Protective Effect of Pituitary Adenylate Cyclase-Activating Polypeptide-38 Against Radiation-Induced Myocardial Injury in Mice
Article Information
Huan Li#, Pei-Qiang Yi#, Qian Zhu, Lu Cao, Cheng Xu, Min Li*, Jia-Yi Chen*
1Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
*Corresponding author: Jiayi Chen, Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Min Li, Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
#These authors have contributed equally to this work.
Received: 22 May 2023; Accepted: 30 May 2023; Published: 20 June 2023
Citation: Huan Li, Pei-Qiang Yi, Qian Zhu, Lu Cao, Cheng Xu, Min Li, Jia-Yi Chen. Protective Effect of Pituitary Adenylate Cyclase-Activating Polypeptide -38 Against Radiation-Induced Myocardial Injury in Mice. Cardiology and Cardiovascular Medicine. 7 (2023): 178-187.
View / Download Pdf Share at FacebookAbstract
Background: Radiation-Induced Heart Disease (RIHD) restricts the survival benefit of radiotherapy for thoracic tumors. Myocardial fibrosis and remodeling are late consequences of the long-term development of RIHD, and pituitary adenylate cyclase-activating polypeptide-38 (PACAP38) could ameliorate RIHD.
Methods: An animal model of heart irradiation was prepared from mice, and subjected to X-ray exposure. Mice were pretreated with PACAP38 in vivo before cardiac irradiation. The cardiac function and pathological changes of myocardium were evaluated by animal echocardiography, micro PET-CT and histological staining, respectively.
Results: Murine heart exposed to a single dose of 20 Gy X-ray resulted in a significant increases pathological injury and average standard uptake value (SUV) of 18F-FDG induced by irradiation in mice. The cardiac function that displays systolic parameter LVEF and diastolic parameter E/e' of mice did not change significantly after irradiation at 6 months. PACAP38 (10 μg/100μl, i.p.) significant inhibited the increase of average SUV of 18F-FDG as well as myocardial pathological damage induced by irradiation. The average SUV in the irradiated area of the heart in the control group and irradiation group were 0.76±0.04 and 1.20±0.07, respectively (P<0.05). While in the PACAP38 treatment group, the average SUV decreased to 0.86±0.06 (PACAP38+IR vs. IR, P<0.05), indicating that PACAP38 effectively protected heart function against radiation-induced heart injury.
Conclusions: Our data reveal that PACAP38 significantly alleviated the myocardial fibrosis, remodeling and metabolic dysfunction induced by radiation exposure of heart. The cardioprotective role of PACAP38 provide a new sight for possible intervention of RIHD.
Keywords
Radiation therapy; RIHD; PACAP38; Cardiac injury; Mouse
Radiation therapy articles; RIHD articles; PACAP38 articles; Cardiac injury articles; Mouse articles
Article Details
Introduction
Breast cancer has become the world's leading cancer in thoracic tumors, and the lung cancer, esophageal cancer and Hodgkin's lymphoma were most common cancers with high mortality[1]. Radiotherapy is an important part of the comprehensive treatment for thoracic cancers. Cardiac toxicity is one of the major adverse effects associated with thoracic irradiation (IR), which to certain extent, limits the survival benefit of radiotherapy. Studies have indicated that the radiation-Induced Heart Diseases (RIHD) of thoracic tumors significantly affects its long-term survival benefits[2-6]][[i]][[ii]][[iii]][. However, there is currently no effective manner to prevent or cure RIHD available so far. The cardiac injury associated with radiotherapy was first evaluated in breast cancer and Hodgkin lymphoma due to their high survival rate. The risk of fatal cardiovascular events increased significantly after irradiation. In recent years, with the improvement of the survival rate of non-small cell lung cancer (NSCLC), researchers pay more attention to not only the occurrence of radiation induced pneumonia and esophagitis during lung cancer irradiation, but also the detriment of RIHD. Several studies have demonstrated that the cardiac dose and volume induced by radiation exposure in the treatment of lung cancer are highly related to the development of cardiac injury which reduced the survival benefits of NSCLC treatments[7,8]. However, the decrease of radiation dose and irradiation volume will not solve the clinical issues of RIHD significantly. The dose threshold of RIHD noticed in the middle of twenty century. The technology of thoracic radiotherapy resulted in a large volume and high-dose radiation exposure to the heart. The threshold of radiation induced heart injury was defined as greater than 30 Gy[9,10]. In the past decades, with the rapid development of radiotherapy technology, the cardiac irradiation dose and volume have decreased dramatically, to the mean dose as low as 2 Gy. It is still impossible to avoid the occurrence of RIHD in the era of inverse intensity modulated radiation therapy (IMRT)[11,12]. The report showed that the long-term follow-up of Japanese nuclear bomb survivors found that the massive low-dose coverage of the heart irradiation significantly increased the risk of death related to the cardiac events after 10 years[13]. Because of the increasing of multiple and various chest tumors, the optimal dose and volume parameters of the heart irradiation cannot be identical. Moreover, the diversification of fractionation in IMRT and the development of proton therapy, as well as the 3D conformal radiotherapy (3DCRT), the dose and volume parameters of heart irradiation could not be unified. Therefore, it requires clinical approach to investigate cardiotoxicity caused by thoracic radiation exposure and to evaluate the ability of intervention strategies for prevention of cardiac injury during radiotherapy.
The prevention and treatment strategies of RIHD follow the management standards for the conventional heart diseases. There are currently no therapeutic targets of radiation induced heart injury. The incubation period of RIHD occurs as long as 10-20 years and the appearance of most symptoms represents heart organic changes. There is no specific or effective manner of intervention currently available[14]. A few studies have shown that β-receptor blockers and ACEI could alleviate radiation induced heart injury, but there is no evidence of large-scale phase III randomized controlled clinical trials[15]. Pituitary Adenylate Cyclase-Activating Polypeptide-38 (PACAP38) is a 38-amino-acid pleiotropic neuroendocrine peptide originally isolated from ovine hypothalamic tissues with anti-apoptotic and antioxidant activities[16]. Early studies have found that PACAP38 showed significant neuroprotective and renoprotective effects both in vivo and in vitro[17,18]. In previous studies we established an animal model of cardiac irradiation and preliminarily explored the protective effect of PACAP38 on radiation-induced early heart injury in rat cardiomyocytes and in mice[19]. This study is to investigate the efficacy of PACAP38 as a cardioprotectant for radiation-induced late cardiac injury in mice.
Materials and Methods
Establishment of heart irradiation animal model and PACAP38 intervention
Experimental animal
All animal experiments and procedures were conducted in accordance with the approval of the Shanghai Jiaotong University School of Medicine Institutional Animal Care and Use Committee. C57BL/6J male mice (20-25g, 6-8weeks) were purchased from Shanghai Shrek Experimental Animal Co., Ltd. The feeding conditions were SPF level, sufficient sunshine, 12 hour circadian rhythm, appropriate temperature (about 22-27°C) and humidity (40%-60%). A total of 48 male C57BL/6J mice were divided into four groups: solvent control group, PACAP38 control group, irradiation group (IR) and irradiation+PACAP38 group (IR+PACAP38).
Heart irradiation in mice
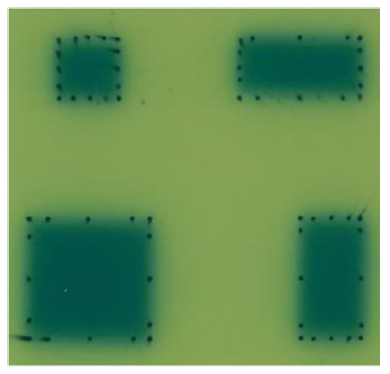
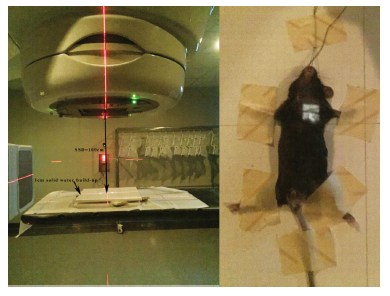
Mouse heart was performed with X-ray irradiation on Varian trilogy medical linear accelerator (Varian, USA). In preliminary experiment, the position of the heart relative to the body surface and the chest depth of the heart were determined by dissecting the chest of mice to determine the conditions of heart irradiation: X-ray energy: 6-MV; dose/fraction: 20Gy/1Fx; dose rate: 300cGy/min; Source Surface Distance (SSD): 100cm; dose build-up: 1cm solid water; radiation field: 1×1cm2. We applied 1×1cm2 irradiation field to avoid both lungs irradiation. The film verification of 1×1cm2, 1×2cm2, 2×2cm2 and 2×1cm2 radiation field on medical linear accelerator (Figure 1) showed that the penumbra of 1×1cm2 radiation field did not increase significantly, and had good dose coverage and uniformity. Before irradiation, each mouse (including all groups) was anesthetized by intraperitoneal injection of 0.1 ml/10g body weight of 4% chloral hydrate. After anesthesia, it was fixed on solid water. The limbs and tail were fixed with adhesive tape, and the incisors were pulled and fixed on solid water with surgical thread. 1×1cm2 irradiation field was aligned with the position of the anterior heart (the position of the heart is determined mainly according to the anatomical experience and touching the apical beat) with 1 cm solid water build-up (Figure 2).
Note: Top left: 1×1 cm2; Top right:1×2 cm2;Bottom left:2×2 cm2;Bottom right:2×1 cm2.
Note: The left figure showed the mouse heart irradiation model, parameters: SSD: 100cm; Compensation: 1cm solid water; Irradiation field: 1×1cm2. The right figure showed example diagram of mouse positioning.
PACAP38 treatment
In PACAP38 intervention group, PACAP38 (10µg/100µL, Sigma Aldrich, USA) was injected intraperitoneally (i.p.) 2 hours before irradiation, 24 hours and 48 hours after irradiation. The control group and irradiation group were injected intraperitoneally with normal saline of the same volume and times. Normal feeding was continued after irradiation. Small animal echocardiography and Micro PET-CT were performed 6 months later, and then specimens were obtained after sacrifice.
Histological evaluation
Hematoxylin and Eosin (HE) staining
Fresh mouse heart tissue was fixed in 4% paraformaldehyde for more than 24 hours, and then dehydrated with concentration gradient ethanol, soaked in wax and embedded. Then, the sections were dewaxed in gradient concentration alcohol. Next, the nuclei and cytoplasm were stained with hematoxylin and eosin staining, and finally dehydrated and sealed. The cardiac histopathological changes between each group were observed under microscope (Zeiss AxioVert A1, Jena, Germany).
MASSON trichrome stain
Fresh heart tissue was embedded in paraffin and sliced. Firstly, hematoxylin was used to dye the nucleus, then the sections were stained with fuchsin red and aniline blue respectively, and finally dehydrated and sealed. Microscopic examination was performed to observe the pathological changes of heart tissue between each group. Among them, collagen fibers were blue, cardiomyocytes were red, and the nucleus was blue black. The degree of myocardial fibrosis can be semi-quantified according to the proportion of blue area in all areas of the visual field using ImageJ software (NIH, Bethesda, MD).
Immunohistochemistry (IHC)
The paraffin-embedded tissues were cut into 3-5μm sections. Firstly, the sections were deparaffinized, hydrated, and placed in a buffer containing citrate to retrieve antigen. Then, the sections were washed with PBS and incubated with 3% hydrogen peroxide to remove endogenous catalase. After blocking with 5% BSA solution, the sections were mixed with the primary anti-α-SMA antibody (Cell Signaling Technology, Danvers, MA, USA) and incubated at 4°C overnight. The secondary antibody was Biotinylated goat anti-rabbit antibody (EnVision + System HRP anti rabbit (K4002, Dako, Tokyo, Japan)), which was used to incubate at room temperature for 30 minutes. The average optical density (AOD) of α-SMA was measured with each image by ImageJ software (NIH, Bethesda, MD).
Animal echocardiography
The mice in each group were fed for 6 months, and the left ventricular systolic and diastolic functions were evaluated by small animal echocardiography (vevo2100, Fuji film, Japan). Firstly, the mouse was anesthetized in a small room filled with 2% isoflurane gas, and the heart rate was maintained at 350-450 beats/min. The ultrasonic imaging system was visual sonic VEVO 2100, with MS-400 mouse probe and detection frequency of 30MHz. Adjust the probe angle of MS-400 to collect 2D images, which displayed the left ventricular short axis section at the level of parasternal papillary muscle, and obtain the M-type motion curve of left ventricular wall. More than three stable cardiac cycle images were collected and analysed. Interventricular septal end diastole and end systole, Left ventricular posterior wall end diastole and end systole, and Left ventricular internal diameter end diastole and end systole were measured. The left ventricular ejection fraction (LVEF) was measured by the instrument's software. The probe angle and the position of the examination table were readjusted to find the four chamber cardiac section at the lower end of the mouse sternum. The mitral orifice was identified by B-mode ultrasound, the mitral orifice blood flow spectrum was recorded by pulse spectrum Doppler module, and the early diastolic blood flow peak (e peak) and late diastolic blood flow peak (a peak) were measured. The motion spectrum of ventricular septal mitral annulus was recorded by tissue Doppler imaging, and the peak velocities e' and a' in early and late diastole were measured. The evaluation parameter of left ventricular diastolic function was E/e'.
Micro positron emission tomography-CT
Myocardial metabolism imaging was performed in mouse of each group after 6 months of feeding with 18F-FDG PET-CT (SNPC-103, Kunshan life science and Technology). The irradiation related injury was evaluated by measuring the uptake of 18F-FDG in mouse myocardium. The equipment model used in this experiment was Supernova PET-CT (Life Medical Technology Company, Jiangsu, China). Before scanning, the mice were anesthetized by inhaling 2% isoflurane/oxygen with a flow rate of 0.7 L/min. During the whole examination, 1% isoflurane and oxygen were used to maintain the mice in a light anesthesia state. Then, 18F-FDG of 7.4-11.1 MBq (200-300uCi) was injected into the caudal vein. PET scan was performed 40 minutes after injection, which took about 20 minutes. CT scan was performed after PET scan. The PET image was reconstructed by OSEM algorithm, and the attenuation was corrected with reference to CT image. Using avatar 1.5 software, the heart was selected as the region of interest (ROI) on the PET image. The standard uptake value (SUV) of the heart was obtained by calculating the average radioactivity in ROI and the weight of mice.
Statistical analysis
The data were presented as the means ± SEM. One-way ANOVA with Bonferroni’s multiple comparison test was performed using GraphPad Prism version 5.0c for analyses, with statistical significance set at P<0.05.
Results
Effect of PACAP38 on the physical condition of irradiated mice
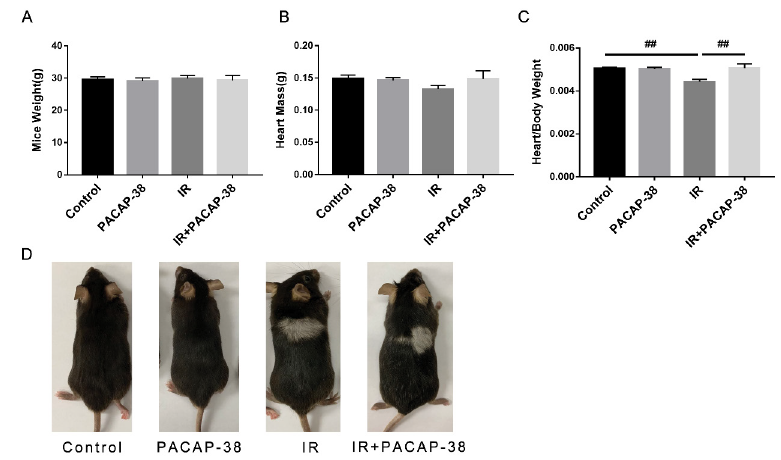
After six months of irradiation and PACAP38 intervention, the mice were sacrificed to collect heart tissue. The body weight and heart mass of mice did not change, as shown in Fig. 3A and B. However, the heart/body weight ratio reduced significantly in the irradiation group (control vs IR, 0.0050 vs 0.0044, P<0.01), the heart/body weight ratio reversed in the combined group (IR vs IR+PACAP-38, 0.0044 vs 0.0051, P<0.01) (Figure 3C). We observed hair whitening on the back of irradiated mice and combined group (Figure 3D), and its location and size correspond to the radiation field of the heart, which indicated the accuracy of irradiation.
Note: After Six months of irradiation and PACAP38 intervention, the mice were sacrificed to collect heart tissue. The body weight(A) and heart mass(B) of mice were measured. C. The heart/body weight ratio reduced significantly in the irradiation group (control vs IR, 0.0050 vs 0.0044, P<0.01). However, the heart/body weight ratio reversed in the combined group (IR vs IR+PACAP-38, 0.0044 vs 0.0051, P<0.01). D. Hair whitening on the back of irradiated mice, and its location and size correspond to radiation field .##P<0.01.
PACAP38 treatment alleviates myocardial histopathological changes in irradiated mice
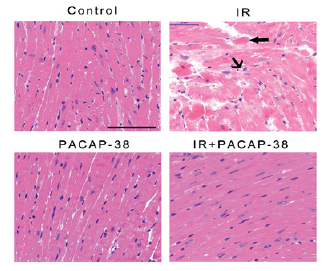
It was found that PACAP38 intervention significantly improved the late irradiation induced myocardial histopathological changes at 6 months after irradiation through H-E staining of mice myocardial tissue, as shown in Figure 4. Compared with the control group, the left ventricular myocardial tissue in the irradiation group showed obvious damaging lesions, including cardiomyocyte degeneration, eosinophilic enhancement, cytoplasmic vacuolation, nuclear pyknosis and myocardial fiber distortion. In PACAP38 treatment group, the above traumatic histopathological changes were significantly relieved.
Note: C57BL/6J mice were intraperitoneally injected with the first dose of PACAP38 2 hours before irradiation, and supplemented with PACAP38 24 hours and 48 hours after irradiation, respectively. Six months after irradiation, the mice were sacrificed to collect heart tissue. As shown in the figure 4, there were representative H-E staining micrographs of left ventricular myocardium in each group 6 months after irradiation. Obvious injury changes were observed in the irradiation (IR) group: cardiomyocyte degeneration, eosinophilic enhancement (thick arrow), cytoplasmic vacuolation (middle arrow), nuclear pyknosis and myocardial fiber distortion (middle arrow). The above injury changes were significantly relieved in IR + PACAP38 group.
Effect of PACAP38 intervention on myocardial fibrosis in irradiated mice
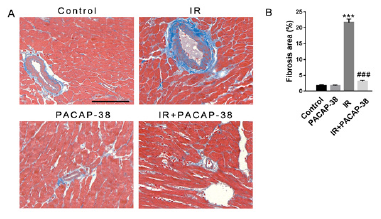
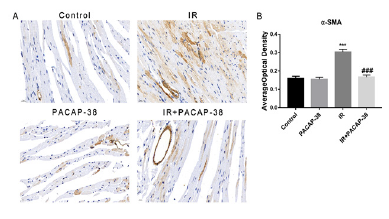
Masson staining of mice myocardial tissue showed that PACAP38 pretreatment could prevent and reduce radiation-induced myocardial fibrosis, as shown in Figure 5. Six months after irradiation, it was found that the intervention of PACAP38 significantly reduced the deposition of collagen fibers between myocardial tissues and around blood vessels (3.1±0.35%, n=6, P<0.001 vs. 21.63±1.09%, n=6 in the irradiation group). As a well-accepted marker of myofibroblast differentiation, α-SMA expression increased significantly in irradiated mice, which was reversed with PACAP38 intervention in IR+PACAP38 group (0.304±1.40%, n=6, P<0.001 in IR group vs. 0.167±1.20%, n=6, P<0.001 in IR+PACAP38 group), as shown in Figure 6. There were no significant histopathological changes in the PACAP38 control group, indicating the safety of PACAP38 intervention.
Note: A. Representative masson staining micrograph of left ventricular myocardium in each group 6 months after irradiation. Blue represents fibrous tissue. B. Semi-quantitative analysis of myocardial fibrosis. ***P<0.001 vs. Control group; ###P<0.001 vs. IR group. Magnification: ×400, Scale bar: 100 µm. There were six mice in each group.
Note: A. Immunohistochemistry with anti-α-SMA in heart tissues in each treatment group. The expression of α-SMA increased significantly in irradiated mice, which was reversed with PACAP38 intervention in IR+PACAP38 group. B. The expression of α-SMA was shown by the average optical density (AOD). ***P<0.001 vs. Control group; ###P<0.001 vs. IR group. Magnification: ×400, Scale bar: 20 µm. There were six mice in each group.
Effect of PACAP38 on cardiac systolic and diastolic function in murine heart after radiation exposure
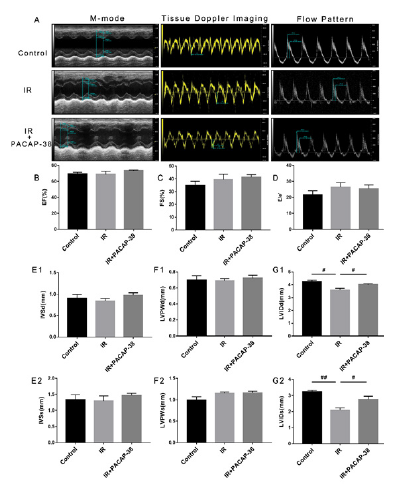
Cardiac ultrasound is the most commonly used monitoring method of cardiac injury related to tumor treatment, and LVEF is the most commonly used detection index. In recent years, studies have found that left ventricular diastolic function is more sensitive than LVEF to detect cardiac dysfunction. The effects of PACAP38 intervention on cardiac LVEF, LVFS and left ventricular diastolic function (E/e’) were detected by echocardiography 6 months after irradiation. However, the results showed that LVEF, LVFS and E/e' of mice hearts did not change significantly half a year after irradiation, as shown in Fig. 7A-D. Ventricular dimensions were also measured by echocardiogram (Fig. 7E-G), including interventricular septal end diastole and end systole (IVSd and IVSs), left ventricular posterior wall end diastole and end systole (LVPWd and LVPWs) and left ventricular internal diameter end diastole and end systole (LVIDd and LVIDs). IVS and LVPW are measured to evaluate left ventricular hypertrophy, There was no significant difference among the three groups (control, IR and IR + PACAP38) (Fig. 7E and F). Previous studies have found that LVID is related to cardiac diastolic dysfunction[20]. We found that LVIDd and LVIDs decreased significantly in the IR group (LVIDd: control vs IR, 4.252mm vs 3.575mm, P<0.05; LVIDs: control vs IR, 3.232mm vs 2.073mm, P<0.01). However, the LVIDd and LVIDs both returned to normal in the PACAP38 treatment group (LVIDd: IR vs IR+PACAP-38, 3.575mm vs 4.018mm, P<0.05; LVIDs: IR vs IR+PACAP-38, 2.073mm vs 2.772mm, P<0.05) ( Fig. 7G1 and G2).
Note: A. Example diagram of left ventricular systolic and diastolic function in mice detected by small animal echocardiography. Left ventricular systolic function was measured by M-ultrasound, and the parameter was EF (A-left); Detection of mitral annular motion spectrum by tissue Doppler imaging. The parameters are early diastolic peak velocity e' and late diastolic peak velocity a' (A-middle); The pulse spectrum Doppler module recorded the blood flow spectrum of mitral valve orifice, and measured the early diastolic blood flow peak (E peak) and late diastolic blood flow peak (A peak) (A-right); The evaluation parameter of left ventricular diastolic function is E/e'. B,C. Statistical analysis of left ventricular systolic function evaluation parameter Ejection Fraction (EF) and Fractional Shortening (FS). D. Statistical analysis of left ventricular diastolic function evaluation parameter E/e'. There was no significant difference among the three groups (control, IR and IR + PACAP38). E1,E2. IVSd and IVSs - Interventricular septal end diastole and end systole. F1,F2. LVPWd and LVPWs - Left ventricular posterior wall end diastole and end systole. G1,G2. LVIDd and LVIDs - Left ventricular internal diameter end diastole and end systole. n=6/group. #P<0.05; ##P<0.01.
Protective effect of PACAP38 on myocardial metabolic function in irradiated mice
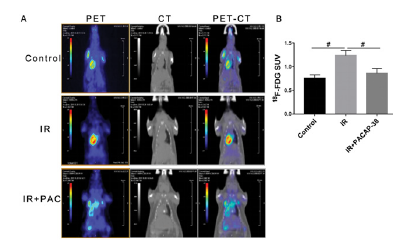
Six months after irradiation, 18F-FDG Micro PET-CT was performed for myocardial metabolism imaging to evaluate the level of myocardial injury. The changes of SUV can be used for early monitoring of radiation-induced cardiac injury. Results are shown in Fig. 8. The average SUV of myocardial uptake of 18F-FDG increased significantly after cardiac irradiation. The average SUVs in the irradiated area of the heart in the control group and irradiation group were 0.76±0.04 and 1.20±0.07 respectively (P<0.05), while PACAP38 intervention reversed the increase of SUV induced by irradiation, and the average SUV decreased to 0.86 ± 0.06 (PACAP38+IR vs. IR, P<0.05). It showed that PACAP38 effectively improved the myocardial metabolic dysfunction induced by irradiation. Micro PET-CT can be used as another imaging detection method to evaluate cardiac radiation injury, which could be used as a supplement to echocardiography and improve the detection sensitivity.
Note: A. six months after irradiation, micro PET-CT was used to detect the uptake of 18F-FDG in myocardial tissue and evaluate the level of myocardial radiation injury in each treatment group. From left to right are PET image, CT image and PET / CT fusion image. B. Statistical analysis of average SUV value of myocardial tissue in each group. #P < 0.05 reference mark (-).
Discussion
With the continuous improvement of radiotherapy in the comprehensive treatment of thoracic tumors, radiation therapy related heart injury is an important clinical complication which associated with prolonged hospitalization, high morbidity and high mortality[21,22]. Nowadays, there are no specific intervention targets and effective prevention and treatment strategies for RIHD. In past decades, there have been several attempts to explore clinical therapeutic medicine for RIHD, such as amifostine and angiotensin converting enzyme inhibitor. However, its application prospect has been offset by side effects[23,24]. Therefore, it requires to develop effective but non-toxic medicine to prevent and treat radiation induced heart injury. PACAP38 is an endogenous multifunctional bioactive polypeptide which is widely distributed in the brain and peripheral organs in mammals, and reportedly has diverse functions in the endocrine, nervous, gastrointestinal, urinary, immune, and cardiovascular systems[25]. PACAP and its receptors have also been identified in mammalian heart[26]. PACAP binding to its receptors modulates the excitability of intracardiac neurons and has positive inotropic, chronotropic, and dromotoropic effects on cardiomyocytes[27-29]][[iv]][. PACAP is also a potent vasodilator in various organs, including coronary and pulmonary arteries. Moreover, PACAP has been revealed to have cardioprotective capacity by its anti-inflammatory response, anti-apoptotic and anti-oxidant properties. A number of papers have reported that the treatment of PACAP38 has a protective effect on mitomycin or doxorubicin-induced cardiotoxicity[30-32]][[v]][. PACAP has been shown to protect cardiomyocytes in vitro from oxidative or ischemia/reperfusion stress[33]. The receptors of PACAP38 are expressed in the heart, but it is unclear whether PACAP38 exerts its protective effect on the late radiation induced heart injury in vivo. The aim of the present study was to investigate whether PACAP has a cardioprotective effect on radiation-induced cardiac injury in a murine model of RIHD. In this study, it was found that PACAP38 effectively protect or prevent radiation induce advanced cardiac injuries in a mouse model, myocardial fibrosis and perivascular fibrosis, as well as myocardial metabolic dysfunction, which is considered to be an important cause leading to myocardial ischemia and RIHD. In addition to our previous study of PACAP38 against cardiomyocytes irradiation in vitro and acute cardiac injury in vivo[19], the results suggest PACAP38 holding potential for developing a therapeutic agent of RIHD.
Radiation therapy is one of the most effective manner used in the treatment of thoracic cancers, but its dose and volume is often limited because of its marked cardiotoxicity for vascular system, pericardium, myocardium, valves and conduction system[34]. Among them, myocardial tissue is the main substructure and functional unit of the heart exposed to the radiation. The subsequent pathological process displayed edema, fibrosis and calcification of cardiac tissues[35]. Radiation exposure induces a chronic form of cardiomyopathy, which is one of the important causes of heart failure. Our previous results showed that significant apoptosis was observed in the group treated with radiation exposure by TUNEL staining[19]. Treatment with PACAP38 protected mice from histological damage and myocardial apoptosis induced by irradiation. Furthermore, myocardial fibrosis is considered to be the end stage of the long-term development of RIHD, which eventually leads to cardiac dysfunction[36]. The present study showed that the pathological changes in cardiac left ventricular sections from wild-type mice with heart irradiation were significantly different between the saline-treated and PACAP38-treated irradiated mice in HE and Masson’s trichrome stainings, moderate damage and significant fibrosis were observed in radiation exposed mice. PACAP38-treated irradiation mice showed significantly improvement on myocardial morphology and fibrosis. Myocardial fibrosis induces cardiac remodeling, which is characterized by excessive collagen deposition, leading to ventricular sclerosis, diastolic and systolic dysfunction, and ultimately heart failure[37]. Sridharan et al studied brown Norway rats exposed to local heart radiation, in which inflammation and fibrosis were seen at 3 and 6 months after irradiation[38]. Our results showed dramatically reduction of cardiac fibrosis and remodeling would all suggest possible novel cardioprotectant treatments and improve cardiac activity. α-Smooth muscle actin (α-SMA) has been a well-accepted marker of myofibroblast differentiation, which showed a pivotal role in the mechanism of PACAP38 alleviating radiation induced cardiac fibrosis. More work is needed to clarify the regulation mechanism of PACAP38 on α-SMA. Cao et al. found that cardiac diastolic function is more sensitive than left ventricular ejection fraction (LVEF) and can be used as an early monitoring index of radiation induced heart damage by echocardiography[11]. American Academy of Cardiology (ACC) and American Heart Association (AHA) as well as European Society of Cardiology (ESC) recommended echocardiography as a routine monitoring cardiac function and cardiotoxicity of cardiovascular disease[39,40]. Cardiac Magnetic Resonance imaging (CMR) has significant advantage in monitoring myocardial lesions, such as myocardial necrosis, fibrosis, edema and abnormal perfusion[41]. Cardiac radionuclide imaging, such as Single Photon Emission Computed Tomography (SPECT) and Positron Emission Tomography (PET), is more accurate than 2D ultrasound in detecting the asymptomatic decline of LVEF, and correlated with CMR and 3D echocardiography[42,43]. Our results identified that LVEF and E/e 'of the hearts of mice in each group did not change significantly 6 months after irradiation. However, this study found that LVIDd and LVIDs decreased significantly in the IR group, which returned to normal with the intervention of PACAP38. LVID has the potential to become an early marker of cardiac diastolic dysfunction. Micro-PET/CT detection of mouse heart showed that irradiation could significantly improve the heart uptake rate. Significant difference was triggered on the reduction of 18 fludeoxyglucose activity by PACAP38 treatment indicates that PACAP38 reversed cardiac injury and protected heart function. The relationship between SUV value and radiation induced heart injury needs to be further clarified.
Conclusion
In summary, the pathogenesis of radiation-induced cardiotoxicity involves substantial changes in histological damages in irradiated myocardium. PACAP38 prevents and protects heart against later stages of cardiotoxicity in mice exposed to radiation, by inhibiting myocardial remodeling and fibrosis. The use of 18 Fludeoxyglucose PET/CT is helpful for the detection of cardiac radiation injury. PACAP38 could effectively improve the myocardial metabolic dysfunction induced by irradiation, which provides a theoretical basis for PACAP38 to develop a non-toxic and effective protective agent for RIHD.
Author Contributions
Conceptualization, Huan Li, Min Li and Jia-Yi Chen; methodology, Huan Li and Pei-Qiang Yi ; formal analysis, Huan Li and Pei-Qiang Yi; investigation, Huan Li and Qian Zhu; resources, Lu Cao, Cheng Xu and Jia-Yi Chen; data curation, Huan Li; writing—original draft preparation, Huan Li and Pei-Qiang Yi; writing—review and editing, Huan Li, Pei-Qiang Yi, Qian Zhu, Min Li and Jia-Yi Chen; funding acquisition, Qian Zhu, Lu Cao, Min Li and Jia-Yi Chen. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the National Natural Science Foundation of China (81972963) and the National Key Research and Development Program of China (2022YFC2404602).
Institutional Review Board Statement
All animal experiments and procedures were conducted in accordance with the approval of the Shanghai Jiaotong University School of Medicine Institutional Animal Care and Use Committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the fact that we are conducting further experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71 (2021): 209-249.
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366 (2005): 2087-2106.
- McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 100 (2011): 167-175.
- van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med 175 (2015): 1007-1017.
- Fidler MM, Reulen RC, Henson K, et al. British Childhood Cancer Survivor Study Steering, G. Population-Based Long-Term Cardiac-Specific Mortality Among 34 489 Five-Year Survivors of Childhood Cancer in Great Britain. Circulation 135 (2017): 951-963.
- van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 129 (2017): 2257-2265.
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol, 16 (2015): 187-199.
- Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol 35 (2017): 1387-1394.
- Stewart JR, Cohn KE, Fajardo LF, et al. Radiation-Induced Heart Disease A Study of Twenty-five Patients. Radiology 89 (1967).
- Stewart JR, Fajardo LF. Dose response in human and experimental radiation-induced heart disease. Application of the nominal standard dose (NSD) concept. Radiology 99 (1971): 403-408.
- Cao L, Cai G, Chang C, et al. Diastolic Dysfunction Occurs Early in HER2-Positive Breast Cancer Patients Treated Concurrently With Radiation Therapy and Trastuzumab. Oncologist 20 (2015): 605-614.
- Cao L, Hu WG, Kirova YM, et al. Potential impact of cardiac dose-volume on acute cardiac toxicity following concurrent trastuzumab and radiotherapy. Cancer Radiother 18 (2014): 119-124.
- Shimizu Y, Kodama K, Nishi N, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ 340 (2010): b5349.
- Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 128 (2013): 1927-1995.
- van der Veen SJ, Ghobadi G, de Boer RA, et al. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol 114 (2015): 96-103.
- Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61 (2009): 283-357.
- Reglodi D, Vaczy A, Rubio-Beltran E. et al. Protective effects of PACAP in ischemia. J Headache Pain, 19 (2018): 19.
- Li M, Khan AM, Maderdrut JL, et al. The effect of PACAP38 on MyD88-mediated signal transduction in ischemia-/hypoxia-induced acute kidney injury. Am J Nephrol 32 (2010): 522-532.
- Li H, Cao L, Yi PQ, et al. Pituitary adenylate cyclase-activating polypeptide ameliorates radiation-induced cardiac injury. Am J Transl Res 11 (2019): 6585-6599.
- Hamza M, Fatima M, Masood M, et al. Relationship of left ventricular and atrial dimensions with moderate to severe left ventricular diastolic dysfunction (grade II and above). Afr Health Sci 20 (2020): 1749-1753.
- Ng AK. Review of the cardiac long-term effects of therapy for Hodgkin lymphoma. Br J Haematol 154 (2011): 23-31.
- Jaworski C, Mariani JA, Wheeler G, et al. Cardiac complications of thoracic irradiation. J Am Coll Cardiol 61 (2013): 2319-2328.
- Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist 12 (2007): 738-747.
- Demiral AN, Yerebakan O, Simsir V, et al. Amifostine-induced toxic epidermal necrolysis during radiotherapy: a case report. Jpn J Clin Oncol 32 (2002): 477-479.
- Toth D, Szabo E, Tamas A, et al. Protective Effects of PACAP in Peripheral Organs. Front Endocrinol (Lausanne) 11 (2020): 377.
- Sarszegi Z, Szabo D, Gaszner B, et al. Examination of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) as a Potential Biomarker in Heart Failure Patients. J Mol Neurosci 68 (2019): 368-376.
- Merriam LA, Roman CW, Baran CN, et al. Pretreatment with nonselective cationic channel inhibitors blunts the PACAP-induced increase in guinea pig cardiac neuron excitability. J Mol Neurosci 48 (2012): 721-729.
- Clason TA, Girard BM, May V, et al. Activation of MEK/ERK Signaling by PACAP in Guinea Pig Cardiac Neurons. J Mol Neurosci 59 (2016): 309-316.
- Farnham MM, Inglott MA, Pilowsky PM. Intrathecal PACAP-38 causes increases in sympathetic nerve activity and heart rate but not blood pressure in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 300 (2011): H214-222.
- Mori H, Nakamachi T, Ohtaki H, et al. Cardioprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on Doxorubicin-induced cardiomyopathy in mice. Circ J 74 (2010): 1183-1190.
- Racz B, Reglodi D, Horvath G, et al. Protective effect of PACAP against doxorubicin-induced cell death in cardiomyocyte culture. J Mol Neurosci 42 (2010): 419-427.
- Subramaniam V, Chuang G, Xia H, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) protects against mitoxantrone-induced cardiac injury in mice. Peptides 95 (2017) 25-32.
- Roth E, Weber G, Kiss P, et al. Effects of PACAP and preconditioning against ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro. Ann N Y Acad Sci 1163 (2009): 512-516.
- Desai MY, Jellis CL, Kotecha R, et al. Radiation-Associated Cardiac Disease: A Practical Approach to Diagnosis and Management. JACC Cardiovasc Imaging 11 (2018): 1132-1149.
- Spetz J, Moslehi J, Sarosiek K. Radiation-Induced Cardiovascular Toxicity: Mechanisms, Prevention, and Treatment. Curr Treat Options Cardiovasc Med 20 (2018): 31.
- Zhu Q, Kirova YM, Cao L, et al. Cardiotoxicity associated with radiotherapy in breast cancer: A question-based review with current literatures. Cancer Treat Rev 68 (2018): 9-15.
- Roche PL, Filomeno KL, Bagchi RA, et al. Intracellular signaling of cardiac fibroblasts. Compr Physiol 5 (2015): 721-760.
- Sridharan V, Sharma SK, Moros EG, et al. Effects of radiation on the epidermal growth factor receptor pathway in the heart. Int J Radiat Biol 89 (2013): 539-547.
- Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 37 (2016): 2768-2801.
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 70 (2017): 252-289.
- Virani SA, Dent S, Brezden-Masley C, et al. Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy. Can J Cardiol 32 (2016): 831-841.
- Walker J, Bhullar N, Fallah-Rad N, et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol 28 (2010): 3429-3436.
- Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 21 (2000): 1387-1396.