Proton Pump Inhibitors and Cardiovascular Disease: A Review of Clinical Evidence and Biological Mechanisms
Article Information
Syed Adeel Hassan1*, Umar Farooque2, Somia Jamal3, Farah Yasmin4, Naresh Kumar5, Tooba Hussain6, Komal Girdhari7, Jude Ezeh8, Fahad Nawaz Sheikh9, Ali Choudhry10, Ifrah Naeem11, Malik Muhammad Uzair Khan12
1Internal Medicine, Dow University Hospital, Karachi, Pakistan
2Neurology, Dow University of Health Sciences, Karachi, Pakistan
3Internal Medicine, Abbasi Shaheed Hospital, Karachi, Pakistan
4Internal Medicine, Dow University of Health Sciences, Karachi, Pakistan
5Internal Medicine, Dow University of Health Sciences, Karachi, Pakistan
6Internal Medicine, Dow University of Health Sciences, Karachi, Pakistan
7Internal Medicine, Civil Hospital, Sukkur, Pakistan
8Internal Medicine, Newark Beth Israel Medical Center, New Jersey, USA
9Pathology, University of Illinois at Chicago, Illinois, USA
10Internal Medicine, Lahore Medical and Dental College, Lahore, Pakistan
11Internal Medicine, Shalamar Medical and Dental College, Lahore, Pakistan
12Internal Medicine, Shalamar Medical and Dental College, Lahore, Pakistan
*Corresponding Authors: Syed Adeel Hassan, Internal Medicine, Dow University Hospital, Karachi, Pakistan
Received: 17 September 2020; Accepted: 24 September 2020; Published: 05 October 2020
Citation: Syed Adeel Hassan, Umar Farooque, Somia Jamal, Farah Yasmin, Naresh Kumar, et al. Proton Pump Inhibitors and Cardiovascular Disease: A Review of Clinical Evidence and Biological Mechanisms. Cardiology and Cardiovascular Medicine 4 (2020): 611-619.
View / Download Pdf Share at FacebookAbstract
Proton pump inhibitors (PPIs) are amongst the most commonly prescribed medications in the clinical setting. They are commonly used for the treatment of gastroesophageal reflux disease and are readily available over the counter. However, their irrational long term use has been repeatedly discouraged. PPIs are highly lipophilic and may adversely affect multiple physiological pathways of various organs. Over the past few decades, multiple clinical studies have linked PPI use with an increased risk of cardiovascular disease. In this review, we discuss the PPI-induced pathophysiological mechanisms that contribute to cardiovascular disease. Furthermore, we highlight clinical evidence that associates PPI use with an increased risk for various cardiovascular manifestations.
Keywords
Proton pump inhibitors; Cardiovascular disease; Gastroesophageal reflux disease; Clinical studies; Pathophysiology; Mechanisms
Article Details
1. Introduction & Background
Since their Introduction in 1989, proton pump inhibitors (PPIs) have become the gold standard for the short-term therapy of various acid-related disorders. In the United States, over 100 million PPIs have been prescribed annually [1]. From a global perspective, an estimated 113 million PPI prescriptions are issued every year [1]. Their profound clinical efficacy and easy availability account for the staggering expenditure annually. Therefore, PPIs are one of the most commonly prescribed medications in the United States [2].
When used as recommended, PPIs are generally considered a safe class of medications. The food and drug administration (FDA) originally evaluated PPIs for short-term treatment. More specifically, they were approved for a treatment period of 4 weeks with no more than 2 treatment cycles per year [3]. However, in modern clinical practice, the inadvertent long term use and prescription of PPIs are quite common [4]. As a consequence, accumulating evidence from several clinical studies have associated several adverse events with long term PPI use. Adverse sequelae include cardiovascular disease, dementia, bone fractures, renal failure, nutritional deficiencies, enteric infections, and antiplatelet drug interactions [1,3]. In this review, we discuss possible pathophysiological mechanisms leading to the development of the cardiovascular disease. Furthermore, we also discuss the spectrum of cardiovascular involvement backed by clinical evidence.
2. The spectrum of cardiovascular involvement based on clinical evidence
In a meta-analysis, 14 observational studies were carefully analyzed to determine the association between proton pump inhibitor (PPI) use and cerebro-cardiovascular outcomes [5]. The study concluded that PPI use was associated with an increased risk of ischemic stroke (OR: 1.22, 95% CI: 1.08-1.36), myocardial infarction (MI; OR: 1.23, 95% CI: 1.14-1.32), and cardiovascular death (OR: 1.83, 95% CI: 1.69-1.98) [5]. In a Danish registry study, 214,998 patients were assessed for the risk of first-time ischemic stroke and myocardial infarction (MI) in long term PPI users [6]. Furthermore, the duration and dose of PPI use were also analyzed [6]. Follow up over the years revealed that PPI exposure was associated with an increased risk of ischemic stroke (HR 1.13; CI 1.08 -1.19; p <0.001 ) and MI (HR 1.31; CI 1.23 -1.39; p <0.001) [6]. A significant relationship was noted between the PPI dose and the occurrence of outcomes. More specifically, a low-dose PPI was not associated with any of the adverse outcomes [6]. However, high-dose PPI was significantly associated with increased rates of both outcomes [6]. Therefore, based on these findings, PPI use increases the risk for a first-time ischemic stroke. This risk is more commonly seen with the long term use of PPIs. Furthermore, a dose-response relationship can be appreciated between PPI use and stroke. Therefore, long term high dose PPI use is associated with the greatest risk for stroke.
In a meta-analysis conducted by Cheungpasitporn et al., 3 cohort studies, 5 cross-sectional studies, and a case-control study were analyzed cumulatively [7]. Its results showed that the pooled risk ratio of hypomagnesemia in patients with PPI use was 1.63 (95% CI, 1.14-2.23) [7]. More specifically, in these patients, the magnesium levels were noted below 1.6-1.7 mg/dL [7]. Park et al. also conducted a meta-analysis cumulatively of 115,455 patients [8]. Among patients taking PPI, 27.1% developed hypomagnesemia [8]. Whereas, 18.1% of patients who developed hypomagnesemia were not taking PPIs [8]. Therefore, it is quite evident that PPIs are notorious for causing life-threatening hypomagnesemia. Although not common, the physicians must monitor magnesium levels prior to and during PPI therapy administration. It has been hypothesized that PPIs disrupt the active uptake of magnesium across the bowel wall [9]. Furthermore, excessive losses in the gut have also been postulated as a possible mechanism [9]. These patients also present with other electrolyte abnormalities such as hypokalemia and hypocalcemia. Clinically, patients usually present with the classical signs of tremors, cramps, weakness, irritability, seizures, and carpopedal spasms. Furthermore, electrophysiological conduction defects such as torsades de pointes, ventricular tachycardias, and QT prolongation are evident.
The concomitant use of anticoagulants and PPIs has also gained much attention. Several clinical studies have implicated antagonistic interactions between clopidogrel and certain types of PPI [10-11]. The metabolism of both classes of drugs is dependent on cytochrome (CYP) 2C19 [10]. Co-administration results in competition for CYP2C19 and as a consequence reduced effective plasma therapeutic dose and clinical benefit. This accounts for the possible increase in the risk of major adverse cardiac events (MACE). It is vital to note that the increased risk of adverse events is not due to metabolic interactions between esomeprazole and clopidogrel. This is evident from a meta-analysis of 23 studies conducted by Kwok and colleagues [12]. It was noted that a 30% increase in MACE was seen in patients being administered PPIs independently of clopidogrel use [12]. The increased risk of MACE was more evident with long term PPI administration (> 6 months) [12]. Furthermore, an increased risk of recurrent myocardial infarction (MI) has been reported in patients hospitalized for acute MI [13]. In the General Population, PPI use is independently linked to the first time cardiovascular event. Casula et al. identified 17,832 cases of new PPI users [14]. From which, 11,616 cases were hospitalized for coronary artery disease and 6467 for ischemic stroke [14]. Further analysis revealed that the risk for cardiovascular events was higher in the current (OR 1.61; 95%CI 1.55–1.68) and recent users (OR 1.15; 95%CI 1.06–1.26) [14]. Furthermore, this association was also noted in clinical subgroups based on exposure duration, statin use, and antithrombotic medication use [14].
Several clinical studies have also indicated an increased risk of short term mortality in patients with concomitant use of clopidogrel and PPI after a primary cutaneous intervention (PCI) [15,16]. Bundhun et al. analyzed a total of 11 clinical studies that cumulatively involved 84,729 patients [15]. Of the total, 29,235 patients belonged to the PPI group. Whereas, 55,494 patients belonged to the non-PPI group. The risk of short term mortality and target vessel revascularization was higher in the PPI group (OR 1.55) [15]. Furthermore, the incidence of long term MACE, stent thrombosis, and myocardial infarction post-PCI was also higher in the PPI group [15]. PPI should be cautiously prescribed to patients taking aspirin and clopidogrel. Combination therapy can result in increased rates of MACE, stent thrombosis, and revascularization [16]. However, this combination therapy has proven to be quite effective in reducing the risk of GI bleeding. Therefore, in such patients, GI benefits should be weighed against the risk of MACE [16].
3. Pathophysiological mechanisms
Several underlying mechanisms have been postulated as the culprit of cardiovascular dysfunction with PPI use. These include the increased synthesis of chromogranin A (CgA) levels, reduced nitric oxide, endothelial cell dysfunction, endothelial senescence, hypomagnesemia, electrolyte imbalance, reduced clopidogrel bioactivation, reduced vitamin B12 absorption, and decreased dimethylarginine dimethylaminohydrolase (DDAH) activity. A summary of the proposed mechanisms has been depicted in Figure 1 below.
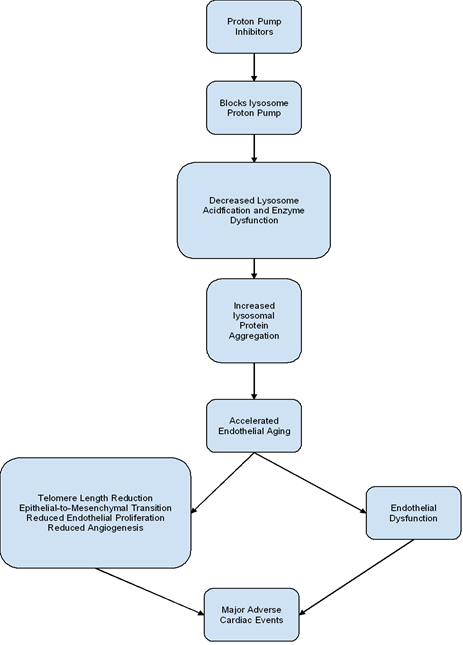
Long-term administration of PPIs has been associated with increased production of free radicals [17]. In turn, this is thought to arise as a consequence of PPI-induced inhibition of the endothelial lysosome proton pump [17]. This leads to protein aggregation due to reduced lysosomal acidification and enzyme function. Downstream biological effects of protein aggregation result in endothelial senescence [17]. Endothelial aging is thought to arise due to telomere shortening, epithelial-to-mesenchymal transition, reduced epithelial proliferation, and angiogenesis (Figure 2) [17]. Impaired nitric oxide synthase synthesis and increased generation of reactive oxygen species also aid in endothelial cell dysfunction [18]. Furthermore, upon exposure to PPI, senescent cells undergo a change in its secretory phenotype [19]. More specifically, the downregulation of genes encoding anti-atherogenic proteins in senescent cells has been noted [19]. As a consequence, PPIs tend to promote pro-atherogenic tendencies in senescent endothelial cells.
Proton pump inhibitors impair the absorption of vitamin B12, vitamin C, iron, and calcium [20]. Reduced levels of ascorbic acid prevent antioxidative protection of nitric oxide [21]. As a consequence, nitric oxide tends to be degraded. The impairment in vitamin b12 absorption results in the failure of converting homocysteine to cysteine [22]. Therefore, consistent elevations in homocysteine levels are known to downregulate the function of dimethylarginine dimethylaminohydrolase (DDAG) [22]. The subsequent elevation in asymmetric dimethylarginine (ADMA) inhibits endogenous nitric oxide synthase and reduces nitric oxide generation at the vascular cellular level [22]. Furthermore, the elevated levels of homocysteine impose an oxidative burden by favoring the formation of reactive oxygen species by nitric oxide synthase [22]. This leads to the uncoupling of endothelial nitric oxide synthase [23]. As a result, decreased nitric oxide synthesis and widespread endothelial cell dysfunction occurs. In a study conducted by Ghebremariam et al., omeprazole inhibited the production of both the active and inactive protein components of nitric oxide synthase [24]. Therefore, it can also be concluded that via a direct mechanism, PPIs tend to directly influence the expression of nitric oxide synthase.
Dietary derived nitrites and nitrates produced by the oral flora get converted to nitrous acid [25]. Spontaneous release of nitric acid from nitrous acid follows [25]. PPIs via inhibition of gastric proton pumps decrease gastric acidity. As a consequence, the spontaneous release of nitric oxide from nitrous acid is hindered [25]. In addition to the regular pathway, nitric oxide can also be synthesized via the enterosalivary pathway. It is also known as the nitrate-nitrite-nitric oxide pathway [26]. In this pathway, the endogenous nitric oxide can be recycled to nitrate via oxidation [27]. The nitrate is then actively concentrated in the saliva, where it can be converted to nitrite by the oral flora [27]. As it is quite evident from our discussion above, PPIs reduced nitric oxide production. Therefore, the downstream levels of nitrite and nitrate should also be expected to reduce. In summary, one can expect reduced levels of nitric oxide in the circulatory reservoir.
Usually Implicated as a biomarker of neuroendocrine neoplasias, chromogranin A levels are also upregulated with PPI use [28]. At the molecular level, chromogranin A exerts adaptive vasodilatory and cardio-regulatory effects [29]. Chromogranin A is the precursor to vasostatins and catestatins [29]. Vasostatins act as a vasorelaxant to promote negative lusitropic and negative ionotropic effects [30]. Furthermore, they also inhibit the release of catecholamines [30]. Chromogranin A also upregulates the release of endothelin-1 [31]. Being a potent vasoconstrictor, endothelin-1 via endothelial dysfunction promotes pro-inflammatory and pro-atherogenic pathways [32].
4. Conclusion
As per the review of the literature, it is quite evident that PPIs increase the risk of stroke, arrhythmias, and MACE. More specifically, PPI increases the risk for the first time ischemic stroke and has a dose-response relationship. The greatest risk of MACE is noted in patients taking PPIs for > 6 months. On a molecular level, these cardiovascular effects are mediated by nitric oxide insufficiency, accelerated endothelial aging, endothelial dysfunction, and increased synthesis of chromogranin A.
Conflict of Interest Statement:
All authors have no conflict of interest to declare.
Funding:
None
Acknowledgments:
None
References
- Ren D, Gurney E, Hornecker J. Appropriate use and stewardship of proton-pump inhibitors. US Pharm 44 (2019): 25-31.
- Shah NH, LePendu P, Bauer-Mehren A, et al. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One 10 (2015): e0124653.
- Ariel H, Cooke JP. Cardiovascular Risk of Proton Pump Inhibitors. Methodist Debakey Cardiovasc J 15 (2019): 214-219.
- Eid SM, Boueiz A, Paranji S, Mativo C, Landis R, Abougergi MS. Patterns and predictors of proton pump inhibitor overuse among academic and non-academic hospitalists. Intern Med 49 (2010): 2561-2568.
- Li S, Liu F, Chen C, et al. Real-World Relationship Between Proton Pump Inhibitors and Cerebro-Cardiovascular Outcomes Independent of Clopidogrel. Int Heart J 60 (2019): 910-918.
- Sehested TSG, Gerds TA, Fosbøl EL, et al. Long-term use of proton pump inhibitors, dose-response relationship and associated risk of ischemic stroke and myocardial infarction. J Intern Med 283 (2018): 268-281.
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail 37 (2015): 1237-1241.
- Park CH, Kim EH, Roh YH, Kim HY, Lee SK. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One 9 (2014): e112558.
- Cundy T, Mackay J. Proton pump inhibitors and severe hypomagnesaemia. Curr Opin Gastroenterol 27 (2011): 180-185.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 301 (2009): 937-944.
- Wedemeyer RS, Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf 37 (2014): 201-211.
- Kwok CS, Jeevanantham V, Dawn B, Loke YK. No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: meta-analysis. Int J Cardiol 167 (2013): 965-974.
- Valkhoff VE, 't Jong GW, Van Soest EM, Kuipers EJ, Sturkenboom MC. Risk of recurrent myocardial infarction with the concomitant use of clopidogrel and proton pump inhibitors. Aliment Pharmacol Ther 33 (2011): 77-88.
- Casula M, Scotti L, Galimberti F, et al. Use of proton pump inhibitors and risk of ischemic events in the general population. Atherosclerosis 277 (2018): 123-129.
- Bundhun PK, Teeluck AR, Bhurtu A, Huang WQ. Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: a systematic review and meta-analysis of recently published studies (2012 - 2016). BMC Cardiovasc Disord 17 (2017): 3.
- Hu W, Tong J, Kuang X, Chen W, Liu Z. Influence of proton pump inhibitors on clinical outcomes in coronary heart disease patients receiving aspirin and clopidogrel: A meta-analysis. Medicine (Baltimore) 97 (2018): e9638.
- Yepuri G, Sukhovershin R, Nazari-Shafti TZ, Petrascheck M, Ghebre YT, Cooke JP. Proton Pump Inhibitors Accelerate Endothelial Senescence. Circ Res 118 (2016): e36-e42.
- Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens 23 (2005): 233-246.
- Costarelli L, Giacconi R, Malavolta M, et al. Different transcriptional profiling between senescent and non-senescent human coronary artery endothelial cells (HCAECs) by Omeprazole and Lansoprazole treatment. Biogerontology 18 (2017): 217-236.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 104 (2009): S5-S9.
- S A Kyrtopoulos, Ascorbic acid and the formation of N-nitroso compounds: possible role of ascorbic acid in cancer prevention, The American Journal of Clinical Nutrition, Volume 45 (1987): 1344–1350.
- Stühlinger MC, Oka RK, Graf EE, et al. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation 108 (2003): 933-938.
- Dayal S, Lentz SR. ADMA and hyperhomocysteinemia. Vasc Med 10 (2005): S27-S33.
- Ghebremariam YT, LePendu P, Lee JC, et al. Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation 128 (2013): 845-853.
- Pinheiro LC, Amaral JH, Tanus-Santos JE. Letter by Pinheiro et al. Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation 129 (2014): e427.
- Weitzberg E, Hezel M, Lundberg JO. Nitrate-nitrite-nitric oxide pathway: implications for anesthesiology and intensive care. Anesthesiology 113 (2010): 1460-1475.
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7 (2008): 156-167.
- Giusti M, Sidoti M, Augeri C, Rabitti C, Minuto F. Effect of short-term treatment with low dosages of the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. Eur J Endocrinol 150 (2004): 299-303.
- Fragasso G. Editorial commentary: Pathophysiological effects of proton pump inhibitors in cardiac patients: Time for a critical reappraisal. Trends Cardiovasc Med 29 (2019): 361-362.
- D'amico MA, Ghinassi B, Izzicupo P, Manzoli L, Di Baldassarre A. Biological function and clinical relevance of chromogranin A and derived peptides. Endocr Connect 3 (2014): R45-R54.
- Chen Y, Mahata M, Rao F, et al. Chromogranin A regulates renal function by triggering Weibel-Palade body exocytosis. J Am Soc Nephrol 20 (2009): 1623-1632.
- Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 76 (2007): 8-18.