recurrence of VF.
VF by aortic declamping is obviously a potentially undesirable event during cardiac surgery. It may enhance the metabolic demands while potentiating impairments in myocardial contractility due to countershock mediated injury. For these reasons, it becomes clear that VF has to be avoided, especially in patients with preexisting systolic dysfunction. RAN has been experimentally and clinically shown to possess antiarrhythmic activity against atrial and ventricular arrhythmias associated with myocardial ischemia and reperfusion [8, 9]. This drug has also been given to patients with reduced LVEF without significant side effects on hemodynamic or electrocardiographic parameters [10]. The results from the present study and from recent clinical investigations confirm postulations regarding the minor side effects of oral RAN at therapeutic plasma concentrations (5-10 µM/L) [11, 12].
Our results showing the anti-fibrillatory effects of RAN, are in line with previous experimental and clinical research findings. That is, RAN has been shown to possess significant antiarrhythmic properties that became even stronger in heart failure without favoring ventricular proarrhythmias [12, 13]. Furthermore, RAN has been shown to enhance the antiarrhythmic action of amiodarone in converting postoperative atrial fibrillation earlier than amiodarone alone and to reduce the number of ventricular extrasystoles in patients with ischemic heart disease [14, 15]. Experimental investigations strengthened the role of the increased late Na+ current subsequent to oxidative stress after ischemia-reperfusion injury, in ventricular arrhythmogenesis [16]. According to these investigations, RAN prevented pacing-induced reentrant and multifocal VF by blocking the late Na+ current in experimental animal models of myocardial ischemia. Ca2+-dependent early afterdepolarizations have been suggested as a main mechanism triggering these arrhythmias [17]. In the present study, VF occurring under ischemia-reperfusion conditions, was related, at least partially, to such mechanisms that were depressed by RAN.
Despite the generally accepted multifactorial origin of intraoperative VF during cardiac surgery, aortic declamping-induced reperfusion of the ischemic ventricle remains the main proposed mechanism promoting the initiation and recurrence of this serious arrhythmia. Collectively, candidates for AVR that chronically received oral RAN showed significantly fewer episodes of VF during surgery. VF when manifested in these patients was more readily terminated with internal defibrillation in terms of the number and the energy of countershocks.
5. Limitations
There were no direct comparisons of ECG variables between the two groups in the respective data prior to the operation. This inevitably allows comparative evaluations only between the postoperative measurements in the control and the RAN groups. Another limitation is that there were difficulties in confirming documentation regarding the history of miscellaneous cardiac arrhythmias and/or silent myocardial infarction of the patients. Such events might have limited, at least in part, a clear conclusion on the incidence of spontaneous VF during the surgical procedure. The underlying valvular disease itself and the type of prosthetic valves used in the two groups could have also influenced the course of arrhythmogenesis.
6. Conclusion and Clinical Impact
Our data suggest that RAN is safe and effective in preventing surgical VF even in heart failure patients, implicating that this medication could be a promising measure in preventing reperfusion arrhythmias in cardiac surgery. Recurrent VF could be associated with electrical remodeling and direct injury to the heart due to internal defibrillations. This may worsen the postoperative outcome especially in patients with systolic dysfunction. Larger prospective studies are necessary to confirm our observations on the protective effect of RAN against ventricular arrhythmias in the setting of cardiac surgery.
Acknowledgements
We thank the personal of the ICU and Cardiovascular Clinic for the kind cooperation in registering postoperative data related to the current study protocol.
Conflict of Interest
None declared.
References
- Spanos PK, Brown AL Jr, McGoon DC. The significance of intraoperative ventricular fibrillation during aortic valve replacement. J Thorac Cardiovasc Surg 73 (1977): 605-610.
- Mauermann WJ, Pulido JN, Barbara DW, et al. Amiodarone versus lidocaine and placebo for the prevention of ventricular fibrillation after aortic crossclamping: a randomized, double-blind, placebo-controlled trial. J Thorac Cardiovasc Surg 144 (2012): 1229-1234.
- Praeger P, Kay R, Moggio R, et al. Prevention of ventricular fibrillation after aortic declamping during cardiac surgery. Tex Heart Inst J 15 (1988): 98-101.
- Mauermann WJ, Pulido JN, Barbara DW, et al. Amiodarone versus lidocaine and placebo for the prevention of ventricular fibrillation after aortic crossclamping: a randomized, double-blind, placebo-controlled trial. J Thorac Cardiovasc Surg 144 (2012): 1229-1234.
- Baraka A, Kawkabani N, Dabbous A, et al. Lidocaine for prevention of reperfusion ventricular fibrillation after release of aortic cross-clamping. J Cardiothorac Vasc Anesth 14 (2000): 531-533.
- Aidonidis I, Brachmann J, Seller H, et al. Cardiac sympathetic nervous activity during myocardial ischemia, reperfusion and ventricular fibrillation in the dog--effects of intravenous lidocaine. Cardiology 80 (1992): 196-204.
- Aidonidis I, Brachmann J, Rizos I, et al. Lidocaine converts inducible ventricular fibrillation into sustained ventricular tachycardia in conscious dogs with recent myocardial infarction. Cardiology 85 (1994): 378-387.
- Dhalla AK, Wang WQ, Dow J, et al. Ranolazine, an antianginal agent, markedly reduces ventricular arrhythmias induced by ischemia and ischemia-reperfusion. Am J Physiol Heart Circ Physiol 297 (2009): 1923-1929.
- Kloner RA, Dow JS, Bhandari A. The antianginal agent ranolazine is a potent antiarrhythmic agent that reduces ventricular arrhythmias: through a mechanism favoring inhibition of late sodium channel. Cardiovasc Ther 29 (2011): 36-41.
- Frommeyer G, Rajamani S, Grundmann F, et al. New insights into the beneficial electrophysiologic profile of ranolazine in heart failure: prevention of ventricular fibrillation with increased postrepolarization refractoriness and without drug-induced proarrhythmia. J Card Fail 18 (2012): 939-949.
- Bazoukis G, Yeung C, Wui Hang Ho R, et al. Association of QT dispersion with mortality and arrhythmic events-A meta-analysis of observational studies. J Arrhythm 36 (2019): 105-115.
- Simopoulos V, Hevas A, Hatziefthimiou A, et al. Amiodarone plus Ranolazine for Conversion of Post-Cardiac Surgery Atrial Fibrillation: Enhanced Effectiveness in Reduced Versus Preserved Ejection Fraction Patients. Cardiovasc Drugs Ther 32 (2018): 559-565.
- Simopoulos V, Tagarakis G, Daskalopoulou S, et al. Ranolazine enhances the antiarrhythmic activity of amiodarone by accelerating conversion of new-onset atrial fibrillation after cardiac surgery. Angiology 65 (2014): 294-297.
- Tsanaxidis N, Aidonidis I, Hatziefthimiou A, et al. Ranolazine Added to Amiodarone Facilitates Earlier Conversion of Atrial Fibrillation Compared to Amiodarone-Only Therapy. Pacing and Clinical Electrophysiology 40 (2017): 372-378.
- Nanda S, Levin V, Martinez MW, et al. Ranolazine--treatment of ventricular tachycardia and symptomatic ventricular premature beats in ischemic cardiomyopathy. Pacing Clin Electrophysiol 33 (2010): 119-120.
- Morita N, Lee JH, Xie Y, et al. Suppression of re-entrant and multifocal ventricular fibrillation by the late sodium current blocker ranolazine. J Am Coll Cardiol 57 (2011): 366-375.
- Ogawa T, Honjo H, Yamazaki M, et al. Ranolazine Facilitates Termination of Ventricular Tachyarrhythmia Associated With Acute Myocardial Ischemia Through Suppression of Late INa-Mediated Focal Activity. Circ J 81 (2017): 1411-1428.
