Quercetin Inhibits the Formation of Atherosclerosis Plaque by Protecting Vascular Endothelial Cells
Article Information
Ying Zhang1*, Fangfang Dou2
1Department of Endocrinology, Shanghai children’s hospital, Shanghai Jiaotong University, Shanghai, P.R. China
2Basic Research Department, Shanghai Geriatric Institute of Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, P.R. China
*Corresponding Author: Ying Zhang, Department of Endocrinology, Shanghai children’s hospital, No. 355, Luding Road, Putuo district, Shanghai, 200062, P.R. China
Ying Zhang and Fangfang Dou contributed equally to this work
Received: 14 October 2019; Accepted: 18 November 2019; Published: 11 December 2019
Citation:
Ying Zhang, Fangfang Dou. Quercetin Inhibits the Formation of Atherosclerosis Plaque by Protecting Vascular Endothelial Cells. J Pharm Pharmacol Res 3 (2019): 116-127.
View / Download Pdf Share at FacebookAbstract
Background: Quercetin and its derivatives are the most widely distributed flavonoids, which have many pharmacological activities, such as anti-oxidation, anti-aging and anti-inflammatory, Quercetin inhibits the occurrence of atherosclerosis (AS) due to its antioxidant activity, but the mechanism is unknown.
Results: Our research found that quercetin plays a role in reducing the formation of atherosclerotic plaque by protecting vascular endothelial cell injury. In an atherosclerotic mouse model, quercetin inhibited the formation of atherosclerotic plaques and downregulated the level of endothelial cell protein C receptors (EPCR), thrombomodulin (TM), von willabrand factor (vWF) and intercellular cell adhesion molecule-1(ICAM-1) in peripheral blood of AS mouse, because of increasing the number of vascular endothelial cells at the atherosclerotic plaque. In vitro model of human vascular endothelial cells (HUVECs) injury by H2O2, we found that quercetin significantly reduced endothelial cell apoptosis, and it can prevent vascular endothelial cell injury by inhibiting the secretion of chemokines (CXCL1 and CXCL8) and inflammatory (TNF-α, IL-β, IL-6 and IL-12) factors and reducing macrophage infiltration, at the same time, quercetin promotes the secretion of pro-angiogenic chemokines (CXCL4 and CXCL10) from vascular endothelial cells. Real-time PCR and western-blot assay found that the quercetin protects vascular endothelial cells by inhibiting apoptotic signaling pathway proteins (Bax and Bcl-2) and inflammatory regulatory pathway proteins (IκBα and p65).
Conclusion: Consequently, we concluded that the role of quercetin in the protection of vascular endothelial cells against atherosclerotic plaques may be through the inhibition of vascular endothelial cell apoptosis.
Keywords
Quercetin, Human vascular endothelial cells, Endothelial cell injury, Atherosclerosis
Article Details
1.Introduction
Endothelial cells are important metabolic and endocrine organs, which plays an important role in regulating vascular function. Vascular endothelial injury is common in AS, hypertension, diabetic vascular disease and other cardiovascular and cerebrovascular diseases, and it was considered as the first step in these diseases [1, 2]. Many studies have shown that the mechanism of endothelial cell damage mainly involves inflammation and oxidative stress [3]. Through the reduction of predisposing factors, inhibition of inflammatory reactions and oxidative stress, a variety of chemicals and traditional Chinese medicine delay endothelial cell senescence and play a role in endothelial protection [4, 5]. On the other hand, endothelial cells have the ability to proliferate and self-healing [6]. Therefore, we will further investigate whether quercetin inhibits the formation of atherosclerotic plaque by protecting endothelial cells and promoting endothelial cell repair.
Quercetin and its derivatives are the most widely distributed flavonoids found in all plant, which is widely distributed in plant flowers, leaves and fruits, it is also the most important bioflavonoid in the human diet. Recent studies found that quercetin have many pharmacological activities, such as anti-oxidation, anti-inflammatory, anti-hypertensive, anti-platelet aggregation, anti-cancer, anti-aging, anti-mutation and anti-AS. Some research indicated that the role of anti-aging, anti-mutation and anti-AS of quercetin were all related to the antioxidant effect [7, 8]. It has been reported quercetin could reduce the hydrogen peroxide content of normal human neutrophil, its antioxidant activity was related to the number of hydroxyl [9]. Quercetin can also prevent daunomycin-induced oxidative damage in rat myocardial mitochondria, decrease the secretion of adenylate cyclase and glutathione reductase which induced by daunomycin, at the same time, quercetin can prevent the decline of glutathione peroxidase [10]. Therefore, we observed whether quercetin inhibits plaque formation by protecting endothelial cells in in vivo and in vitro models of AS.
2. Materials and Methods
2.1 Establishment of atherosclerotic mouse model
60 ApoE-/- C57BL/6 mice were randomly divided into 4 groups of 15 mice each. Mice of control group were fed with normal feed, and the remaining mice are all fed with high-fat diet (normal mouse feed +21% lard +0.15% cholesterol) (SLAC, China) for 12 weeks. 100 μg/ml quercetin was dissolved in DMSO, on the day of AS model preparation, 100 μg/ml quercetin and 400 μg/ml α-tocopherol were intragastrically administered to mice everday. The mice in the control group and the model group were given the same dose of sterilized double distilled water for gastric gavage for 12 weeks respectively.
2.2 HE staining and endothelial cell immunofluorescence in left ventricular outflow tract of mouse
Paraffin sections of the left ventricular outflow tract were made from 4% paraformaldehyde-perfused mice. After deparaffinization and antigen retrieval of the sections, the blocking solution was blocked at room temperature for 30 minutes, mouse CD31 antibody (Abcam, USA) was diluted with primary antibody dilutions (Beyotime, China) and incubated overnight at 4°C, the goat anti-mouse 546 fluorescent secondary antibody (Beyotime, China) was incubated for 1 hour at 37°C after sections were washing with PBS for 3 times at room temperature, finally, the tissue was covered with a DAPI dye-containing sealing agent (Beyotime, China). After dewaxing and antigen retrieval, tissue sections were also stained with hematoxylin and eosin (Beyotime, China), and the morphology and number of endothelial cells were observed under a fluorescence microscope.
2.3 Primary HUVEC culture and H2O2 -induced HUVEC injury
HUVECs were purchased from Beijing Qinyuanhao Biological Technology Co., Ltd (Beijing China). HUVECs were cultured in 10 cm dishes with EBM-2 (Lonza, USA) at 37°C in a humid 5% CO2 atmosphere, the culture medium was replaced every other day and cells were passaged when cells density was more than 90%. In order to observe the hydrogen peroxide (H2O2) damage and the protective effect of quercetin to HUVECs, we seeded HUVECs in 6-well dishes, 10 μM quercetin or 200 μM α-tocopherol was added into the culture system after 24 hours, and then cells were exposed to 100 μM H2O2 for 4 hours to detect HUVEC injury and related markers.
2.4 Examine the level of EPCR, TM, vWF in peripheral blood of AS model
EPCR (Yanke, China), TM (R&D, USA), vWF (Abcam, USA) and ICAM-1 (Abcam, USA) were detected by corresponding Elisa kits, detailed experimental steps refer to the corresponding operation manual. Briefly, the obtained fresh mouse blood was stored in a heparin-containing anticoagulant tube, the upper serum was aspirated after centrifugation at 12000 rpm at 4°C for 20 minutes, and serum can be stored at -80°C for assay at the end of reaction. Dilute serum samples into sample diluent NS according to the different concentration, and the final absorbance at 450 were measured by microplate reader, the final concentration of the sample is calculated based on absorbance and standard concentration.
2.5 Detection of endothelial cell apoptosis by TUNEL kit
The culture medium of H2O2-treated HUVECs was removed and washed once with phosphate buffered solution (PBS) (Gibco, USA). Cells were fixed with 4% paraformaldehyde for 30 minutes at room temperature, after paraformaldehyde was removed and washed twice with PBS, PBS containing 0.3% Triton X-100 was added and incubated for 5 minutes at room temperature. Prepared TUNEL test solution was added and cells were incubated at 37ºC for 60 minutes in the dark. After two washes in PBS, the cell staining was observed under a fluorescence microscope.
2.6 Chemokine and inflammatory factor level in mouse and cell model
We detected the chemokine level of CXCL1, CXCL8, CXCL4 and CXCL10 in the atherosclerotic mouse model and cell model, the elisa kits of chemokines all purchased from Abcam company. Inflammatory factor including tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), IL-6 and IL-12 of aorta were detected by corresponding Elisa kits (R&D, USA). Detailed experimental method also reference to protocol booklets, serum of AS mouse or supernatant of HUVECs were collected, aorta tissue was cut into small pieces by eye scissors, then put the tissue in saline for homogenization and sonicate, collected sample can be stored at -80°C for assay at the end of reaction. The final concentration of the sample was measured at 450 nm.
2.7 Transwell assay
Transwell assay of Raw264.7 cells (Chinese Academy of Sciences Cell Bank, Shanghai, China) was measured using a transwell chamber with 6.5 mm polycarbonate membrane (8 μm pore size) (Cornning, NY, USA). Normal Raw264.7 (2 × 104 cells/well) cells plated on the upper chamber, HUVECs (5 × 104 cells/well) with fresh medium containing 100 μM H2O2 or 100 μg/ml quercetin and 400 μg/ml α-tocopherol plus H2O2 plated on the lower chamber. The Raw264.7 cells were loaded at to the upper chambers and allowed to migrate for 24 hr at 37°C. Then the cells were fixed with 4% paraformaldehyde for 10 minutes before washed with PBS for 3 times, migrated Raw264.7 cells were stained with crystal violet for 1 hour at 37°C, non-migrating cells on the upper surface of the membrane were removed using a cotton swab. The migrated Raw264.7 cells were counted under the microscopy (CX43 Biological Microscope, Olympus, Japan). Lastly, we calculated the average of 10 fields in each group and compared differences between groups.
2.8 RNA isolation and Real-time PCR assay
Total RNA from HUVEC cells was prepared using Trizol reagent (Invitrogen, CA, USA), according to the manufacturer's instructions. Reverse transcription was performed using a ReverTra Ace qPCR RT kit (TOYOBO, Japan), according to the manufacturer's instructions, and a SYBR Green Realtime PCR Master Mix (TOYOBO, Japan) system was used for all real-time PCR reactions. The comparative threshold cycle (ΔCT) method was used to quantify target mRNA levels of different groups. Target mRNA levels were normalized to an endogenous reference GAPDH and a control. The following primer pairs were used: Bax (sense: 5’-TCCACCAAGA AGCTGAGCGAG -3’ and anti-sense: 5’-GTCCAGCCCATGATGGTTCT -3’), Bcl-2 (sense: 5’-TTCTTTGAGTTCGGTGGGGTC-3’ and anti-sense: 5’-TGCATATTTGTT TGGGGCAGG -3’), IκBα (sense: 5’- TACGTCCCAGGGTCAGAGAG -3’ and anti-sense: 5’- CTCTCTGACCCTGGGACGTA -3’), p65 (sense:5’-ATGCCCAA GGAGTTGAAATTG
T-3’ and anti-sense: 5’-ACACCAAAGTCCCATGTCT
TTTT-3’), and GAPDH (sense: 5’- CTTT GTCAAGCT
CATTTCCTGG-3’ and anti-sense: 5’- TCTTGCTCAG
TGTCCTTGC-3’).
2.9 Western-blot assay
In brief, total protein was extracted from cultured HUVEC cells using RIPA lysis buffer (Beyotime, China) with cocktail tablets (Roche, Germany). Equal amounts of protein were subjected to SDS-PAGE and electro-transferred to 0.45 μm polyvinylidene fluoride filter membrane (Millipore, MA, USA). The membrane was blocked with 1.5 % bovine serum albumin (BSA) in Tris–HCl buffered saline (PH 7.5) for 30 minutes at room temperature and incubated with the primary antibodies against Bax (Cell signaling technology, USA), Bcl-2 (Cell signaling technology, USA), IκBα and phospho- IκBα (Cell signaling technology, USA), p65 and phospho-p65 (Cell signaling technology, USA) or β-actin (Cell signaling technology, USA), all the primary antibody dilution is 1:2000. After a secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit/mouse immunoglobulin (Ig) G antibody (1:2,000, Cell signaling technology, USA) incubated for 2 hour at room temperature, signals were detected by the electro-chemi-luminescence (ECL) substrate (Beyotime, China) and quantified using Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA).
2.10 Statistical analysis
Statistical analysis was performed with SPSS 17.0 software (SPSS Inc., Chicago, USA). Data are presented as mean ± SD of five independent experiments. Student’s t-test for unpaired variable was used to determine statistical significance of differences between groups, and a P-value less than 0.05 was considered statistically significant.
3. Results
3.1 Quercetin inhibits AS plaque formation by protecting vascular endothelial cell in ApoE-/- mice
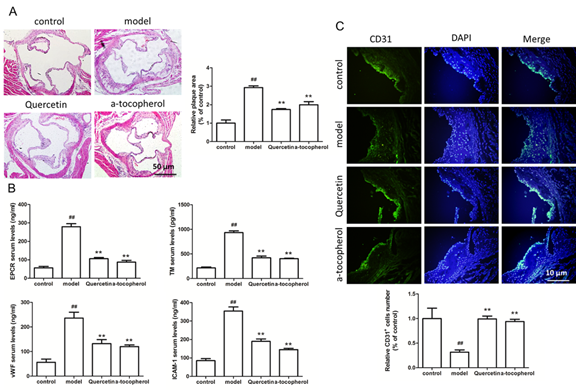
Firstly, we confirmed whether quercetin inhibited the formation of atherosclerotic plaques in ApoE-/- mice. HE staining showed that atherosclerotic plaque area in ApoE-/- mice with the high-fat diet plus quercetin was significantly smaller than that of the mice fed with the high-fat diet alone (Figure 1A), the result confirmed that quercetin indeed inhibited the formation of atherosclerotic plaques, however, how is quercetin achieve this effect? Endocalocytes act as the first line of defense against the formation of atherosclerotic plaque, elevated plasma EPCR, TM and vWF levels are considered hallmarks of vascular injury or dysfunction, therefore, we examined molecular markers of HUVECs damage including EPCR, TM, vWF and ICAM-1 in the serum of mouse, results of Elisa showed that EPCR, TM, vWF and ICAM-1 levels were significantly increased in ApoE-/- mice with high-fat diet, however, the EPCR, TM, vWF and ICAM-1 expression were significantly decreased in ApoE-/- mice with quercetin and high-fat diet (Figure 1B), in addition to detecting the above endothelial cell injury marker, we examined the number of vascular endothelial cells in the left ventricular outflow tract of ApoE-/- mice, results of CD31 fluorescent staining showed that CD31 positive staining of left ventricular outflow tract of ApoE-/- mice with high-fat diet was weaker than that of ApoE-/- mice with normal diet, it indicated that ApoE-/- mice with high fat diet have occurrence with endothelial cell injury, however, ApoE-/- mice given a high-fat diet with quercetin have a large number of CD31-positive cells, it indicated that quercetin has the function of protecting endothelial cells, we also performed a statistical analysis of the number of CD31-positive cells in the left ventricular outflow tract of ApoE-/- mice, Figure 1C showed that compared with the normal control, the number of endothelial cells of ApoE-/- mice with high-fat diet was significantly reduced, quercetin significantly increased the number of endothelial cells in high-fat diet ApoE-/- mice, these results demonstrated that quercetin inhibited the formation of atherosclerotic plaques by inhibiting vascular endothelial cells damage and protecting vascular endothelial cells.
3.2 Quercetin inhibits oxidative damage and apoptosis of HUVECs
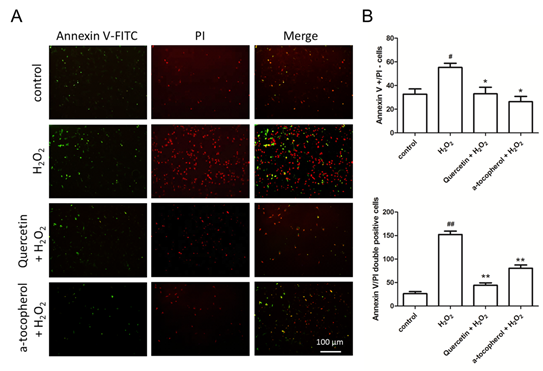
In order to detect the protective ability of quercetin on HUVECs with H2O2 injury, we examined the inhibition ability of quercetin on endothelial cell apoptosis, annexin V and propidium iodide (PI) staining was used to observe the number of apoptosis cells (Figure 2A). Then, we separately counted the number of annexin V positive cells with PI negative cells and annexin V / PI double positive cells, we found that H2O2 promoted early apoptosis of HUVECs which were stained by annexin V only, quercetin reduced the number of early apoptotic cells; similarly, quercetin also reduced annexin V / PI double positive staining of late apoptotic cells (Figure 2B). Therefore, we hypothesize that quercetin protects vascular endothelial cells by inhibiting apoptosis of HUVECs.
3.3 Regulatory effect of quercetin on chemokine secretion of HUVECs
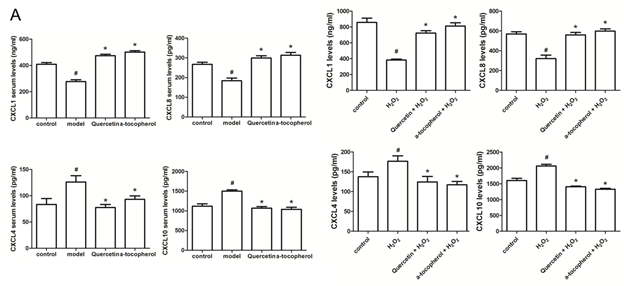
After clarifying that quercetin inhibits apoptosis of HUVECs, we examined the effect of quercetin on the secretion of chemokines. Different types of chemokines play different funcitons on regulating the formation of blood vessels of vascular endothelial cells. We examined the chemokines CXCL (Chemokine (C-X-C motif) ligand) 1 and CXCL8 that promote angiogenesis, as well as the chemokines CXCL4 and CXCL10 that inhibit angiogenesis, our results showed that the levels of CXCL1 and CXCL8 in the serum of the model group were significant lower than those of the control group, and the levels of CXCL4 and CXCL10 were significantly increased contrast to the control group. Compared with the model group, quercetin significantly promoted the serum levels Of CXCL1 and CXCL8 and inhibited the serum levels of CXCL4 and CXCL10 (Figure 3A). We also got the same conclusion in HUVECs cultured in vitro (Figure 3B). These results indicate that quercetin protects vascular endothelial cells and promotes angiogenesis. Since vascular endothelial cells are the first line of defense to protect blood vessels from damage, protecting vascular endothelial cells plays an important role in preventing atherosclerosis.
3.4 Quercetin reduces the recruitment of macrophages by vascular endothelial cells
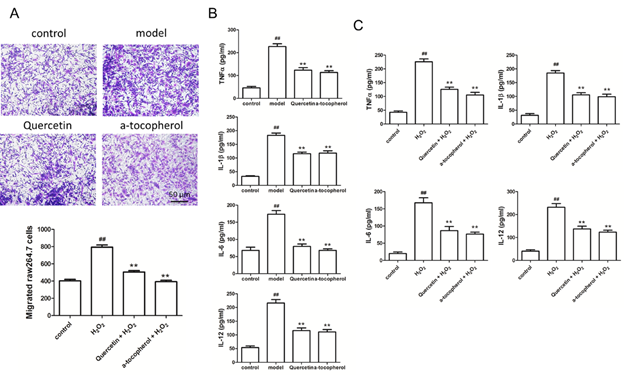
Based on the above findings, we would further confirmed that quercetin have the effect of preventing atherosclerotic plaque formation. At the first, we used Transwell assay to observe the chemotaxis of HUVECs to macrophages, we found that when HUVECs were administered with hydrogen peroxide damage, a large number of macrophages were induced to migrate to HUVECs, while quercetin plus hydrogen peroxide were both added to the supernatant of HUVECs, the number of macrophages chemotaxis to HUVECs was greatly reduced (Figure 4A). The above results indicate that the damage can promote the migration of a large number of macrophages to endothelial cells, thereby promoting the formation of atherosclerotic plaques. Quercetin delays the migration of macrophages by inhibiting endothelial cell damage, thus delaying atherosclerosis formation. Macrophages that migrate to the injury site release a large amount of inflammatory factors, exacerbating inflammatory response. Accordingly, we detected the inflammatory cytokines levels of aorta tissue and cultured HUVECs, the level of TNF-α, IL-1β, IL-6 and IL-12 were all increased in ApoE-/- mice with high-fat diet (Figure 4B) or HUVECs stimulated by hydrogen peroxide (Figure 4C), and quercetin similarly inhibited the expression of inflammatory cytokine. The above results showed that quercetin could protect vascular endothelial cells by inhibiting apoptosis and inflammatory response, and these results also indicated that quercetin further protected endothelial cells from atherosclerotic plaque by protecting vascular endothelial cells, reducing chemokine release, and inhibiting macrophage migration.
3.5 Mechanism of quercetin inhibiting apoptosis and inflammation
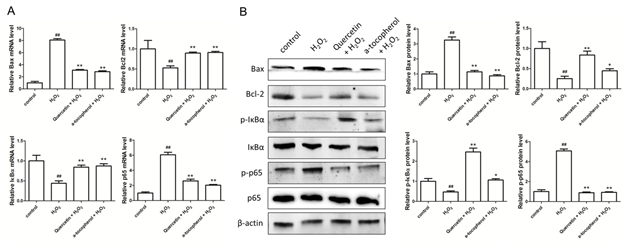
In order to study how quercetin affects apoptosis and inflammatory factor secretion we examined the expression of signaling molecules involved in apoptosis and inflammation if HUVECs in vitro. We first tested the expression of proteins associated with apoptosis (Bax and Bcl2) and inflammation (IκBα and NFκB(p65)) at the mRNA level, hydrogen peroxide promotes the expression of Bax and P65 and inhibits the expression of Bcl2 and IκBα at mRNA level, quercetin inhibits the expression of Bax and P65 and promotes the expression of Bcl2 and IκBα at mRNA level (Figure 5A). Bax, Bcl2, IκBα and p65 protein level was confirmed by western-blot, hydrogen peroxide similarly promotes the expression of Bax and phosphorylated p65 and inhibits the expression of Bcl2 and phosphorylated IκBα, and quercetin inhibits the expression of Bax and phosphorylated p65 and promotes the expression of Bcl2 and phosphorylated IκBα (Figure 5B). Therefore, we have determined that quercetin can inhibit apoptosis and inflammatory response of vascular endothelial cells and protect blood vessels from formatting atherosclerotic plaque by inhibiting expression of Bax and phosphorylation of p65, and by promoting the expression of Bcl2 and phosphorylated IκBα.
Figure 1: Quercetin inhibits atherosclerotic plaque formation and protects vascular endothelial cells. (A) HE staining of left ventricular outflow tract of ApoE-/- mice and relative plaque area of eft ventricular outflow tract of ApoE-/- mice; (B) Elisa detects the serum level of EPCR, TM, vWF and ICAM-1 of ApoE-/- mice which were fed with normal diet, high-fat diet, high-fat diet plus quercetin, high-fat diet plus α-tocopherol; (C) CD31 immunofluorescence staining of left ventricular outflow tract of ApoE-/- mice and relative CD31 positive cells of of left ventricular outflow tract of ApoE-/- mice. Data represent the mean ± SD from 5 independent experiments. Scale bar: 10 μm or 50 μm. ##, ** p<0.01, #: vs control, *: vs model.
Figure 2: Quercetin inhibits H2O2-induced HUVECs apoptosis. (A) Apoptosis of HUVECs stained by Annexin V and PI. Scale bar: 100 μm; (B) The number of Annexin V positive staining and PI negative staining HUVECs; (C) The number of Annexin V and PI double positive staining HUVECs. Data represent the mean ± SD from 5 independent experiments. Data represent the mean ± SD from 5 independent experiments. #, *p<0.05, ##, **p<0.01, #: vs control, *: vs H2O2 group.
Figure 3: Effect of quercetin on the secretion of chemokines. (A) Elisa detects the serum level of CXCL1, CXCL8, CXCL4 and CXCL10 of ApoE-/- mice which were fed with normal diet, high-fat diet, high-fat diet plus quercetin, high-fat diet plus α-tocopherol; (B) Elisa detects the level of CXCL1, CXCL8, CXCL4 and CXCL10 of HUVECs stimulated by H2O2, quercetin and α-tocopherol. Data represent the mean ± SD from 5 independent experiments. #, * p<0.05, #: vs control, *: vs H2O2 group.
Figure 4: Quercetin inhibits macrophage migration and inflammatory factor secretion. (A) Transwell assay detects the migrated macrophage induced by HUVECs cells under different stimuli; (B) Elisa detects the aortic tissue level of TNF-α, IL-1β, IL-6 and IL-12 of ApoE-/- mice which were fed with normal diet, high-fat diet, high-fat diet plus quercetin, high-fat diet plus α-tocopherol; (C) Elisa detects the level of TNF-α, IL-1β, IL-6 and IL-12 of HUVECs stimulated by H2O2, quercetin and α-tocopherol. Data represent the mean ± SD from 5 independent experiments. Scale bar: 50 μm. #, * p<0.05, ##, ** p<0.01, #: vs control, *: vs model.
Figure 5: Effect of quercetin on the expression of apoptosis and inflammatory regulatory protein. (A) Real-time PCR examins the level of Bax, Bcl2, IκBα and p65 at mRNA level; (B) Western blot detects the expression of Bax, Bcl2, phosphorylated IκBα and phosphorylated p65 at protein level. Data represent the mean ± SD from 5 independent experiments (#, * p<0.05, ##, ** p<0.01, #: vs control, *: vs model).
4. Discussion
Endothelial cell injury is the initiating factor of atherosclerotic lesions. Studies confirm that there is endothelial dysfunction in the subclinical stage of atherosclerosis [11]. Therefore, inhibition of endothelial cell injury, or protection of damaged endothelial cells is of great importance for the delay of the occurrence and development of AS. Quercetin is widely found in vegetables, fruits and botanicals, such as onion, honey and wine, and quercetin is easy to extract, separate and detect. In recent years, the research of quercetin is increasing in domestic and foreign pharmaceutical region. It was found that quercetin have many pharmacological activities, including anti-oxidation, anti-inflammatory, lowering blood pressure, anti-platelet aggregation, anti-cancer, anti-aging, anti-mutation and anti-atherosclerosis [12, 13]. Therefore, we firstly observed whether quercetin could inhibite the formation of atherosclerotic plaque, and whether the role of preventing atherosclerosis of quercetin was realized by protecting vascular endothelial cells. Since quercetin has antioxidant function in vascular endothelial cells, we continued to investigate whether quercetin inhibited apoptosis of HUVECs, results showed that quercetin does reduce the number of apoptotic cells in HUVECs induced by H2O2. Some studies have found that quercetin can effectively improve mouse experimental colitis through anti-inflammatory and anti-oxidation mechanisms [14]. Studies have shown that quercetin improved tunicamycin-induced endoplasmic reticulum stress by regulating the stress response genes glucose regulated protein 78 (GRP78) and C / EBP homologous protein (CHOP) [15]. Therefore, quercetin could inhibit oxidative stress in different ways and protect cells from damage.
Vascular endothelium is not only an important barrier of the intima, but also has a secretory function. Normal endothelial function has an important protective effect on the vascular wall, and vascular endothelial cell injury is the initial stage of atherosclerosis and many diseases. Functional changes that occur after endothelial cell injury are important factors that lead to the development of cardiovascular diseases such as hypertension, heart failure, coronary heart disease, postoperative restenosis, and primary pulmonary hypertension, endothelial cell injury also involved in the pathological processes of kidney disease, diabetes and other refractory diseases [16, 17]. The traditional view believed that the pathological lipid deposition in the artery wall is a key factor in the development of AS, however, the present study found that dysfunctional endothelial cells play a pivotal role in the formation of atherosclerotic plaques [18, 19]. Therefore, we examined whether quercetin protected endothelial cells at the left ventricular outflow tract of ApoE-/- mice on a high-fat diet, results showed that the number of endothelial cells in ApoE-/- mice with high-fat diet was significantly reduced compared with that in normal diet mice, it shows that high-fat diet leads to vascular endothelial cell injury, and the number of endothelial cells in ApoE-/- mice fed high-fat diet and quercetin was significantly increased, these results indicated that quercetin also has the function of protecting endothelial cells in mice. Since quercetin protects vascular endothelial cells, does it prevent the formation of atherosclerotic plaques by protecting the vascular endothelium? HE staining showed that atherosclerotic plaque in ApoE-/- mice fed high-fat diet and quercetin was significantly decreased, it indicated that protection of endothelial cells could indeed reduce the development of atherosclerotic plaque. Studies on the role of quercetin in protecting vascular endothelial cells through the mechanism of anti-oxidative injury in vitro AS model were more in-depth [20-22]. However, little is known about the protective effects of quercetin on vascular endothelial cells in an in vivo model of atherosclerotic mice. Therefore, we further clarified the role of quercetin in inhibiting vascular endothelial cell injury and anti-atherosclerosis in vitro and in vivo experimental model, we confirmed that quercetin inhibited the development of atherosclerotic plaques by protecting vascular endothelial cells.
5. Conclusion
Our research showed that quercetin exerts potent anti-oxidant, anti-apoptosis and anti-inflammatory effects, thus exerting the protective effect against on vascular endothelial cells injury, therefore, quercetin in the protection of vascular endothelial cells against atherosclerotic plaques may be through the inhibition of vascular endothelial cell apoptosis.
Conflict of Interest
None
Acknowledgement
The present study was supported by grants from the Shanghai Municipal Commission of Health and Family Planning (program no. 20174Y0007), from Natural Science Foundation of China (program no. 81704129), and from the Shanghai Health Bureau Youth Fund (program no. 201640232).
References
- Schwartz BG, Economides C, Mayeda GS, et al. The endothelial cell in health and disease: its function, dysfunction, measurement and therapy. International journal of impotence research 22 (2010): 77-90.
- Gimbrone MA, Jr., Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circulation research 118 (2016): 620-636.
- Higashi Y. Mechanisms of impairment of endothelial cell. Nihon rinsho Japanese journal of clinical medicine 70 (2012): 1519-1523.
- Ming GF, Tang YJ, Hu K, et al. Visfatin attenuates the ox-LDL-induced senescence of endothelial progenitor cells by upregulating SIRT1 expression through the PI3K/Akt/ERK pathway. International journal of molecular medicine 38 (2016): 643-649.
- Tsai KL, Huang PH, Kao CL, et al. Aspirin attenuates vinorelbine-induced endothelial inflammation via modulating SIRT1/AMPK axis. Biochemical pharmacology 88 (2014): 189-200.
- Watt SM, Athanassopoulos A, Harris AL, et al. Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. Journal of the Royal Society, Interface 6 (2010): 731-751.
- Ferraresi R, Troiano L, Roat E, et al. Essential requirement of reduced glutathione (GSH) for the anti-oxidant effect of the flavonoid quercetin. Free radical research 39 (2005): 1249-1258.
- De Oliveira MR, Nabavi SM, Braidy N, et al. Quercetin and the mitochondria: A mechanistic view. Biotechnology advances 34 (2016): 532-549.
- Zielinska M, Kostrzewa A, Ignatowicz E. Antioxidative activity of flavonoids in stimulated human neutrophils. Folia histochemica et cytobiologica 38 (2000): 25-30.
- Lopez-Revuelta A, Sanchez-Gallego JI, Hernandez-Hernandez A, et al. Membrane cholesterol contents influence the protective effects of quercetin and rutin in erythrocytes damaged by oxidative stress. Chemico-biological interactions 161 (2006): 79-91.
- Choi BJ, Prasad A, Gulati R, et al. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. European heart journal 34 (2013): 2047-2054.
- Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 33 (1995): 1061-1080.
- Miles SL, McFarland M, Niles RM. Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human disease. Nutrition reviews 72 (2014): 720-734.
- Guazelli CF, Fattori V, Colombo BB, et al. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. Journal of natural products 76 (2013): 200-208.
- Suganya N, Bhakkiyalakshmi E, Suriyanarayanan S, et al. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell proliferation 47 (2014): 231-240.
- Hirase T, Node K. Endothelial dysfunction as a cellular mechanism for vascular failure. American journal of physiology Heart and circulatory physiology 302 (2012): 499-505.
- Rajendran P, Rengarajan T, Thangavel J, et al. The vascular endothelium and human diseases. International journal of biological sciences 9 (2013): 1057-1069.
- Mannarino E, Pirro M. Endothelial injury and repair: a novel theory for atherosclerosis. Angiology 59 (2008): 69-72.
- Briasoulis A, Tousoulis D, Antoniades C, et al. The role of endothelial progenitor cells in vascular repair after arterial injury and atherosclerotic plaque development. Cardiovascular therapeutics 29 (2011): 125-139.
- Hung CH, Chan SH, Chu PM, et al. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Molecular nutrition and food research 59 (2015): 1905-1917.
- Yang D, Liu X, Liu M, et al. Protective effects of quercetin and taraxasterol against H2O2-induced human umbilical vein endothelial cell injury in vitro. Experimental and therapeutic medicine 10 (2015): 1253-1260.
- Cai X, Bao L, Ding Y, et al. Quercetin alleviates cell apoptosis and inflammation via the ER stress pathway in vascular endothelial cells cultured in high concentrations of glucosamine. Molecular medicine reports 15 (2017): 825-832.