Radical versus Local Surgical Excision for Early Rectal Cancer: A Systematic Review and Meta-Analysis
Article Information
Sarah El-Nakeep1, Samragnyi Madala2, Anusha Chidharla5, Balarama Krishna Surapaneni2, Subhrajit Saha4, Benjamin Martin3, Anup Kasi5
1Faculty of Medicine, Ain Shams University, Cairo, Egypt
2University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA, United States; 52242
3Division of Colorectal Surgery, University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS, United States: 66160
4Division of Radiation Oncology, University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS, United States: 66160
5Division of Medical Oncology, University of Kansas Cancer Center, 2650 Shawnee Mission Pkwy, Westwood, KS, United States 66205
*Corresponding Author: Dr. Anup Kasi, University of Kansas Cancer Center, 2650 Shawnee Mission Pkwy, Westwood, KS, United States 66205.
Received: 26 September 2023; Accepted: 03 October 2023; Published: 20 January 2024
Citation:
Sarah El-Nakeep, Samragnyi Madala, Anusha Chidharla, Balarama Krishna Surapaneni, Subhrajit Saha, Benjamin Martin, Anup Kasi. Radical versus Local Surgical Excision for Early Rectal Cancer: A Systematic Review and Meta- Analysis. Archives of Internal Medicine Research. 7 (2024): 01-11.
View / Download Pdf Share at FacebookAbstract
Background:
Radical excision (RE) for rectal cancer carries a higher risk of mortality and morbidity, while local excision (LE) could decrease these postoperative risks. However, the long-term benefit of LE is still debatable.
Aim:
To study the effectiveness of LE versus RE in T1 and T2 rectal cancer.
Methods:
A systematic review and meta-analysis was conducted using key databases like PubMed and ClinicalTrials.gov. Only cohort studies and randomized controlled trials were included. RevMan 5.4 tool was used for data analysis. Both clinical and statistical heterogeneity of the studies were assessed, and I2 >75% was considered as highly heterogeneous. The primary outcomes being measured were 5-year overall survival (OS) and 5-year disease free survival (DFS). A subgroup analysis of patients with T1-only was also conducted, without adjuvant chemo/radiotherapy.
Results:
A total of 18 studies were included for final meta-analysis. Four were RCTs, while the other 15 were retrospective cohort studies. One included study had data from both RCT and non-RCT study groups. Nine studies were multicentered or national studies while nine were unicentral. There was no difference in risk ratio (RR) between OS: RR 0.95, 95% Confidence Interval (CI) [0.91, 0.99] and DFS: RR 0.93, 95% CI [0.87, 1.01]. There were lower hazards ratios in OS: RR 1.41, 95% CI [1.14, 1.74] and DFS: RR 1.95, 95% CI [1.36, 2.78] with radical, as compared to LE. Lower recurrence rate was associated with RE. Random effect model was used due to clinical heterogeneity between studies (different surgical procedures, tumor staging, adjuvant chemo or radiotherapy).
Conclusions:
LE for early-stage rectal cancer has lower 5-year OS and DFS than RE, with higher local recurrence rate. However, LE is associated with lower early postoperative mortality, morbidity and length of stay as compared to RE.
Keywords
Early rectal cancer; T1-T2 rectal cancer; Metaanalysis; Radical excision; Local excision
Early rectal cancer articles; T1-T2 rectal cancer articles; Metaanalysis articles; Radical excision articles; Local excision articles
Article Details
List of abbreviations:
CI: Confidence Interval; CRC: colorectal cancer; CRT: Chemoradiotherapy; DFS: Disease Free Survival
HR: Hazards Ratio; LAR: Low Anterior Resection; LE: Local Excision; OVS: Overall Survival
RCTs: Randomized Controlled Trials; RE: Radical Excision; RR: Risk Ratio; RT: Radiation Therapy
TAMIS: Transanal Minimally Invasive Surgery; TEM: Transanal Endoscopic Microsurgery
TME: Total Mesorectal Excision
Introduction
Colorectal cancer (CRC) is the third most common malignancy in the United States. Rectal cancer occurs in 40,000 people annually in the United States with the incidence rising among the younger population [1].
Rectal cancer is associated with a higher overall risk of recurrence than colon cancer with a similar stage. The local recurrence in rectal cancer is believed to be secondary to the anatomic location and inherent challenge for surgeons to achieve clear, circumferential margins. The standard of care surgery for mid-to-low rectal cancers currently is total mesorectal excision (TME) with RE of the rectum and the draining lymph nodes. This encompasses the surgical techniques of low anterior resection (LAR) and abdominoperineal resection (APR). For select patients LE can be done via transanal excision, transanal endoscopic microsurgery (TEM), or transanal minimally invasive surgery (TAMIS) [2].
LE is an organ preserving surgery compared to RE with TME for patients with stage I rectal cancer. Data regarding local recurrence and long term patient outcomes for patients who underwent LE for T2 tumors is limited [3]. The long-term oncologic outcomes of LE are still debatable. Currently there is no standard treatment consensus for early rectal cancers and the best standard of care treatment for early rectal cancers has yet to be defined. Therefore, we aim to do a systematic review and meta-analysis to study the effect of LE versus RE in T1 and T2 rectal cancer.
Radical excision has a higher risk of morbidity and mortality, while LE could reduce the postoperative complications. Generally, stage II and III rectal cancers are treated with trimodality therapy with chemotherapy, radiation therapy (RT) and surgery unlike colon cancer [4]. The determination of an optimal treatment plan for a rectal cancer patient is complex. Additionally, consideration should be given to the postoperative complications after surgery like restoring and maintaining bowel function, preservation of genitourinary functions and anal incontinence, which can affect quality of life.
Risk of local recurrence is higher in patients with rectal cancer compared to colon cancer. Treatment decision should be made carefully in each patient with regards to use of multimodality therapy involving chemotherapy, radiation treatment, and surgery. For early-stage rectal cancers local excision is attractive because of sphincter preservation and decreased morbidity. However, there is some contradictory data in the literature about local recurrence and overall survival when compared to radical excision [5].
Methods:
We conducted the search on 15 June 2021 (from inception until 15 June 2021), using the following keywords and PICOTS as shown in the table.
We searched PubMed, CENTRAL, and ClinicalTrials.gov databases, using relevant keywords. We included only cohort studies and randomized controlled trials.
All included trials contained at least one of the primary outcomes.
Data analysis:
We collected data for our predesigned primary outcomes:
- Overall survival at 5 years (RR and HR)
- Disease free survival after 5 years (RR and HR)
- Local recurrence rate
- Total postoperative 30-days mortality
- Post operative hospital stay in days - postoperative
- All adverse events
Statistical calculation:
We used Revman desktop version 5.4 for data analysis. For continuous outcomes we used standardized mean difference (SMD), the only continuous outcome data were for hospital stay in days- postoperative. We used standardized mean difference, as the data presented in the studies as mean and standard deviation, or interquartile range. In case of IQR we used the median as mean, and divided IQR/1.35.
For survival data outcomes we calculated both the risk ratio (RR) and hazard ratio (HR). We calculated both hazards ratio (HR) and risk ratio (RR) for the 5-years survival analyses, with their 95% confidence intervals (CI). We extracted HR from the studies if mentioned, or else from Kaplan Meyer’s curves using the methods presented in Tierney et al [6].
We assessed both clinical and statistical heterogeneity of the studies, and considered I2 >75% as highly heterogeneous, we used random effect model if highly heterogeneous.
For mortality outcome, we used Peto’s odds ratio, as some studies showed zero mortality, so we were unable to calculate odds or risk ratios.
We used COVIDENCE online software which is used primarily for systematic reviews and meta-analysis for the screening process. We used Excel sheets for data extraction. We used Revman desktop version 5.4 for data analysis.
Subgroup analysis: We did subgroup analysis of T1 only patients who did not receive mandatory pre or postoperative adjuvant chemo or radiotherapy.
Sensitivity Analysis:
- We used HR and RR for the same outcome five-year OS and we found a difference between both statistical calculations.
- We compared fixed effect model with random effect model: we didn’t find any change in the statistical direction of the outcomes, but the difference was slightly higher in the RE than the FE model.
Unit of analysis:
We planned to use data before the crossover, but there was no cross over trials in our finally included studies.
PICOTS and keywords used in the search.
|
PICOTS: Population: Patients with T1 and T2 rectal cancer Intervention: minimal surgery Comparative: standard surgery Outcome: · Primary outcomes: survival- cure- time to relapse · Secondary outcomes: side effects- hospitalization days post operative Time: Time to primary outcomes Setting: Hospital for Surgery Search strategy key words: Total mesorectal excision OR Low anterior resection OR Abdominoperineal resection OR Laparoscopic OR Robotic OR Open Transanal TME AND Local Excision" OR Transanal Endoscopic Microsurgery OR Transanal Minimally Invasive Surgery" OR "Robotic transanal minimally invasive surgery OR Transanal Excision OR transanal endoscopic operation OR robotic transanal excision AND Rectal OR Anal Limitations for all: Title and abstract |
CENTRAL search:
#1 (Local Excision OR Transanal Endoscopic Microsurgery OR Transanal Minimally Invasive Surgery OR Robotic transanal minimally invasive surgery OR Transanal Excision OR transanal endoscopic operation OR robotic transanal excision) AND (Total mesorectal excision OR Low anterior resection OR Abdominoperineal resection OR Laparoscopic OR Robotic OR Open Transanal TME):ti,ab,kw AND (Rectal OR anal):ti,ab,kw (Word variations have been searched)
Results:
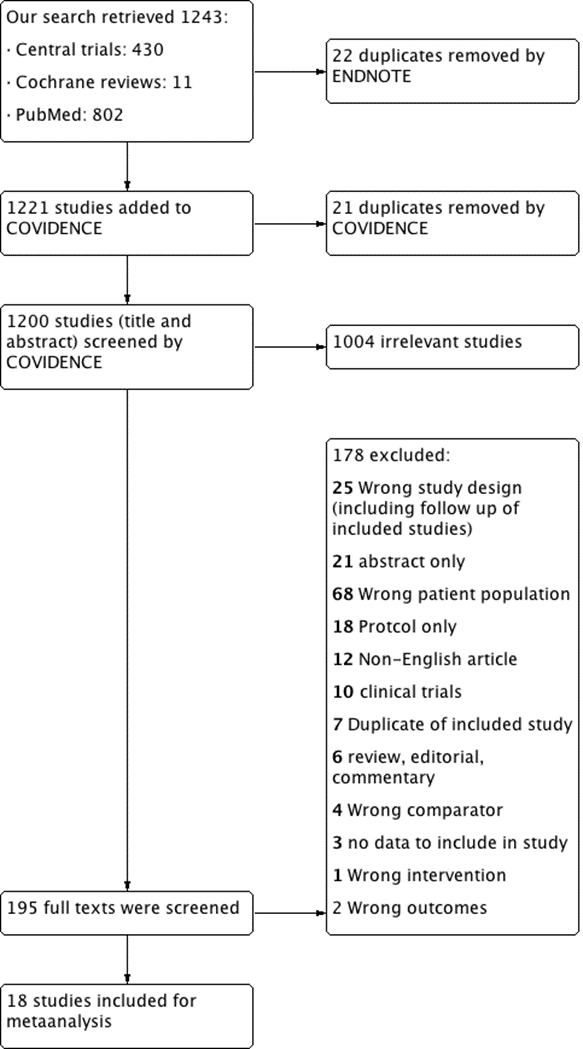
- Our search retrieved 1243 studies.
- Central trials: 430
- Cochrane reviews: 11
- PubMed: 802
Two authors screened independently the title, abstracts, and full text articles using COVIDENCE online program. Any discrepancies between the authors were resolved through a consensus between them after discussion.
22 duplicates removed by Endnote.
- 1221 studies screened through COVIDENCE:
21 duplicates removed by COVIDENCE
- 1200 studies (title and abstract) screened using COVIDENCE
Exclusion after title and abstract screening: 1004 irrelevant
- 195 full texts were screened:
Excluded after full text screening: 176
25 Wrong study design (including follow up of included studies)
21 abstracts only
68 Wrong patient population
18 Protocol only
12 non-English articles
10 clinical trials
6 reviews, editorial, commentary
7 Duplicate of included study
4 Wrong comparator
3 no data to include in the study
1 Wrong intervention
2 Wrong outcomes
- Included: 18 studies.
Flow chart:
We retrieved from the search strategy 1243 records. After entering the data in COVIDENCE, we excluded 21 duplicates. Endnote application desktop version X7.8 removed 22 duplicates. Then first two authors screened independently the titles and abstracts for exclusion of irrelevant records. Both authors screened independently 195 reports of studies in their full text for final inclusion. Any discrepancy or disagreements were resolved through a consensus with the principal investigator. (Figure 1)
We finally included 18 studies [7-24]. One of the included studies [9] had two cohorts; an RCT and a non-RCT, we treated the data of each study separately.
Results:
We used random effect model due to clinical heterogeneity between studies (different surgical procedures, tumor staging, adjuvant chemo or radiotherapy). Six studies showed absent 30-days postoperative mortality in both groups, so we used peto-odds ratio. Postoperative mortality and morbidity were higher with RE than LE.
|
Outcome or Subgroup |
Number of Studies |
Number of Participants |
I2 |
Statistical methods |
Estimate effect [CI] |
|
|
1- Overall survival in 5-years (HR) |
14 |
23717 |
64% |
Hazard Ratio (IV, Random, 95% CI) |
1.41 [1.14, 1.74] |
|
|
2- Overall survival in 5-years (RR) |
15 |
27037 |
77% |
Risk Ratio (M-H, Random, 95% CI) |
0.95 [0.91, 0.99] |
|
|
3- Disease Free Survival in 5-years (HR) |
10 |
2568 |
18% |
Hazard Ratio (IV, Random, 95% CI) |
1.95 [1.36, 2.78] |
|
|
4- Disease Free Survival in 5-years (RR) |
10 |
3541 |
59% |
Risk Ratio (M-H, Random, 95% CI) |
0.93 [0.87, 1.01] |
|
|
5- Local recurrence rate |
13 |
6952 |
40% |
Risk Ratio (M-H, Random, 95% CI) |
2.85 [1.86, 4.36] |
|
|
6- All-cause mortality in 30-days |
10 |
8800 |
13% |
Peto Odds Ratio (Peto, Fixed, 95% CI) |
0.36 [0.22, 0.59] |
|
|
7- Total post-operative morbidity |
7 |
671 |
71% |
Risk Ratio (M-H, Random, 95% CI) |
0.38 [0.18, 0.80] |
|
|
8- Hospital stay post-operative |
5 |
3336 |
99% |
Std. Mean Difference (IV, Random, 95% CI) |
-2.23 [-3.64, -0.83] |
|
|
9- Subgroup analysis: Overall survival T1 only- without obligatory CRT |
6 |
14275 |
79% |
Hazard Ratio (IV, Random, 95% CI) |
1.46 [1.08, 1.99] |
|
Table 1: showing the statistical results of the metaanalysis
We did not find any difference in risk ratio (RR) between OS and DFS. But there were lower hazards ratios in OS and DFS with radical, as compared to local excision. Lower recurrence rate was associated with RE, as shown in table 1.
Four of the included studies were RCTs (Bach 2021 [9]; DeGraaf 2009 [10]; Lezoche 2012 [17]; Winde 1997 [23]).
Only one study reported the quality of life as an outcome for the interventions (endoluminal locoregional resection (ELRR) versus TME) in Leroche et al.[25]. We excluded this study because of absence of data on our primary or secondary assigned outcomes.
We excluded Ellis et al. as the authors only reported the 7 years survival with no reported data on the 5-years OS or DFS [26].
From the included studies: nine were single center studies and nine were multicenter studies as shown in table 2.
|
Study ID |
Stage of the tumor |
Number of patients in the study |
Age of the patients |
Number of centers |
Country of the conducted study |
|
Olsheski 2013 |
T1-T2 N0M0 |
2391 |
APR 68 vs LE alone 68 vs LE+RT 67 |
Multicenteral |
USA |
|
Tokunaga 2017 |
T1T2 |
11 |
63±11 (TLE) 65±13 (47-76) (TME) |
Single center |
Japan |
|
Saraste 2013 |
T1T2N0M0 |
3694 |
median age AR 70 y vs APR 71y vs LE 75 y |
Multicentral |
Sweden |
|
Melnitchouk 2020 |
T1T2 |
2996 |
Multicentral |
USA |
|
|
Stornes 2016 |
T1T2 |
2136 |
T1: (TEM)72.5 vs 68.2 (TME); T2: 81.7 vs 69.9 |
Multicentral |
Norway |
|
Witjes 2021 |
T1T2N0M0 |
112 |
72 (EMR), 67 (TEM/TAMIS), 62 (LAR/APR) |
Multicentral |
UK |
|
Allaix 2012 |
T2 |
78 |
72 (38–91) TEM vs 65 (34–90) LR |
Single center |
Italy |
|
Atallah 2020 |
T1 |
10053 |
Multiple groups (<55, 55-64, 65-74, >75) |
Multicentral |
USA |
|
Bach 2021 |
T1T2 |
55 RCT; 68 Non RCT |
65 (52–79) LE vs 65 (49–83) RR |
Multicentral |
UK |
|
DeGraaf 2009 |
T1 |
155 |
TEM 71 (44-92) vs TME 67(48-83) |
Single center |
Netherlands |
|
Debove 2017 |
T1 |
91 |
62 +/- 12 |
Single center |
France |
|
Elmessiry 2014 |
T1T2 |
153 |
68.7 +/-11.9 LE vs 65.3 +/-15.3 TME |
Single center |
USA |
|
Endreseth 2005 |
T1T2 |
291 |
68 TME vs 77 LE |
Single center |
Norway |
|
Hwang 2019 |
T1 |
268 |
58.0 ± 9.5 TAE vs 59.0 ± 9.6 TME |
Single center |
Korea |
|
Lai 2019 |
T1T2 |
173 |
59.7 ± 13.9 LE vs 63.0 ± 12.9 TME |
Single center |
Taiwan |
|
Lee 2017 |
T2 |
4822 |
65.6 LE+Neoadj CRT vs 65.9 LE+ Adj CRT vs 66 RR |
Multicentral |
USA |
|
Lezoche 2012 |
T2N0M0 |
100 |
ELRR 66 vs 66 TME |
Multicentral |
Italy |
|
Winde 1997 |
T1 |
54 |
Single center |
Germany |
Table 2: showing the characteristics of the included studies
Forest plot for different outcomes:
Clinical trials registered on the topic:
It is shown in Supplementary table 1 that two trials are in the recruiting phase and two trials completed the recruiting of their designed number of patients but have not published their results yet. Two out of the four trials are open label with no blinding of participants or the physicians. Only one trial will restrict the population included to the T1N0 stage of rectal cancer and will recruit 326 patients in both arms. It will complete recruitment in May 2023.
Discussion:
Previously, local excision was performed in patients who couldn’t undergo radical surgery. Due to high rate of survival and lower postoperative complications, it is now being used as a standard procedure for early rectal cancer. Though the patient is eligible for radical surgery, local excision is becoming the preferred technique for most patients [3].
In a retrospective population-based study of 2391 patients, there was no difference in disease specific survival between LE with RT vs APR, however overall survival was better in patients with APR for T2 disease [19]. As per Melnitchouk et al, in a study of 2084 patients with T1 tumors and 912 patients with T2 tumors, there was no difference in survival in patients who underwent LE and APR in T1 patients. Also, there was no difference in survival in T2 tumors between LE with chemotherapy and APR [18]. In a small sample study by Tokunaga et al, and colleagues, early rectal cancer patients who underwent chemoradiotherapy followed by LE were compared to TME. Disease-free survival and overall survival were not significantly different between the groups, however the study included only 5 patients in each cohort [27]. In a phase 2 trial of clinical stage T2N0 rectal cancer patients the three-year disease-free survival was 88% in the neoadjuvant chemotherapy followed by local excision compared to the radical excision group [28]. Currently prospective trials are being conducted for early distal rectal cancers comparing LE with neoadjuvant treatment to radical excision [17].
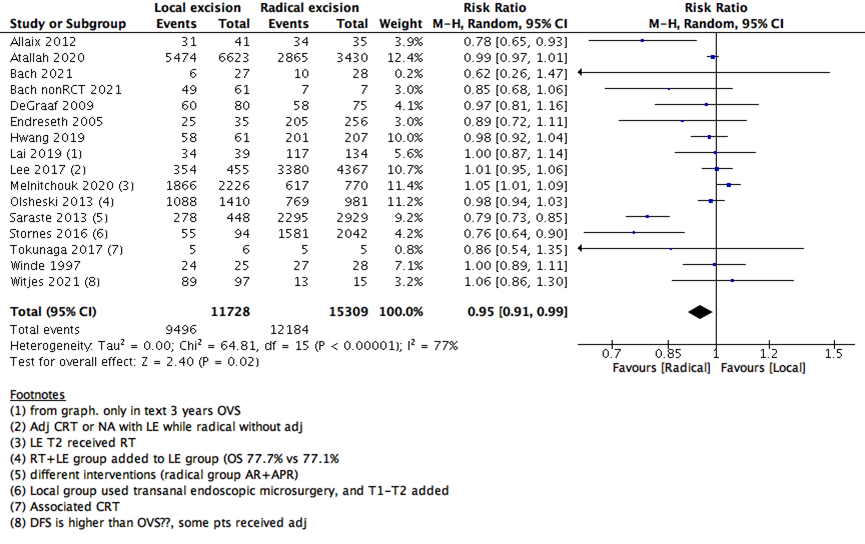
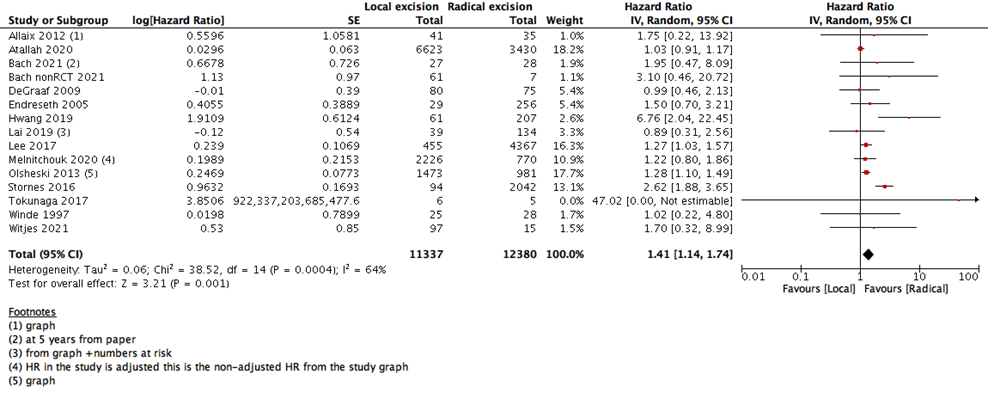
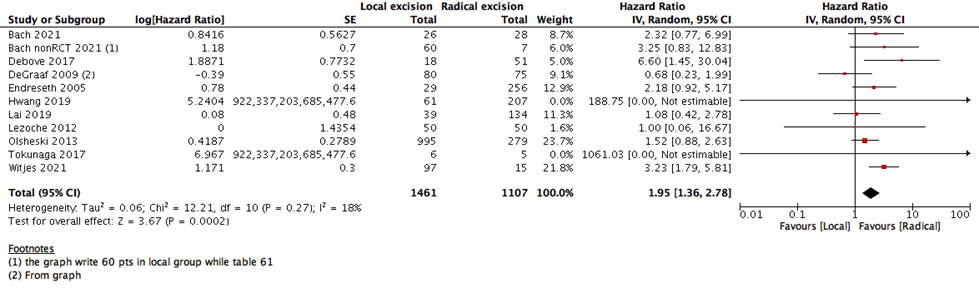
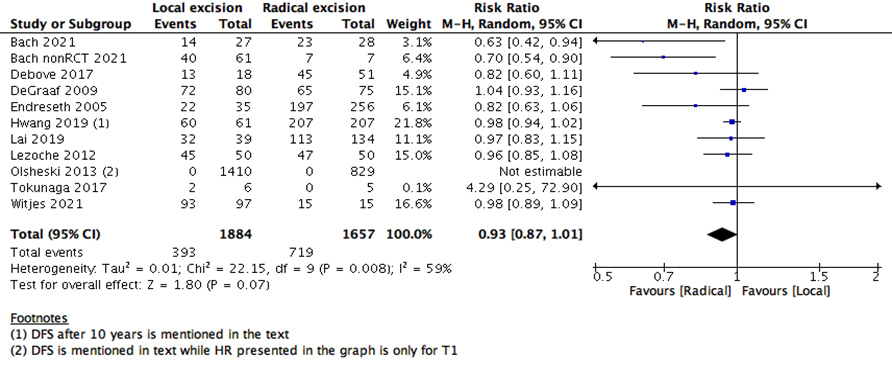
We measured survival analysis in two different statistical ways: hazards ratio depicting the decline of number of patients across time (5- years survival), and risk ratio depicting the difference between the number of patients at the start and end of the 5 years period. We found no difference in RR between LE versus RE (15 studies with 27037 participants; RR: 0.95; 95% CI: 0.91 to 0.99) (Figure 2). However, the HR showed a higher survival effect with RE as compared to LE (14 studies with 23717 participants; HR: 1.95, 95% CI: 1.36 to 2.78) (Figure 3). HR is a better indicator of the survival than RR.
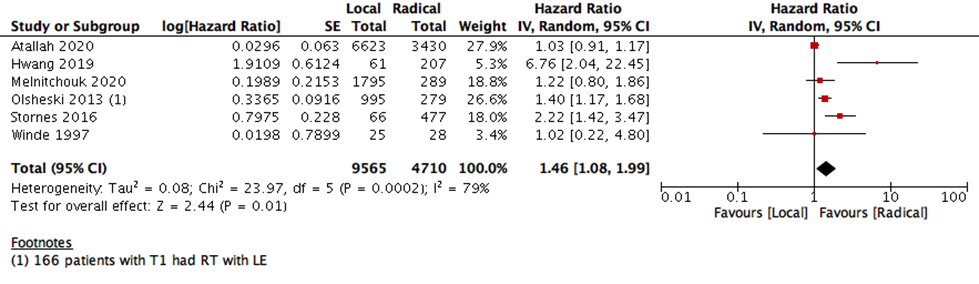
This statistical preference of RE as opposed to LE maintained after subgroup analysis, removing all participants with T2 stage, or those who received CRT. We found that T1 rectal cancer patients show higher survival with RE than LE (6 studies with 14275 participants; HR 1.46; 95% CI 1.08- 1.99) (Figure 4). The same was found with DFS, as there was no difference between the two interventions when using the RR (10 studies with 3541participants; RR 0.93; 95% CI 0.87 to 1.01) (Figure 6). However, when using the HR for the same outcome we find that RE has higher survival than LE (10 studies with 2568 participants; HR 1.95; 95% CI 1.36 to 2.78) (Figure 5).
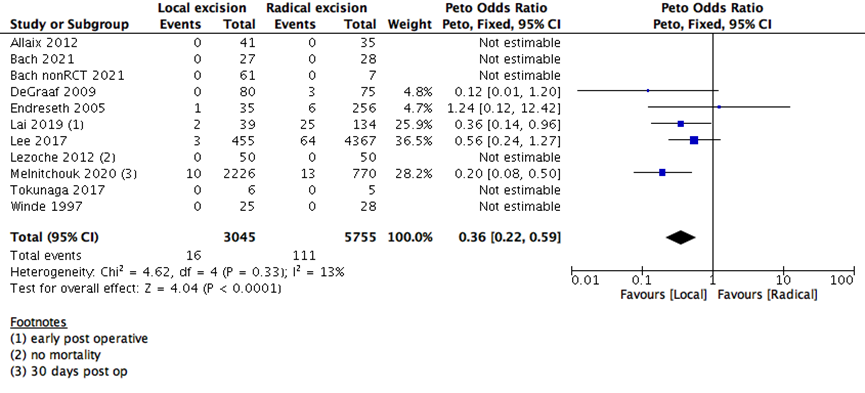
We found that early postoperative all-cause mortality (30 days postoperative) was higher in RE as compared to LE group (11 studies with 8800 participants; Peto’s OR 0.36; 95% CI 0.22- 0.59) (Supplementary Figure 1). This is consistent with the hazard of major surgery as opposed to a minor surgery.
A recent metaanalysis compared only TME to TEM surgical procedures for the outcome (local recurrence) and found only three RCTs for inclusion. They reported that there was no difference between the two interventions as regards to local recurrence or postoperative complications [29]. In our metaanalysis we included two of the three RCTs but unfortunately we didn’t find the full manuscript of Chen et al [30] to extract the data from, so it was not included on our final analysis.
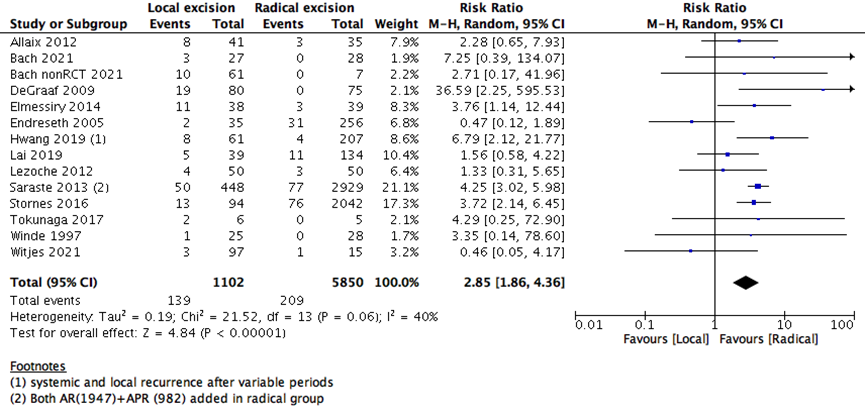
The risk of local tumor recurrence was higher in LE than RE group (13 studies with 6952 participants; RR 2.85; 95% CI 1.86 to 4.36) (Supplementary Figure 2). This may raise the question, whether proceeding with a major surgery in early rectal cancer may benefit the patients more as it decreases the risk of recurrence and increase OS and DFS. Even though major surgeries are associated with more patient reservations, and higher risk of adverse events.
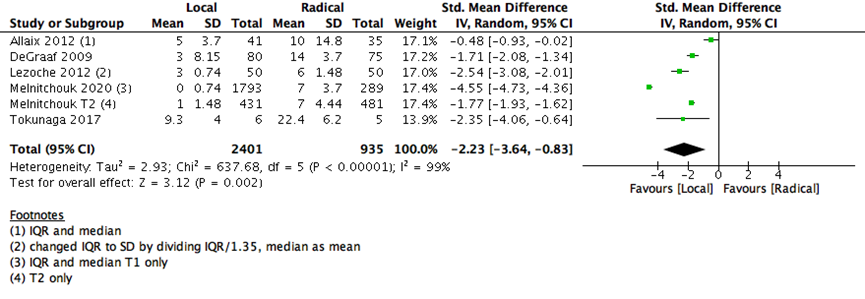
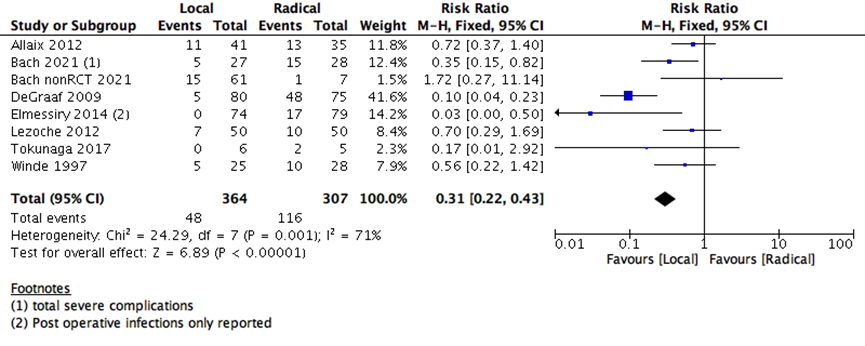
The number of days spent in the hospital in early postoperative stage were lower with LE than RE (5 studies with 3336 participants-standard mean difference -2.23 (95% CI: -3.64 to -0.83) (Supplementary Figure 3). The risk of early postoperative morbidity was higher with RE than LE group (7 studies with 671 participants; RR 0.31; 95% CI 0.22- 0.43) (Supplementary Figure 4). Both adverse events (postoperative hospitalization days and morbidity) and early postoperative 30 days mortality are consistent with the outcome of any major surgery, as RE is substantially carries more surgical risk and need more postoperative time for healing than the LE surgeries.
The techniques of the surgeries used in local excision in the included studies versus the techniques used the radical excision are mentioned in Supplementary table 2 in detail.
We found a Cochrane review on the topic [31] which measured the OS as a dichotomous outcome using odds ratio, and found similar results to our study. They found no difference between LE and TME. This concludes the fact that selecting the most appropriate statistical method could play a role in understanding the risk and benefit for the patients, but sometimes could be misleading.
A meta analysis including 29 RCTs with 6237 participants showed laparoscopic, robotic, and open TME had no difference in the intra and post operative morbidity, need for reoperation or presence of anastomotic leaks. However, the need for blood transfusion was lower with laparoscopic TME as compared to the other two. In addition, the risk of post operative infections and morbidity was lower with laparoscopic surgery as compare to open [32]. We didn’t compare between different kinds of surgical intervention in the same intervention group as this was beyond the scope of our study.
Atallah et al [8] carries the largest population weight in our study (51.3%), this may have an impact on our final results. It is a retrospective cohort- national study conducted in the United States using the National Cancer Database (NCDB). It is also included in our subgroup analysis as all patients in this study were T1 stage with no CRT.
Conclusion:
LE for early-stage rectal cancer could decrease the risk of early postoperative mortality, hospitalization, and morbidity as compared to RE. However, RE could increase the 5-year OS, 5-year DFS and decrease the local recurrence. More large scale and national RCTs are needed to fully understand the benefit and risk of each of the surgical approaches. Currently a personalized approach to the patient’s condition is recommended to decide the best surgical and medical plan to the patient with early rectal cancer. The addition of chemo and radiotherapy for early rectal cancer needs further studies.
Declarations
Author contributions:
Sarah El-Nakeep: Formulated the search strategy, conducted the search, screening of the studies, data extraction, data analysis, and writing of the first draft of the manuscript.
Samragnyi Madala: Conducted the screening of studies, data extraction, and revised the manuscript.
Anusha Chidharla: Contributed to writing the first draft.
Benjamin Martin: Formulated the research question, revised the manuscript, provided expertise on surgical aspects related to the study.
Subhrajit Saha: Revision of manuscript
Anup Kasi: Formulated the research question, oncology expert on the topic, revised the results, and the last version of the manuscript.
All Authors revised and accepted the final submitted manuscript.
Conflict-of-interest statement:
All the authors report no relevant conflicts of interest for this article.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials:
Data can be obtained if requested from the first author of the article.
Ethics Approval:
Not applicable
Consent to participate:
Not applicable
References
- Quinn TJ, Kabolizadeh P. Rectal cancer in young patients: incidence and outcome disparities. J Gastrointest Oncol 11 (2020): 880-93.
- Rajput A, Bullard Dunn K. Surgical management of rectal cancer. Semin Oncol 34 (2007): 241-249.
- You YN, Baxter NN, Stewart A, et al. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Annals of surgery 245 (2007): 726-33.
- Morino M, Risio M, Bach S, et al. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc 29 (2015): 755-73.
- Chang AJ, Nahas CS, Araujo SE, Nahas SC, Marques CF, Kiss DR, et al. Early rectal cancer: local excision or radical surgery? J Surg Educ 65 (2008): 67-72.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8 (2007): 16.
- Allaix ME, Arezzo A, Giraudo G, et al. Transanal endoscopic microsurgery vs. laparoscopic total mesorectal excision for T2N0 rectal cancer. J Gastrointest Surg 16 (2012): 2280-2287.
- Atallah C, Taylor JP, Lo BD, et al. Local excision for T1 rectal tumours: are we getting better? Colorectal Dis 22 (2020): 2038-2048.
- Bach SP, Gilbert A, Brock K, et al. Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): a randomised, open-label feasibility study. The lancet Gastroenterology & hepatology 6 (2021): 92-105.
- De Graaf EJ, Doornebosch PG, Tollenaar RA, et al. Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol 35 (2009): 1280-1285.
- Debove C, Svrcek M, Dumont S, et al. Is the assessment of submucosal invasion still useful in the management of early rectal cancer? A study of 91 consecutive patients. Colorectal Dis 19 (2017): 27-37.
- Elmessiry MM, Van Koughnett JA, Maya A, et al. Local excision of T1 and T2 rectal cancer: proceed with caution. Colorectal Dis 16 (2014): 703-709.
- Endreseth BH, Myrvold HE, Romundstad P, et al. Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum 48 (2005): 1380-1388.
- Hwang Y, Yoon YS, Bong JW, et al. Long-term Transanal Excision Outcomes in Patients With T1 Rectal Cancer: Comparative Analysis of Radical Resection. Ann Coloproctol 35 (2019): 194-201.
- Lai IL, You JF, Chern YJ, et al. Survival analysis of local excision vs total mesorectal excision for middle and low rectal cancer in pT1/pT2 stage and intermediate pathological risk. World J Surg Oncol 17 (2019): 212.
- Lee L, Kelly J, Nassif GJ, et al. Chemoradiation and Local Excision for T2N0 Rectal Cancer Offers Equivalent Overall Survival Compared to Standard Resection: a National Cancer Database Analysis. J Gastrointest Surg 21 (2017): 1666-1674.
- Lezoche E, Baldarelli M, Lezoche G, et al. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 99 (2012): 1211-1218.
- Melnitchouk N, Fields AC, Lu P, et al. Local versus Radical Excision of Early Distal Rectal Cancers: A National Cancer Database Analysis. Ann Surg Oncol 27 (2020): 2169-2176.
- Olsheski M, Schwartz D, Rineer J, et al. A population-based comparison of overall and disease-specific survival following local excision or abdominoperineal resection for stage I rectal adenocarcinoma. J Gastrointest Cancer 44 (2013): 305-312.
- Saraste D, Gunnarsson U, Janson M. Local excision in early rectal cancer-outcome worse than expected: a population based study. Eur J Surg Oncol 39 (2013): 634-639.
- Stornes T, Wibe A, Nesbakken A, et al. National Early Rectal Cancer Treatment Revisited. Dis Colon Rectum 59 (2016): 623-629.
- Tokunaga T, Nishi M, Higashijima J, et al. Feasibility of Transanal Local Excision Following Chemoradiation for Lower Rectal Cancer. Anticancer Res 37 (2017): 5617-5622.
- Winde G, Blasius G, Herwig R, et al. Benefit in therapy of superficial rectal neoplasms objectivized: transanal endoscopic microsurgery (TEM) compared to surgical standards. Minimally invasive therapy and allied technologies 6 (1997): 315-323.
- Witjes CDM, Patel AS, Shenoy A, et al. Oncological outcome after local treatment for early stage rectal cancer. Surg Endosc (2021).
- Lezoche E, Paganini AM, Fabiani B, et al. Quality-of-life impairment after endoluminal locoregional resection and laparoscopic total mesorectal excision. Surg Endosc 28 (2014): 227-234.
- Ellis TC, Charlton ME, Stitzenberg KB. Patient-Reported Roles, Preferences, and Expectations Regarding Treatment of Stage I Rectal Cancer in the Cancer Care Outcomes Research and Surveillance Consortium. Dis Colon Rectum 59 (10): 907-915.
- Tokunaga T, Nishi M, Higashijima JUN, et al. Feasibility of Transanal Local Excision Following Chemoradiation for Lower Rectal Cancer. Anticancer Research 37 (2017): 5617.
- Garcia-Aguilar J, Renfro LA, Chow OS, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 16 (2015): 1537-1546.
- Ahmad NZ, Abbas MH, Abunada MH, et al. A Meta-analysis of Transanal Endoscopic Microsurgery versus Total Mesorectal Excision in the Treatment of Rectal Cancer. Surg J (N Y). 7 (2021): e241-e50.
- Chen YY, Liu ZH, Zhu K, et al. Transanal endoscopic microsurgery versus laparoscopic lower anterior resection for the treatment of T1-2 rectal cancers. Hepatogastroenterology 60 (2013): 727-32.
- Vennix S, Pelzers L, Bouvy N, et al. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database of Systematic Reviews 4 (2014).
- Simillis C, Lal N, Thoukididou SN, et al. Open Versus Laparoscopic Versus Robotic Versus Transanal Mesorectal Excision for Rectal Cancer: A Systematic Review and Network Meta-analysis. Ann Surg 270 (2019): 59-68.
Supplementary data
Supplementary Table 1: show ongoing and finished clinical trials on ClinicalTrials.gov
|
Study ID |
Surgeries performed |
|
Olsheski 2013 |
LE versus APR |
|
Tokunaga 2017 |
LE vs TME |
|
Saraste 2013 |
LE, AR, APR, Hartman? |
|
Melnitchouk 2020 |
LE versus APR |
|
Stornes 2016 |
TEM vs TME |
|
Witjes 2021 |
TEM; TAMIS; ESD; EMR vs APR ; TME |
|
Allaix 2012 |
TEM vs RT + TEM vs LR |
|
Atallah 2020 |
LE (open or laparoscopic) vs RR |
|
Bach 2021 |
RT followed by TEM vs LAR |
|
DeGraaf 2009 |
RT before TEM vs TME alone |
|
Debove 2017 |
LE vs TME vs LE+TME |
|
Elmessiry 2014 |
TME vs LE |
|
Endreseth 2005 |
Transanal Exicison vs Major Surgery (AR,APR,Hartmann) |
|
Hwang 2019 |
TAE vs TME (some patients received chemo and radio) |
|
Lai 2019 |
LE vs TME |
|
Lee 2017 |
NA-CRT+LE vs LE+adj CRT vs RS |
|
Lezoche 2012 |
Endoluminal locoregional resection (ELRR) by TEM vs laparoscopic TME (All patients had chemoradiotherapy) |
|
Winde 1997 |
TEM vs AR TME |
Supplementary Table 2: showing the surgical procedures