Prospective Observational Study to Assess Effectiveness and Safety of Intravenous Ferric Carboxymaltose 1000 mg in Patients of Chronic Heart Failure with Iron DeficiEncy - PRIDE-HF Study
Article Information
Bagirath Raghuraman1, Rajendra Kumar Jain2, Rajesh Surendra Badani3, Sachin Narendra Patil4, Nanette Suares5, Dhammdeep Dabhade6, Rishikesh Shewale6*, Sachin Suryawanshi6, Vikneswaran. G7
1Department of Heart transplant, Narayana Institute of Cardiac Sciences, Bommasandra Industrial Area, Anekal Taluk, Hosur Road, Bangalore-560099, Karnataka
2Head of Department & Consultant Cardiologist, Krishna Institute of Medical Sciences, Minister road, Secunderabad- 500003, Telangana
3Department of Cardiology, Aditya Birla Memorial Hospital, Thergaon, Chinchwad, Pune- 411033, Maharashtra
4Sachin Superspeciality Clinic, The Icon, 2nd floor, Rajarampuri 6th lane, Near Sriram High School, Kolhapur-416008, Maharashtra
5Medical Services, Emcure Pharmaceuticals Ltd., Phase-1, Rajiv Gandhi ITBT Park, M.I.D.C., Hinjewadi, Pune-411057, Maharashtra
6Medical Services, Emcure Pharmaceuticals Ltd., Unit No. D-5119-5148, D-Wing, Oberoi Garden Estates, Chandivali, Andheri East, Mumbai – 400 072, Maharashtra
7Narayana Hrudayalaya Limited, No.258/A, Bommasandra Industrial Area, Anekal Taluk, Hosur Road, Bangalore-560099, Karnataka
*Corresponding author: Dr. Rishikesh Shewale, Medical Services, Emcure Pharmaceuticals Ltd., Unit No. D-5119-5148, D-Wing, Oberoi Garden Estates, Chandivali, Andheri East, Mumbai – 400 072, Maharashtra
Received: 04 September 2023; Accepted: 12 September 2023; Published: 05 December 2023.
Citation: Bagirath Raghuraman, Rajendra Kumar Jain, Rajesh Surendra Badani, Sachin Narendra Patil, Nanette Suares, Dhammdeep Dabhade, Rishikesh Shewale, Sachin Suryawanshi, Vikneswaran. G. Prospective Observational Study to Assess Effectiveness and Safety of Intravenous Ferric Carboxymaltose 1000 mg in Patients of Chronic Heart Failure with Iron DeficiEncy - PRIDE-HF Study. Cardiology and Cardiovascular Medicine. 7 (2023): 349-354.
View / Download Pdf Share at FacebookAbstract
Objectives: The present study aims to assess the effectiveness and safety profile of intravenous (IV) ferric carboxymaltose (FCM) administration in patients with chronic heart failure (HF) having iron deficiency (ID) in real-world clinical practice.
Methods: This was a prospective, multi-center, non-interventional, observational study that included patients aged 18 years and above of either gender. The included patients belonged to New York Heart Association (NYHA) class II – III who had ID. The primary objective of the study was to evaluate the effectiveness of treatment with injection FCM in patients of chronic HF with ID by assessing improvement in the symptoms at week 12. The study was registered with clinical trial registry of India (CTRI) [CTRI/2021/02/031307].
Results: A total of 98 subjects were enrolled in the study from 4 centers. The mean age of the study population was 61.08 ± 12.95 years with 58% being male. Primary endpoint of the study was a change in patients' symptoms assessed using NYHA class. There was a significant improvement in NYHA class from baseline to 12 weeks, with 56.1% of patients belonging to NYHA Class III as compared to only 8.4% at 12 weeks (P value < 0.001). No adverse events or hospitalizations were reported during the study period.
Conclusion: Rectifying ID in chronic HF using IV FCM leads to substantial improvement in disease symptoms and haematological parameters which in turn may lead to favourable disease outcomes and lessen HF-related hospitalization. The study highlights IV FCM's potential as a promising therapy in HF management.
Keywords
Ferric carboxymaltose; Heart failure; Iron deficiency; Reduced ejection fraction
Ferric carboxymaltose articles; Heart failure articles; Iron deficiency articles; Reduced ejection fraction articles.
Article Details
1. Introduction
Heart failure (HF) is a complex clinical syndrome marked by high morbidity and mortality, low functional capacity, and poor quality of life [1]. Globally, HF affects around 64 million people [1]. According to the Trivandrum Heart Failure Registry, Indian HF patients have a 5-year mortality rate of 59% and a median survival of 3.1 years [2]. Iron is crucial for the transport of oxygen, not just through haematopoiesis but also in the metabolism of cardiac and skeletal muscles [3]. All of these elements contribute to the decreased exercise capacity in HF [3]. Regardless of the left ventricular ejection fraction or haemoglobin levels, iron deficiency (ID) is frequently observed in individuals with chronic heart failure and is independently linked to more severe symptoms, reduced exercise tolerance, and a higher risk of hospitalisation and mortality [4]. The frequency of ID in HF is estimated to be around 50% irrespective of the anaemic status [5]. When it comes to heart failure, ID is defined as ferritin levels of less than 100 mcg/L or between 100 and 299 mcg/L with a transferrin saturation of less than 20% [6]. One of the characteristics of chronic HF is an intolerance to physical activity, which is further diminished in the presence of an iron deficit [3]. Oral iron supplementation is the primary approach employed to restore iron levels in patients [5]. Although, trials like IRON-HF do not advocate the use of the oral route for iron replenishment in HF patients with reduced ejection fraction owing to failure in demonstration of improvement in the exercise capacity [7]. Simultaneously, reports indicate that patients with stable, symptomatic, iron-deficient heart failure (HF) who received intravenous iron such as ferric carboxymaltose (FCM) experienced improvements in their symptoms, quality of life, and functional capacity. Furthermore, they demonstrated a reduced risk of the first hospitalization for HF exacerbation [3], [8-10]. Adults with iron deficiency anaemia who cannot accept or could not be successfully treated with oral iron supplements are given iron intravenously [11]. A new-generation intravenous iron formulation called FCM allows for the safe administration of up to 1000 mg (20 mg/kg) of iron in a single 15-min infusion with a very low risk of life-threatening allergic responses [12]. Even though intravenous iron therapy seems to help iron-deficient stable HF patients, there are still concerns regarding its usage in HF, including whether the benefits will last over the long term, if it will be safe, and whether it will have significant outcomes [13, 14]. Moreover, there is limited data available on the effectiveness and safety of IV FCM in HF patients in the Indian context. The present Prospective ObseRvational Study to Assess Effectiveness and Safety of Intravenous Ferric Carboxymaltose 1000 mg in Patients of Chronic Heart Failure with Iron DeficiEncy (PRIDE-HF) study was designed to assess the effectiveness and safety profile of IV FCM administration in real-world clinical practice.
2. Materials and Methods
2.1 Study Design
PRIDE-HF was a prospective, multicentre, non-interventional, observational study to evaluate the real-world effectiveness and safety of IV FCM in HF patients. The 4 participating sites from India were Narayana Institute of Cardiac Sciences, Bangalore; Krishna Institute of Medical Sciences, Secunderabad; Sachin Superspeciality Clinic, Kolhapur and Aditya Birla Memorial Hospital, Pune. The ethics committee of each participating centre gave its approval to the study protocol. For study participation, each subject voluntarily provided written informed consent. The study been registered with CTRI as CTRI/2021/02/031307.
The inclusion and exclusion criteria for enrolling participants are mentioned in Table 1.
Table 1: Eligibility Criteria
|
Inclusion criteria |
Exclusion criteria |
|
· Adult (> 18 years of age) patients of either gender. |
· Patients not willing to sign the Informed Consent Form and provide medical data. |
|
· Stable symptomatic ambulatory patients diagnosed with chronic heart failure of New York Heart Association (NYHA) class II – III and ID. |
· Hypersensitivity to ferric carboxymaltose injection. |
|
· Patients willing to give informed consent for participation in the study. |
· Participation in any other clinical trial in the past 3 months |
|
· Treatment with other IV Iron preparations within 4 weeks before study enrolment. |
|
|
· Any condition that, in the opinion of the investigator, does not justify the patient’s inclusion in the study |
2.2 Study procedure
Eligible patients as confirmed by inclusion/exclusion criteria and by the investigator were enrolled in the study. After acquiring the required consent from the participants, their data was collected in a predesigned case report form (CRF). Patient’s demographic characteristics, disease profile, significant medical history, treatment details, and concomitant medications details were recorded in the CRF. Ferric carboxymaltose (FCM) was administered at a dose of 1000 mg as an infusion with 250 ml of 0.9% normal saline over 15 min. All the participants were followed up at 4 weeks and then at the end of the study period. The details of assessments were recorded.
Laboratory assessments were performed for haemoglobin, serum ferritin, transferrin saturation (TSAT) and total iron binding capacity (TIBC) levels at baseline and 12th week. NYHA class of the enrolled patients were assessed at baseline, 4th and 12th week and, ejection fraction was assessed at baseline and 12th. Additionally, self-reported Patient Global Assessment (PGA) Score was evaluated at 4th and 12th week of the study. Any adverse event, serious adverse event, hospitalisation, and death, if any, were recorded at each visit of the participant.
2.3 Study outcomes
The primary objective of this study was to evaluate the effectiveness of treatment with IV FCM in patients of chronic HF with ID by assessing improvement in the symptoms at week 12. ID was diagnosed when the serum ferritin level was less than 100 mcg/L or was between 100 and 299 mcg/L when the transferrin saturation (TSAT) was less than 20%. The study outcomes are outlined in Table 2.
Table 2: Study outcomes
|
Primary outcome |
· Change in the NYHA class from baseline to Week 12 (±1 week). |
|
Secondary outcome |
· Mean self-reported PGA score at Week 12 (±1 week). · Number and nature of serious and non-serious adverse events throughout study duration. · Rate of any hospitalization, rate of hospitalization for any cardiovascular reason, and rate of hospitalization due to worsening HF as assessed up to Week 12 (±1 week). · Time to first hospitalization for any reason, time to first hospitalization for any cardiovascular reason and time to first hospitalization due to worsening HF as assessed up to Week 12 (±1 week). · Mean change in the haematological parameters [haemoglobin, serum ferritin, TIBC and TSAT] from baseline to week 12 (±1 week). · Change in the NYHA class and mean self-reported PGA score from baseline at week 4. Change in ejection fraction from baseline to week 12 (±1 week). |
NYHA New York Heart Association, PGA Patient Global Assessment, TIBC Total Iron Binding Capacity, TSAT Transferrin Saturation.
2.4 Statistical analysis
Data were collected in REDCap and exported to IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp. Baseline characteristics were expressed using mean and standard deviation or median with range for continuous variables; frequency and percentage for categorical variables.
Any patient who signed informed consent form and received at least one injection of FCM was included in the descriptive analysis, while only those data available at baseline as well as 12 weeks were included for comparative analysis. Wilcoxon Signed rank test was used to study the change in NYHA class, haematological parameters, self-reported patient global assessment between baseline and 12 weeks. For statistical analysis P value less than 0.05 was considered statistically significant. A convenient sample size of 100 was chosen for the study. The sample size was not based on any calculation.
3. Results
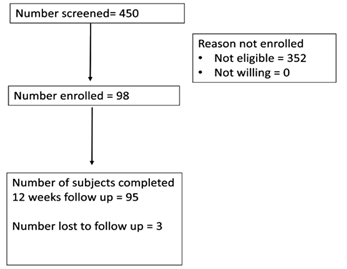
A total of 98 subjects were enrolled for the study from 4 centres, of which 3 patients were lost to follow-up. The study outline is depicted in Figure 1.
The baseline demographic characteristics and laboratory parameters are mentioned in Table 3 and Table 4 respectively. The mean age of the study participants was 61.08 ± 12.95 years with 58.2% of them being male. Almost 80% of the participants were found to have HF with reduced ejection fraction.
Table 3: Baseline Demographic characteristics
|
Age (years) |
61.08 ± 12.95 |
|
Gender-Male (%) |
57 (58.2%) |
|
Female (%) |
41 (41.8%) |
|
Height (cms) |
159.6 ± 7.82 |
|
Weight (kgs) |
63.31 ± 10.68 |
|
SBP (mm Hg) |
124.36 ± 18.78 |
|
DBP (mm Hg) |
74.84 ± 11.79 |
|
HR (beats/ min) |
78.69 ± 13.68 |
|
Type of heart failure |
|
|
HFrEF |
78 (79.6%) |
|
HFpEF |
20 (20.4%) |
|
Etiology of Heart failure |
|
|
MI |
22 (22.4%) |
|
Hypertension |
19 (19.4%) |
|
AF |
2 (2%) |
|
Valvular heart disease |
6 (6.1%) |
|
Cardiomyopathy |
31 (31.6%) |
|
Others |
18 (18.4%) |
|
Duration of heart failure* (years) |
4 (1,8) |
SBP Systolic blood pressure, DBP Diastolic blood pressure, HR Heart rate, HFrEF Heart failure with reduced ejection fraction, HFpEF Heart failure with preserved ejection fraction, MI Myocardial infarction, AF Atrial fibrillation. *Represented as Median
Table 4: Baseline laboratory investigations
|
Parameter |
Value |
|
Haemoglobin (g/dL) |
10.69 ± 2.22 |
|
Serum ferritin (mcg/L) |
54.70 ± 45.32 |
|
TSAT (%) |
11.33 ± 3.86 |
|
TIBC (mcg/dL) |
346.71 ± 58.75 |
TSAT transferrin saturation, TIBC total iron binding capacity
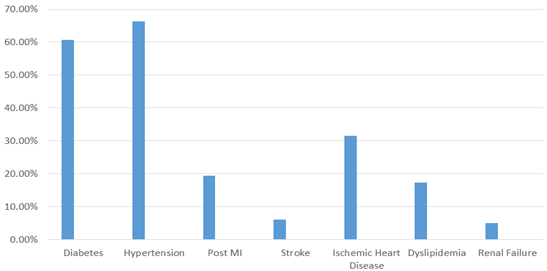
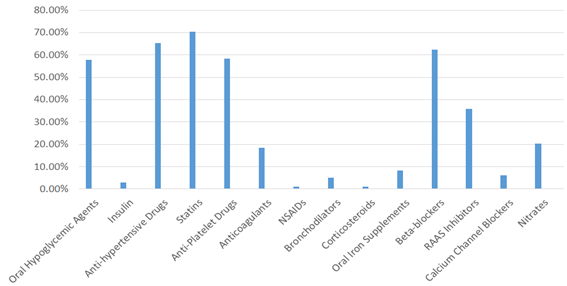
Details about comorbid illness and concomitant medications are described in Figure 2 and Figure 3 respectively. Hypertension was the most common comorbidity observed in 66.30% of the participants. This was followed by diabetes (60.76%) and ischemic heart disease (31.6%).
MI Myocardial infarction
NSAID Non-steroidal anti-inflammatory drugs, RAAS Renin angiotensin aldosterone system
The efficacy-related endpoints are summarized in Table 5, Table 6, and Table 7. The primary endpoint of the study was change in the patient’s symptoms which was evaluated utilising the NYHA classification. A significant improvement in NYHA class from baseline to 12 weeks was observed in the patients (P< 0.001). At baseline, 56.10% of the participants belonged to NYHA class III which was reduced to 8.40% by end of the study. The haematological parameters also improved significantly from baseline to 12 weeks for haemoglobin (10.69 ± 2.22 g/dL to 11.41 ± 6.68 g/dL; P value= 0.54), serum ferritin (54.70 ± 75.32 mcg/L to 226.72 ± 145 mcg/L; P value < 0.001), TSAT (11.33 ± 3.86% to 24.03 ± 9.78%; P value < 0.001) and TIBC (346.71 ± 58.75 mcg/dL to 294.97 ± 54.43 mcg/dL; P value= 0.002). The self-reported patient global assessment score was used to assess patient response to treatment. There was a significant improvement in the score when the values were compared for week 4 and week 12 (P< 0.001) (Table 7). The changes in ejection fraction from baseline to week 12th is represented in Table 8 where no significant changes were observed (P value= 0.68). No adverse events or hospitalisations were reported during the study period.
Table 5: Comparison of participants based on NYHA class during the study period
|
|
Baseline |
4 weeks |
12 weeks |
P value |
|
NYHA class |
Class II -43 (43.9%) |
Class I -10 (10.5%) |
Class I -29 (30.5%) |
< 0.001* |
|
Class III -55 (56.1%) |
Class II- 65 (68.4%) |
Class II-58 (61.1%) |
||
|
Class III 20 (21.1%) |
Class III 8 (8.4%) |
|||
* Comparing week 4 with baseline and week 12 with week 4
Table 6: Comparison of change in haematological parameters at the end of the study period
|
Parameter |
Baseline |
12 weeks |
P value |
|
Haemoglobin (g/dL) |
10.69 ± 2.22 |
11.41 ± 6.68 |
0.54 |
|
Serum ferritin (mcg/L) |
54.70 ± 45.32 |
226.72 ± 145.12 |
<0.001 |
|
TSAT (%) |
11.33 ± 3.86 |
24.03 ± 9.78 |
< 0.001 |
|
TIBC (mcg/dL) |
346.71 ± 58.75 |
294.97 ± 54.43 |
0.002 |
TSAT transferrin saturation, TIBC total iron binding capacity
Table 7: Assessment of Self-reported patient global assessment
|
Week 4 |
Week 12 |
P value |
|
|
Much improved |
15 (15.8%) |
32 (33.7%) |
< 0.001 |
|
Moderately improved |
32 (33.7%) |
37 (38.9%) |
|
|
A little improved |
39 (41.1%) |
21 (22.1%) |
|
|
Unchanged |
8 (8.4%) |
3 (3.2%) |
|
|
A little worse |
1 (1.1%) |
1 (1.1%) |
|
|
Moderately worse |
0 |
1 (1.1%) |
|
|
Much worse |
0 |
0 |
Table 8: Comparison of change in ejection fraction
|
Baseline |
12 weeks |
P value |
|
|
Ejection fraction (%) |
36.11±6.5 |
37.22±11.21 |
0.68 |
|
(Mean ±SD) |
4. Discussion
The present study reports the effects of IV FCM in chronic HF patients with NYHA class II and III along with ID. The important findings of this study were: (i) There was a significant improvement in NYHA class from baseline to 12 weeks, with 56.1% patients belonging to class III at the baseline as compared to only 8.4% patient at the end of the study period; (ii) Significant improvement in haematological indicators [serum ferritin (P<0.001), TSAT (P< 0.001) and TIBC (P= 0.002)] from baseline to 12 weeks; (iii) Self-reported PGA improved significantly from baseline at week 12 (P< 0.001); (iv) No adverse events or hospitalisations were observed during the study period; and (v) Almost 80% of the study population had heart failure with reduced ejection fraction while 20% had heart failure with preserved ejection fraction. In the iron-sulphur clusters that make up the first three components of the electron transport chain in mitochondria, iron serves as a crucial co-factor for anti-oxidative enzymes [15]. As a result, iron deficiency is identified as a significant comorbidity in the pathological process of heart failure [16, 17]. When iron stores are depleted, an iron deficiency is considered to be "absolute," but it can also be "functional" (or "relative") due to poor iron metabolism, which may be a side effect of inflammatory processes [18]. Ferritin levels are used to identify an iron deficiency in healthy adults, and a cut-off of 30 mcg/l is frequently utilised. The 'normal' range in most laboratories is 30-300 mcg/l, with the mean values being 88 mcg/l for males and 49 mcg/l for women [13]. However, because HF and other chronic disorders are linked to an enhanced activation of both pro- and anti-inflammatory mechanisms, the classification of iron deficiency in these situations is challenging [19]. International HF guidelines define ID as having a serum ferritin level below 100 mcg/L (absolute ID) or having a normal serum ferritin level (100–299 mcg/L) with poor transferrin saturation (<20%) [20]. FCM is the first and only intravenous iron replacement therapy to receive FDA approval for correcting iron deficiency in adult patients with heart failure with reduced ejection fraction [21]. In the current study, iron therapy not only improved the functional capacity of the patients as shown by the primary endpoint of NYHA functional class improvement, but it also had positive effects on patient reported outcomes as assessed by self-reported PGA. The advantage became apparent after 4 weeks and persisted throughout the duration of the study and was statistically significant (P< 0.001). Similar results were reported in FAIR-HF trial as well, where benefits were evident from week 4 onwards and were sustained during the study period [8]. A meta-analysis by Kapoor et al. reported that patients receiving intravenous iron saw significantly fewer hospitalisations and adverse events, as well as improvements in their NYHA class and ejection fraction [22].
Post-intravenous FCM administration, there were no adverse events reported nor did any hospitalisation occur throughout the study period. With no impact on all-cause mortality or CV mortality alone, several studies and meta-analyses have stated that intravenous iron infusion in patients with HF lowers the composite risk of first hospitalisation for HF and CV mortality as well as the risks of first and subsequent hospitalisations for HF [9, 10], [23, 24]. Hospitalisations brought on by HF deterioration are invariably associated with poor outcomes, a reduction in patients' quality of life, and a financial burden on society. Hence, their prevention becomes necessary and new therapies must also be evaluated on these lines. Iron deficiency, which is a frequent occurrence in heart failure is a standalone predictor of worse outcomes [25]. Hence, therapies that improve the iron status in HF patients must be given due importance. The current ESC guidelines recommend that the complete blood count, serum ferritin concentration, and TSAT are routinely used to check all HF patients for anaemia and iron deficiency. If anaemia and/or iron deficiency are detected, it should prompt appropriate investigations to determine their underlying causes [26]. The guideline also states that intravenous iron supplementation with FCM should be taken into consideration in symptomatic patients with Left ventricular ejection fraction less than 45% and iron deficiency, to reduce HF symptoms, enhance exercise capacity, and enhance quality of life [26]. In the current study, IV FCM was administered to the HF patients for the iron-deficient state and there was a serial and significant improvement in the haematological parameters (serum ferritin, TIBC and TSAT) throughout the study duration. The present study shows that IV FCM is an effective and tolerable therapy in chronic HF patients. However, our study has a few limitations. Firstly, this was a single arm study, hence, the comparative efficacy of IV FCM could not be evaluated with other available IV preparations. Secondly, a longer follow-up period or greater exposure dose would have resulted in a more notable improvement in iron reserves and boosted exercise capacity.
Conclusion:
This is a first multicentre study evaluating the clinical utility of IV FCM in Indian HF patients. In conclusion, our study states that the correction of ID in chronic HF with IV FCM results in significant improvement in disease symptoms and haematological parameters. This could lead to a reduction in hospitalisations which may occur due to worsening of HF. The findings of this study provide valuable insights into the benefits of iron deficiency correction in heart failure which adds to the existing body of evidence that emphasizes the potential of IV FCM as a promising therapeutic intervention in HF. Early detection of ID and timely correction with IV FCM can improve heart failure outcomes.
Conflict of Interest:
The author reports no conflicts of interest with anyone with regards to this study. Dr. Nanette Suares, Dr. Dhammdeep Dabhade, Dr. Rishikesh Shewale and Dr. Sachin Suryawanshi are full time employees of Emcure Pharmaceuticals Ltd. which actively markets IV Ferric Carboxymaltose.
References
- G Savarese, PM Becher, LH Lund, et al. “Global burden of heart failure: a comprehensive and updated review of epidemiology,” Cardiovasc. Res 118 (2023): 3272-3287.
- S Harikrishnan. “Five-year mortality and readmission rates in patients with heart failure in India: Results from the Trivandrum heart failure registry,” Int. J. Cardiol 326 (2021): 139-143.
- DJ Van Veldhuisen. “Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Chronic Heart Failure and Iron Deficiency,” Circulation 136 (2017): 1374-1383.
- PR Kalra. “Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): an investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial,” The Lancet 400 (2022): 2199-2209.
- C Rizzo, R Carbonara, R Ruggieri, et al. “Iron Deficiency: A New Target for Patients With Heart Failure,” Front. Cardiovasc. Med 8 (2021): 709872.
- S von Haehling, N Ebner, R Evertz, et al. “Iron Deficiency in Heart Failure: An Overview,” JACC Heart Fail 7 (2019): 36-46.
- GD Lewis. “Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial 317 (2017): 1958.
- SD Anker. “Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency,” N. Engl. J. Med 361 (2009): 2436-2448.
- P Ponikowski. “Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency,” Eur. Heart J 36 (2015): 657-668.
- P Ponikowski. “Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial,” The Lancet 396 (2020): 1895-1904.
- KA Lyseng-Williamson and GM Keating. “Ferric carboxymaltose: a review of its use in iron-deficiency anaemia,” Drugs 69 (2009): 739-756.
- SR Pasricha. “Ferric carboxymaltose versus standard-of-care oral iron to treat second-trimester anaemia in Malawian pregnant women: a randomised controlled trial,” The Lancet 401 (2023): 1595-1609.
- DJ van Veldhuisen, SD Anker, P Ponikowskiet al. “Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches,” Nat. Rev. Cardiol 8 (2011): 485-493.
- T Avni, L Leibovici, and A Gafter-Gvili. “Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis,” Eur. J. Heart Fail 14 (2012): 423-429.
- MF Hoes. “Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function,” Eur. J. Heart Fail 20 (2018): 910-919.
- G Charles-Edwards. “Effect of Iron Isomaltoside on Skeletal Muscle Energetics in Patients With Chronic Heart Failure and Iron Deficiency,” Circulation 139 (2019): 2386-2398.
- V Melenovsky. “Skeletal Muscle Abnormalities and Iron Deficiency in Chronic Heart FailureAn Exercise 31P Magnetic Resonance Spectroscopy Study of Calf Muscle,” Circ. Heart Fail 11 (2018): 004800.
- G Weiss. “Iron metabolism in the anemia of chronic disease,” Biochim. Biophys. Acta 1790 (2009): 682-693.
- S Heymans . “Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology,” Eur. J. Heart Fail 11 (2009): 119-129.
- G Masini. “Criteria for Iron Deficiency in Patients With Heart Failure,” J. Am. Coll. Cardiol 79 (2022): 341-351.
- “FDA Approves Intravenous Iron Replacement Therapy for Heart Failure Patients (2023).
- M Kapoor, MD Schleinitz, A Gemignani, et al. Wu, “Outcomes of patients with chronic heart failure and iron deficiency treated with intravenous iron: a meta-analysis,” Cardiovasc. Hematol. Disord. Drug Targets 13 (2013): 35-44.
- HM Salah, G Savarese, GMC Rosano, et al. “Intravenous iron infusion in patients with heart failure: a systematic review and study-level meta-analysis,” ESC Heart Fail 10 (2023): 1473-1480.
- J Dalal, V Katekhaye, and R Jain. “Effect of ferric carboxymaltose on hospitalization and mortality outcomes in chronic heart failure: A meta-analysis,” Indian Heart J 69 (2017): 736-741.
- H Ismahel and N Ismahel. “Iron replacement therapy in heart failure: a literature review,” Egypt. Heart J 73 (2021): 85.
- TA McDonagh. “2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure,” Eur. Heart J 42 (2021): 3599-3726.