Pulmonary Embolism Secondary to Catheter-Related Thrombosis
Article Information
Géraldine Poenou1, 2*, Emmanuel Toledano1, 2, Yosra Meklhoufi1, Helene Helfer1, Isabelle Mahe1, 2
1Department of Internal Medicine, Louis Mourier-APHP Hospital, Colombes, France
2University of Paris, Paris, France
*Corresponding Author: Géraldine Poenou, Department of Internal Medicine, Louis Mourier-APHP Hospital, Colombes, France
Received: 30 August 2021; Accepted: 07 September 2021; Published: 08 October 2021
Citation: Géraldine Poenou, Emmanuel Toledano, Yosra Meklhoufi, Helene Helfer, Isabelle Mahe. Pulmonary Embolism Secondary to Catheter-Related Thrombosis. Archives of Clinical and Medical Case Reports 5 (2021): 691-698.
View / Download Pdf Share at FacebookAbstract
A central venous catheter is a device used for chemotherapy. Catheters are at high risk for local venous thrombosis. Once a catheter-related thrombosis is formed, the risk of concomitant pulmonary embolism is regularly underestimated. A 58-year-old man with a non-metastatic pulmonary cancer reported a right-side cervical pain and episodes of hemoptysis. A thrombosis of the right internal vein was detected with compressive ultrasound imaging. Because of the presence of abnormal respiratory signs, a CT scan was performed that confirmed a pulmonary embolism. A catheter related thrombosis is associated with a number of clinically relevant complications, including catheter dysfunction, recurrent deep venous thrombosis, post thrombotic syndrome, anticoagulation-associated bleeding and pulmonary embolism. The risk of fatal pulmonary embolism should not be missed.
Keywords
Upper Limb; Deep Venous Thrombosis; Catheter-Related Thrombosis
Upper Limb articles; Deep Venous Thrombosis articles; Catheter-Related Thrombosis articles
Upper Limb articles Upper Limb Research articles Upper Limb review articles Upper Limb PubMed articles Upper Limb PubMed Central articles Upper Limb 2023 articles Upper Limb 2024 articles Upper Limb Scopus articles Upper Limb impact factor journals Upper Limb Scopus journals Upper Limb PubMed journals Upper Limb medical journals Upper Limb free journals Upper Limb best journals Upper Limb top journals Upper Limb free medical journals Upper Limb famous journals Upper Limb Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Deep Venous Thrombosis articles Deep Venous Thrombosis Research articles Deep Venous Thrombosis review articles Deep Venous Thrombosis PubMed articles Deep Venous Thrombosis PubMed Central articles Deep Venous Thrombosis 2023 articles Deep Venous Thrombosis 2024 articles Deep Venous Thrombosis Scopus articles Deep Venous Thrombosis impact factor journals Deep Venous Thrombosis Scopus journals Deep Venous Thrombosis PubMed journals Deep Venous Thrombosis medical journals Deep Venous Thrombosis free journals Deep Venous Thrombosis best journals Deep Venous Thrombosis top journals Deep Venous Thrombosis free medical journals Deep Venous Thrombosis famous journals Deep Venous Thrombosis Google Scholar indexed journals Thrombosis articles Thrombosis Research articles Thrombosis review articles Thrombosis PubMed articles Thrombosis PubMed Central articles Thrombosis 2023 articles Thrombosis 2024 articles Thrombosis Scopus articles Thrombosis impact factor journals Thrombosis Scopus journals Thrombosis PubMed journals Thrombosis medical journals Thrombosis free journals Thrombosis best journals Thrombosis top journals Thrombosis free medical journals Thrombosis famous journals Thrombosis Google Scholar indexed journals Catheter-Related Thrombosis articles Catheter-Related Thrombosis Research articles Catheter-Related Thrombosis review articles Catheter-Related Thrombosis PubMed articles Catheter-Related Thrombosis PubMed Central articles Catheter-Related Thrombosis 2023 articles Catheter-Related Thrombosis 2024 articles Catheter-Related Thrombosis Scopus articles Catheter-Related Thrombosis impact factor journals Catheter-Related Thrombosis Scopus journals Catheter-Related Thrombosis PubMed journals Catheter-Related Thrombosis medical journals Catheter-Related Thrombosis free journals Catheter-Related Thrombosis best journals Catheter-Related Thrombosis top journals Catheter-Related Thrombosis free medical journals Catheter-Related Thrombosis famous journals Catheter-Related Thrombosis Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Hydrocortisone articles Hydrocortisone Research articles Hydrocortisone review articles Hydrocortisone PubMed articles Hydrocortisone PubMed Central articles Hydrocortisone 2023 articles Hydrocortisone 2024 articles Hydrocortisone Scopus articles Hydrocortisone impact factor journals Hydrocortisone Scopus journals Hydrocortisone PubMed journals Hydrocortisone medical journals Hydrocortisone free journals Hydrocortisone best journals Hydrocortisone top journals Hydrocortisone free medical journals Hydrocortisone famous journals Hydrocortisone Google Scholar indexed journals Tuberculomas articles Tuberculomas Research articles Tuberculomas review articles Tuberculomas PubMed articles Tuberculomas PubMed Central articles Tuberculomas 2023 articles Tuberculomas 2024 articles Tuberculomas Scopus articles Tuberculomas impact factor journals Tuberculomas Scopus journals Tuberculomas PubMed journals Tuberculomas medical journals Tuberculomas free journals Tuberculomas best journals Tuberculomas top journals Tuberculomas free medical journals Tuberculomas famous journals Tuberculomas Google Scholar indexed journals venous catheter articles venous catheter Research articles venous catheter review articles venous catheter PubMed articles venous catheter PubMed Central articles venous catheter 2023 articles venous catheter 2024 articles venous catheter Scopus articles venous catheter impact factor journals venous catheter Scopus journals venous catheter PubMed journals venous catheter medical journals venous catheter free journals venous catheter best journals venous catheter top journals venous catheter free medical journals venous catheter famous journals venous catheter Google Scholar indexed journals
Article Details
1. Introduction
A central venous catheter (CVC) is a device used in cancer patients for the management of chemotherapy and supportive therapy. Upper limb deep vein thrombosis (UPDVT) is the most common non-infectious complication of CVC [1]. The incidence of clinically overt catheter-related thrombosis (CRT) varies between 0.3% and 28.3%, whilst the incidence of clinically overt pulmonary embolism (PE) within CRT patients ranges from 15% to 25% [2]. Remarkably, an autopsy-proven PE rate of up to 50% has been reported in those patients with CRT [3]. The high incidence of autopsy-proven PE demonstrates that there is an urgency to explore systematically the risk for presence of PE to prevent the risk of fatal PE in patients with CRT. In the current study, we present a single case of a 58-year-old man who was diagnosed with venous jugular CRT, complicated by a PE revealed by hemoptysis.
2. Case Presentation
A 58-year-old man was diagnosed with a non-metastatic pulmonary cancer after a history of increased dyspnea. The anatomopathological examination showed an epidermoid carcinoma, and the treatment consisted of chemotherapy. His port-a-catheter (PAC) was implanted one month after diagnosis of cancer, and during the first chemotherapy, performed 1month after implantation, the PAC proved functional. On the day of the patient’s second chemotherapy his biological and clinical parameters appeared normal: the patient did not have fever and had a heart rate of 90 bpm, blood pressure of 122/89 mmHg, and an oxygen rate of 96%. During clinical examination, both the chest and PAC examination were ordinary, although the patient reported a right-side cervical pain and episodes of hemoptysis for 3 minutes each. After completion of the regular chemotherapy treatment, an edema of the neck was observed on the side of the PAC, which did not coincide with pain. An X-ray was performed and showed that the tip of the PAC was dispositioned (Figure 1). To investigate the cause of the misplacement, an ultrasound imaging was performed that showed thrombosis within the right internal and external jugular vein (Figure 2). An anticoagulation by enoxaparin 100UI/kg x 2 was started and the interrogation of the patient was renewed, in which he declared an increased dyspnea. Because of the dyspnea and the hemoptysis, PE in addition to the detected thrombosis in the jugular vein was suspected. A CT scan confirmed the jugular vein thrombosis and detected a PE with an endoluminal defect at the right postero-basal segmental pulmonary artery (Figure 3-5).
In the patient’s follow-up, hemoptysis slightly increased, while the dyspnea and the cervical edema decreased. The duration of the anticoagulation will be determined based on the bleeding risk of the patient and the concomitant cancer.
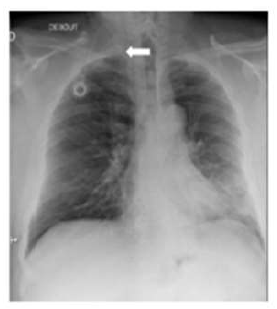
Figure 1: Chest Xray: Disposition of the tip of the port-a-catheter.
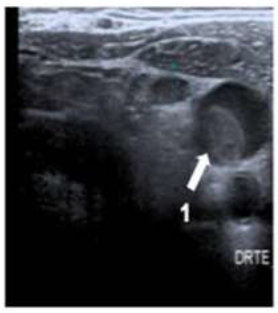
Figure 2: Cervical ultrasoungraphy: 1= internal jugular vein.
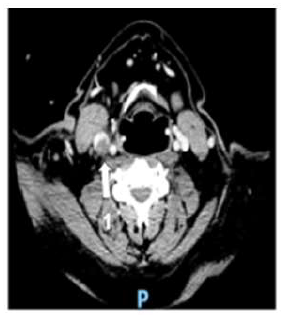
Figure 3: Cervical CT scan axial plane.
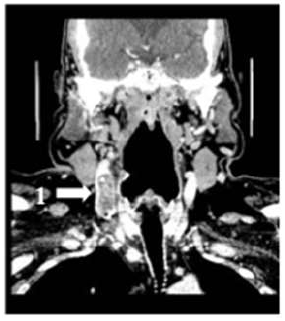
Figure 4: Cervical CT scan coronal plane.
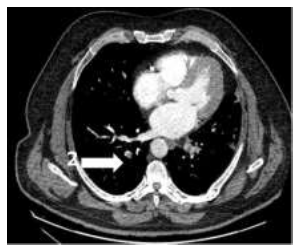
Figure 5: Pulmonary CT scan: 2= right postero-basal segmental pulmonary artery.
3. Discussion
3.1 Generalities
In the general population, UPDVT accounts for only 1 to 4% of all thromboembolic events and can be categorized into idiopathic UPDVT, UPDVT caused by anatomical or biological abnormalities, and UPDVT caused in the presence of equipment [4], such as CRT. The CRT thrombotic model is based on each component of Virchow triad. Endothelial lesions can result from the insertion of the CVC in the vessel, the continuous friction of the CVC against the vessel wall, and the turbulent inflow from the catheter. The presence of a CVC in the vessel lumen hampers blood flow leading to stasis. A state of hyper-coagulability can be introduced by toxic side-effects of some medications, the vessel injury, or synthetic materials [5].
3.2 Presentation
CVCs have considerably improved the management and tolerance of chemotherapy and other supportive care. Data from the RIETE register demonstrated that most cancer patients with CRT were male, younger than 65, and had few additional risk factors [6]. Moreover, death occurring in patients with CRT is mostly PE related [7]. The majority of the patients with CRT were asymptomatic or presented with CVC dysfunction or fever from an associated infection. Symptomatic CRT occurred in 1 to 5% of patients and typically coincided with discomfort, edema, or discoloration at the catheter insertion site or in the ipsilateral upper extremity [8]. As a result of CRT, venous collaterals may be visible in the neck, arm, or chest. Clinicians should examine carefully the catheter entry site for signs of a CVC-related infection, since an infection can result in thrombosis or stimulate thrombosis progression. An important long-term consequence of CRT is the loss of central venous access, which can have significant implications for patient management. CRT is the most common non-malignant cause of superior vena cava syndrome [9]. CRT is associated with a number of clinically relevant complications, including catheter dysfunction, recurrent DVT, PE, post thrombotic syndrome, and anticoagulation-associated bleeding [10]. When examining a patient with CRT, finding symptoms like hemoptysis, shortage of breath, tachycardia should trigger the suspicion of PE.
3.3 Risk factors
Several risk factors for CRT have been reported [11]. Congenital risk factors are a personal history of venous thromboembolism (OR: 2.03; 95% CI: 1.05-3.92) or inherited thrombophilia (Factor V Leiden, OR: 4.6; 95% CI: 2.6-8.1; prothrombin mutation, OR: 4.9; 95% CI: 1.7- 14.3) [2, 12]. Also, the type and the progression of the cancer have to be considered for the evaluation of the risk for CRT (metastases OR: 3.34; 95 % CI 1.17–9.51) [6, 13]. There are other acquired risk factors, such as a body mass index >25 (OR: 51.65 95 % CI 30.72–65.05), age >65 (RR: 2.44 95 % CI 2.05–3.19), or a concomitant infection (RR: 17.6; 95% CI, 4.1 to 74.1) [14-16]. In addition, treatments that can cause damage to the jugular vein or the subclavian vein or an increase of stasis has an impact on the risk of thrombosis (RR: 0.47; 95% CI: 0.23-0.99) [5, 14, 17]. Implanted ports were associated with a significantly lower risk for CRT as compared to PICCs (OR: 0.43; 95% CI: 0.23-0.80) [2]. When the tip of the catheter is located above the proximal superior vena cava, the risk of CRT increases seven times as compared to catheter tips positioned closer to the right atrium (RR: 1.92; 95% CI: 1.22-3.22) [2, 18]. In addition, the size of the catheter’s caliber and the use of some specific materials are reported to be more thrombogenic [19, 20]. If concomitant PE is non-frequent, it is associated with a worse outcome in CRT. In the Newton et al. RIETE study of 1180 patients, recurrent PE after first CRT was confirmed in 13 patients [6]. Although the overall incidence of subsequent PE after CRT was low at 1.2% in the Newton et al. study (n = 13), PE was fatal in 23% of cases (n=3) [6]. On multivariate analysis, recurrent VTE such as PE was predicted by malignant disease (odds ratio, 2.00; 95% confidence interval, 1.04-3.45) and a hemorrhagic event (odds ratio, 2.67; 95% confidence interval, 1.10-6.45). Cancer, obesity, postoperative status, immobility, prior VTE, thrombophilia, and age older than 65 years showed a higher rate of recurrent DVT or PE (7.6%) but non-significantly [6].
3.4 Diagnostic strategy
In this case report, a two-step diagnostic strategy was applied. First, CRT was diagnosed using ultrasound imaging followed by the diagnosis of PE using CT-scan. When suspecting an upper limb DVT, which could be the case for a CRT, the Constans clinical decision algorithm can be practiced [21]. This algorithm results in a score up to 3, based on a combination of 4 clinical factors: The presence of an IV device (+ 1), a localized pain (+ 1), and a localized pain a unilateral edema (+ 1) account for 1 point each, while a negative point is assigned if there is an alternative plausible cause for the specific clinical presentation. In a prospective study that includes 406 patients, a non-relevant D-dimer level with a Constans score below 1 point appeared to be a consistent way to exclude upper limb DVT with no failure after 3 months follow-up (95% CI: 0.0-4.2) [22]. The incidence of clinically overt PE in patients with CRT ranges from 15% to 25%, but an autopsy-proven PE rate of up to 50% has been reported [3]. Clinical prediction rules, such as the Wells score or the Geneva score, do not take the presence of a CRT into consideration, which is by itself associated risk factor for PE. Respiratory signs, such as the reported hemoptysis in our case, should evoke a suspicion of associated PE.
3.5 Catheter related thrombosis treatment
Since PE is a complication of CRT, the initiation and duration of the treatment depends on CRT occurrence and not on the PE occurrence in a patient with cancer. Data from the RIETE registry demonstrate that the rate of recurrent venous thrombosis (independent of the location) during and after therapy was 2.83 and 2.88 per 100 patient years, respectively [10]. Most recurrent thrombotic events occurred within the first 2 months of chemotherapy. French recommendations suggest that symptomatic CRT should be treated for at least 3 months (grade 2), anticoagulation should be continued beyond 3 months when the CVC is kept in place and the cancer is active (grade 2), and CRT should be treated using LVMH or VKA rather than DOACs (grade 2) [23]. In case of a CRT, French recommendations suggest keeping the CVC in place if it is functional (grade 2) and the following conditions are met: The distal end of the CVC is dwelling in a good position at the junction between the upper vena cava and the right atrium, the CVC is necessary for the patient's management, and there is no sign of a CVC infection. If these conditions are not met, it is recommended to remove the CVC (grade 2) [23]. Another reason to remove the CVC includes absence of disappearance of the symptoms with anticoagulation alone.
4. Conclusion
The current case report highlights the importance of acknowledging the high risk of PE associated to CRT, since this clinical situation is structurally underestimated. The case also emphasizes the importance of careful examination by the physician to prevent death of CRT patients from PE.
Competing Interest
No
References
- Sousa B, Furlanetto J, Hutka M, et al. Central venous access in oncology: ESMO Clinical Practice Guidelines. Ann. Oncol 26 (2015): v152–v168.
- Saber W, Moua T, Williams E C, et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective studies: CRT in adult cancer patients. J. Thromb. Haemost 9 (2011): 312-319.
- Verso M, Agnelli G. Venous Thromboembolism Associated With Long-Term Use of Central Venous Catheters in Cancer Patients. J. Clin. Oncol 21 (2003): 3665-3675.
- Hill S L, Berry R E. Subclavian vein thrombosis: a continuing challenge. Surgery 108 (1990): 1-9.
- Scott Evans R, Jamie H Sharp, Lorraine H Linford, et al. Risk of Symptomatic DVT Associated With Peripherally Inserted Central Catheters. Chest 138 (2010): 803-810.
- Daniel H Newton, Manuel Monreal Bosch, Michael Amendola, et al. Analysis of noncatheter-associated upper extremity deep venous thrombosis from the RIETE registry. J. Vasc. Surg. Venous Lymphat. Disord. 5 (2017): 18-24.e1
- Monreal M, Munoz F J, Rosa V, et al. Upper extremity DVT in oncological patients: analysis of risk factors. Data from the RIETE registry. Exp. Oncol 28 (2006): 245-247
- Jacquelyn L Baskin, Ching-Hon Pui, Ulrike Reiss, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. The Lancet 374 (2009): 159-169.
- Geerts W. Central venous catheter–related thrombosis. Hematology 2014 (2014): 306-311.
- Lisa Baumann Kreuziger, Lauren Cote, Peter Verhamme, et al. A RIETE registry analysis of recurrent thromboembolism and hemorrhage in patients with catheter-related thrombosis. J. Vasc. Surg. Venous Lymphat. Disord 3 (2015): 243-250.e1.
- Rajasekhar A, Streiff M B. How I treat central venous access device–related upper extremity deep vein thrombosis. Blood 129 (2017): 2727-2736.
- Dentali F, Gianni M, Agnelli G, et al. Association between inherited thrombophilic abnormalities and central venous catheter thrombosis in patients with cancer: a meta-analysis: Inherited thrombophilic abnormalities in cancer patients with CVC. J. Thromb. Haemost 6 (2007): 70-75.
- Aw A, Carrier M, Koczerginski J, et al. Incidence and predictive factors of symptomatic thrombosis related to peripherally inserted central catheters in chemotherapy patients. Thromb. Res 130 (2012): 323-326.
- Leung A, Heal C, Perera M, et al. A systematic review of patient-related risk factors for catheter-related thrombosis. J. Thromb. Thrombolysis 40 (2015): 363-373.
- Vineet Chopra, Sarah Anand, Andy Hickner, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. The Lancet 382 (2013): 311-325.
- Cornelis J van Rooden, Emile F Schippers, Renée M Y Barge, et al. Infectious Complications of Central Venous Catheters Increase the Risk of Catheter-Related Thrombosis in Hematology Patients: A Prospective Study. J. Clin. Oncol 23 (2005): 2655-2660.
- Timsit J F, Farkas J C, Boyer J M, et al. Central Vein Catheter-Related Thrombosis in Intensive Care Patients. Chest 114 (1998): 207-213.
- Luciani A, Clement O, Halimi P, et al. Catheter-related Upper Extremity Deep Venous Thrombosis in Cancer Patients: A Prospective Study Based on Doppler US. Radiology 220 (2001): 655-660.
- Gallieni M, Pittiruti M, Biffi R. Vascular Access in Oncology Patients. CA. Cancer J. Clin 58 (2008): 323-346.
- Malinoski D. et al. Which central venous catheters have the highest rate of catheter-associated deep venous thrombosis: A prospective analysis of 2,128 catheter days in the surgical intensive care unit. J. Trauma Acute Care Surg 74 (2013): 454-462.
- Joel Constans, Louis-Rachid Salmi, Marie-Antoinette Sevestre-Pietri, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb. Haemost 99 (2008): 202-207.
- Kleinjan A. Safety and Feasibility of a Diagnostic Algorithm Combining Clinical Probability, D -Dimer Testing, and Ultrasonography for Suspected Upper Extremity Deep Venous Thrombosis: A Prospective Management Study. Ann. Intern. Med 160 (2014): 451.
- Sanchez O, Benhamou Y, Bertoletti L, et al. Recommendations for best practice in the management of venous thromboembolic disease in adults. Long version. Rev. Mal. Respir (2019).
- Joffe H V, Kucher N, Tapson V F, et al. Upper-Extremity Deep Vein Thrombosis: A Prospective Registry of 592 Patients. Circulation 110 (2004): 1605-1611.
