Progressive Supranuclear Palsy: A Case Report and Literature Review
Article Information
Samar Ikram MD1, Valeria D. Parra-Payano MD2, Thirumala Keerthi Chandrika Kammaripalle MD3, Abhigyan Datta MD4, Manpreet Kaur MD5, Tanushri Bhushan MD6, Marlliny Sullivan MD7, Abeer Mastoor MBBS8, Humza Haroon MBBS9, Ali Guy MD10, Masum Rahman MD11, Juna Musa MD12, Fjolla Hyseni MD, Ph.D.13, Mumin M Omar14, Essa Mohamed MD15, Ibrahim Bajwa MD16, Peter G. Bernad MD17*
1Department of Neurological Surgery, Mayo Clinic, Rochester, Minnesota
2Universidad Peruana Cayetano Heredia, Lima, Peru
3Apollo Institute of Medical Sciences and Research, Hyderabad, India
4Maulana Azad Medical College, New Delhi, India
5University of Texas Health Science Center, Texas, USA
6Universidade Estácio de Sá, Rio de Janeiro, Brazil
7Omdurman Islamic University, Sudan
8Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar, India
9Jinnah Medical and Dental College, Karachi, Pakistan
10Clinical Assistant Professor, Department of Rehabilitation Medicine, NYU Grossman School of Medicine, New York, USA
11Department of Neurological Surgery, Mayo Clinic, Rochester, Minnesota
12 Department of Surgery, Mayo Clinic, Rochester, MN
13NYU Langone Health, New York, USA
14University of Minnesota, Rochester, MN
15Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN
16Foundation University Islamabad, Punjab, Pakistan
17Clinical Professor, Department of Neurology, George Washington University Hospital, USA
*Corresponding Author: Peter G. Bernad MD, Clinical Professor, Department of Neurology, George Washington University Hospital, Washington, DC 20037, USA
Received: 19 October 2021; Accepted: 08 November 2021; Published: 17 November 2021
Citation: Samar Ikram, Valeria D. Parra-Payano, Thirumala Keerthi Chandrika Kammaripalle, Abhigyan Datta, Manpreet Kaur, Tanushri Bhushan, Marlliny Sullivan, Abeer Mastoor, Humza Haroon, Ali Guy, Masum Rahman, Juna Musa, Fjolla Hyseni, Mumin M Omar, Essa Mohamed, Ibrahim Bajwa, Peter G. Bernad. Progressive Supranuclear Palsy: A Case Report and Literature Review. Archives of Clinical and Medical Case Reports 5 (2021): 838-845.
View / Download Pdf Share at FacebookAbstract
Progressive supranuclear palsy (PSP) is a rare neurodegenerative disease with a prevalence of five to six persons per 100,000 people and characterized by progressive axial rigidity, vertical supranuclear gaze palsy, frontal lobe cognitive decline, and balance issues. The clinical examination findings are diverse and PSP cases are often under-diagnosed or misdiagnosed. We report a case of a 54- year-old male patient that presented primarily with recurrent falls due to postural instability and bilateral leg weakness that had progressively worsened to being wheelchair-bound. An extensive physical examination revealed classical findings indicative of PSP.
Keywords
Progressive supranuclear palsy (PSP); Parkinsonian disease
Progressive supranuclear palsy (PSP) articles; Parkinsonian disease articles
Progressive supranuclear palsy articles Progressive supranuclear palsy Research articles Progressive supranuclear palsy review articles Progressive supranuclear palsy PubMed articles Progressive supranuclear palsy PubMed Central articles Progressive supranuclear palsy 2023 articles Progressive supranuclear palsy 2024 articles Progressive supranuclear palsy Scopus articles Progressive supranuclear palsy impact factor journals Progressive supranuclear palsy Scopus journals Progressive supranuclear palsy PubMed journals Progressive supranuclear palsy medical journals Progressive supranuclear palsy free journals Progressive supranuclear palsy best journals Progressive supranuclear palsy top journals Progressive supranuclear palsy free medical journals Progressive supranuclear palsy famous journals Progressive supranuclear palsy Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals supranuclear palsy articles supranuclear palsy Research articles supranuclear palsy review articles supranuclear palsy PubMed articles supranuclear palsy PubMed Central articles supranuclear palsy 2023 articles supranuclear palsy 2024 articles supranuclear palsy Scopus articles supranuclear palsy impact factor journals supranuclear palsy Scopus journals supranuclear palsy PubMed journals supranuclear palsy medical journals supranuclear palsy free journals supranuclear palsy best journals supranuclear palsy top journals supranuclear palsy free medical journals supranuclear palsy famous journals supranuclear palsy Google Scholar indexed journals Parkinsonian disease articles Parkinsonian disease Research articles Parkinsonian disease review articles Parkinsonian disease PubMed articles Parkinsonian disease PubMed Central articles Parkinsonian disease 2023 articles Parkinsonian disease 2024 articles Parkinsonian disease Scopus articles Parkinsonian disease impact factor journals Parkinsonian disease Scopus journals Parkinsonian disease PubMed journals Parkinsonian disease medical journals Parkinsonian disease free journals Parkinsonian disease best journals Parkinsonian disease top journals Parkinsonian disease free medical journals Parkinsonian disease famous journals Parkinsonian disease Google Scholar indexed journals PSP articles PSP Research articles PSP review articles PSP PubMed articles PSP PubMed Central articles PSP 2023 articles PSP 2024 articles PSP Scopus articles PSP impact factor journals PSP Scopus journals PSP PubMed journals PSP medical journals PSP free journals PSP best journals PSP top journals PSP free medical journals PSP famous journals PSP Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals lymphadenopathy articles lymphadenopathy Research articles lymphadenopathy review articles lymphadenopathy PubMed articles lymphadenopathy PubMed Central articles lymphadenopathy 2023 articles lymphadenopathy 2024 articles lymphadenopathy Scopus articles lymphadenopathy impact factor journals lymphadenopathy Scopus journals lymphadenopathy PubMed journals lymphadenopathy medical journals lymphadenopathy free journals lymphadenopathy best journals lymphadenopathy top journals lymphadenopathy free medical journals lymphadenopathy famous journals lymphadenopathy Google Scholar indexed journals neuropsychiatric articles neuropsychiatric Research articles neuropsychiatric review articles neuropsychiatric PubMed articles neuropsychiatric PubMed Central articles neuropsychiatric 2023 articles neuropsychiatric 2024 articles neuropsychiatric Scopus articles neuropsychiatric impact factor journals neuropsychiatric Scopus journals neuropsychiatric PubMed journals neuropsychiatric medical journals neuropsychiatric free journals neuropsychiatric best journals neuropsychiatric top journals neuropsychiatric free medical journals neuropsychiatric famous journals neuropsychiatric Google Scholar indexed journals Hypoxia articles Hypoxia Research articles Hypoxia review articles Hypoxia PubMed articles Hypoxia PubMed Central articles Hypoxia 2023 articles Hypoxia 2024 articles Hypoxia Scopus articles Hypoxia impact factor journals Hypoxia Scopus journals Hypoxia PubMed journals Hypoxia medical journals Hypoxia free journals Hypoxia best journals Hypoxia top journals Hypoxia free medical journals Hypoxia famous journals Hypoxia Google Scholar indexed journals
Article Details
1. Introduction
Progressive Supranuclear palsy (PSP) is a rare neurological disorder affecting 6 per 100,000 individuals each year [1]. Due to the scarcity of this disease, the etiology of PSP was suspected to be sporadic. However, recent studies have shown that PSP may have an autosomal dominant pattern and that lack of clinical recognition is due to the variable phenotypic expression of this disease [2, 3]. Patients with PSP may have accompanying TDP-43 pathology in the limbic system and PSP-vulnerable regions, such as the subthalamic nucleus, substantia nigra, and pontine tegmentum, but not in the motor neurons [4, 5]. Individuals with PSP are often diagnosed in the fifth decade of life and the disease has a predilection to affect males more than females [1, 5].
The disease was first described by Steele and Richardson in 1964 as a late-onset degenerative disease with a unique constellation of symptoms such as supranuclear palsy affecting vertical gaze, pseudobulbar palsy causing dysarthria, dysphagia, and dystonic rigidity of the neck and upper trunk. Dementia, if present, is usually mild, although patients commonly have neuropsychiatric manifestations such as depression, irritability, and apathy. Neuronal loss, gliosis, and abundant neurofibrillary tangles with neuropil threads are commonly seen in Neuropathology. This specific pattern of nerve change distinguishes the disease from other neurological disorders such as Creutzfeldt Jakob Disease, and Alzheimer’s Disease [6, 7].
PSP is often misdiagnosed because initial symptoms appear vague. The first symptom is usually loss of balance and may be misinterpreted as a cerebellar or inner ear disease. Other early symptoms, such as irritability, and apathy may mistakenly be assumed to be depression. As the disease progresses, patients report blurry vision, frequent blinking, inability to make eye contact, and painful eye movements, especially when looking up. Slurred speech and dysphagia are other early symptoms [3, 7].
Our patient is a Chilean resident and traveled to the United States in the hope of being diagnosed and find treatment for his disease. On history taking and further examination, we deduced his symptoms to be consistent with PSP and followed him with additional tests and radiological imaging to confirm.
2. Case Report
A 54-year-old man from Chile, with a past medical history of hypertension and diabetes mellitus, presented to the office complaining of painful leg muscle spasms, bilateral leg weakness, and involuntary movements that had progressively worsened since the diagnosis, as he is now wheel-chair bound. In the past few months, he started having sensory complaints that consisted of tingling and burning sensation predominantly in the soles, as well as numbness of the entire body below the neck, orthopnea, and dysphagia to solid foods. The disease was diagnosed and treated in Chile, for which he received baclofen 10mg, diazepam 10mg, clonazepam 2mg, acetaminophen, and ketoprofen daily.
Other relevant comorbidities included depression, treated with sertraline; hypertension, treated with enalapril; diabetes mellitus, for which metformin was discontinued due to adverse effects; vertigo due to a middle ear disorder (unclearly stated by the patient), current left inguinal hernia, former smoker, previous tooth reconstruction surgery after a fall in 2015, and an allergic reaction to penicillin. In addition, he had a past family history of hypertension and stroke in his parents and his 3 siblings and Parkinson’s disease in his brother.
On physical examination, he had normal vital signs (blood pressure 131/86 mmHg, pulse 86 bpm, temperature 98.4 F, weight 176 lb and height 70 inches), preserved mental status, spastic facies, and scanning speech. Upon cranial nerves examination, the patient showed bilateral exotropia, painful and slow eye movements, especially upon upward gaze, vertical nystagmus, impaired eye convergence, decreased facial sensation, more marked on the left, bilateral facial weakness, absent gag reflex, normal tongue movements, and absent tongue fasciculations, impaired shoulder shrug, and weak head rotation. He had a widespread reduced sensation on crude touch and prominent upper motor neuron signs, namely spastic quadriparesis (more marked in the lower limbs), generalized hyperreflexia with clonus, suprapatellar reflex, and bilateral Babinski sign. His muscle tropism was preserved in all the extremities and the patient did not show muscle fasciculations (Figure 1). Other neurological findings included major sensing and upper limb dysmetria. Additionally, he had decreased chest expansion with normal breathing sounds; deviation of the phalanges, and equine foot deformity (Figure 2). The patient’s gait and posture could not be assessed due to the referred quadriparesis.
In the absence of lower motor neuron signs, prominent upper motor neuron signs, multiple cranial nerve abnormalities, and cerebellar signs, the main diagnosis considered was progressive supranuclear palsy (PSP). A nerve conduction study (NCV), electroencephalography (EEG), and electromyography (EMG) were performed. The EMG was unremarkable (no peripheral neuropathy, no compressive neuropathy, no evidence of nerve damage, and the sensory amplitudes for median and ulnar nerves were higher than expected, whereas the motor ones were appropriate in size) (Figure 3).
The EEG showed no epileptiform discharges and stage I sleep was noted. The EMG/NCV were normal, with no sensory neuropathy and normal proximal conduction, so overall nerve conduction studies for sensory and motor conduction study were within normal limits. The patient also had a previous brain MRI in 2015, which was normal and showed no white matter lesions. A spinal MRI performed in the same year showed disc pathology in multiple segments in dorsal and lumbar regions. A current brain MRI was performed for assessing classical features of PSP.
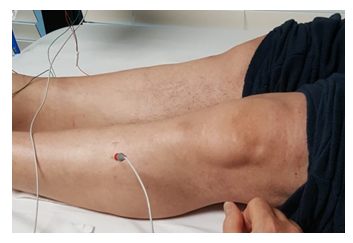
Figure 1: (left) Normal muscle tropism in the lower extremities.
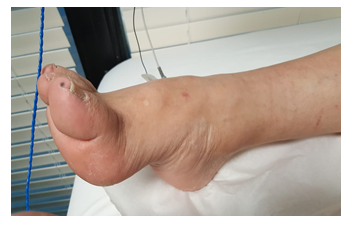
Figure 2: (right) Equine foot deformity.
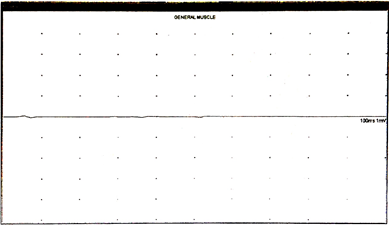
Figure 3: Electromyography performed showed no muscle fibrillation on the general muscle trace.
3. Discussion
PSP is the most common degenerative, atypical parkinsonian disorder, with a prevalence of 6.4 per 100,000 according to Schrag et al. [1, 2] The incidence is reported to increase with age from 1.7 cases per 100,000 at 50-59 years to 14.7 per 100,000 per year at 80-99 years [3, 4]. The mean age of diagnosis is approximately 65 years, with no racial or sex predilection. No significant risk factors for developing PSP have been identified [5].
PSP results from an aggregation of abnormally phosphorylated tau proteins. Tau proteins aid in axonal transport and support neuronal microtubules. Localized accumulation of the irregular tau proteins forms what are known as neurofibrillary tangles [6]. In addition to the tauopathy, PSP involves the degeneration of dopaminergic neurons and cholinergic neurons, leading to loss of basal ganglia, cerebral cortex, and, most clinically characteristic, brainstem structures. Structures within the brainstem that atrophy is the dorsal midbrain, notably the midbrain tegmentum and pedunculopontine nucleus, which leads to postural stability, and the motor nuclei of the cranial nerves in advanced stages of the disease. Given the atrophy of the midbrain tegmentum, the rostral interstitial medial longitudinal fasciculus is greatly affected, which decreases the presence of vertical excitatory burst neurons, and an inability to initiate vertical saccades ensues [7].
Recent epidemiological studies have shown that PSP (Steele–Richardson–Olszewski syndrome) is more common than previously recognized, that it is commonly misdiagnosed, and that it may present to a wide range of hospital specialists [8]. Patients with PSP tend to present with disturbance of balance, a disorder of downward gaze, and levodopa (L-DOPA) unresponsive Parkinsonism, and usually develop progressive dysphagia and dysarthria. Originally, the clinical hallmarks of PSP are vertical gaze palsy, pseudobulbar palsy, axial rigidity, and cognitive impairment [9].
One of the common differentials to keep in mind is Parkinson’s disease. Some of the most distinguishing features in PSP are eye-related in contrast to Parkinson’s disease. Other symptoms of PSP, like difficulty, swallowing, speech issues, rigidity, balance issues, walking issues (gait disturbances), trouble with movement, mask-like facial expression, and monotone speech directly overlap with PD. However, one interesting difference that may be present between those with PSP and those with PD is within their posture. Individuals with PSP may stand up very straight and tip their heads backward when moving, causing them to be off-balance or fall. This is called axial rigidity. On the other hand, those with PD may be more likely to lean over or bend forward when walking. Additionally, individuals with PD will often have a tremor, whereas someone with PSP is less likely to experience this [10-12]. The patient in our case had no tremor and showed axial rigidity ruling out Parkinson’s disease. A trial of Parkinson’s treatment [levodopa]was not given and PD was ruled out purely based on clinical characteristics.
Our patient was reported to be diagnosed with ALS in his home country and presented with painful leg spasms and progressive bilateral leg weakness. Consideration of Progressive supranuclear palsy was done as the patient had no lower motor neuron signs like no muscle fasciculations and we were diagnosed as PSP based on MDS clinical criteria. Our patient met the mandatory inclusion criteria of being over 40 years old when the symptoms first appeared, followed by a gradual progression of the disease with no family history of PSP. None of the exclusion criteria applied to our patient; although he exhibited UMN signs, he had no signs of LMN disease and pure UMN signs are not an exclusion criterion. As per this criteria, our patient had core clinical features categorized at LEVEL -1[ O1, P1] suggesting a high certainty of PSP [13].
|
Level of certainty |
Ocular motor dysfunction |
Postural instability |
Akinesia |
Cognitive dysfunction |
|
Level 1 |
O1: Vertical supranuclear gaze palsy |
P1: Repeated unprovoked falls within 3 years |
A1: Progressive gait freezing within 3 years |
C1: Speech/language disorder, i.e., nonfluent/agrammatic variant of primary progressive aphasia or progressive apraxia of speech |
|
Level 2 |
O2: Slow velocity of vertical saccades |
P2: Tendency to fall on the pull-test within 3 years |
A2: Parkinsonism, akinetic-rigid, predominantly axial, and levodopa resistant |
C2: Frontal cognitive/behavioral presentation |
|
Level 3 |
O3: Frequent macro square wave jerks or “eyelid opening apraxia” |
P3: More than two steps backward on the pull-test within 3 years |
A3: Parkinsonism, with tremor and/or asymmetric and/or levodopa responsive |
C3: Corticobasal syndrome |
There is a marked limitation of the range of voluntary gaze in the vertical more than in the horizontal plane – meeting the criteria of O1. There is a history of more than one unprovoked fall within the past 3 years and is wheelchair-bound now, thus meeting the requirements for P1. He had progressive gait freezing, however, since he was never tried on levodopa, it cannot be ascertained if the patient is resistant to it. Hence, for the time being, he does not meet the requirements for A1 level of certainty for akinesia. Similarly, although he had an effortful, slowed speech with spastic elements, he does not meet the requirements for C1, C2, or C3 levels of certainty of cognitive dysfunction. However, spastic dysarthria and dysphagia are the supportive features in this patient, categorized as CC2 and CC3. Basic features need to be present in a patient in order to be considered for the diagnosis of PSP of any phenotype and at any stage and are present in our patient.
The diagnostic certainty for this patient is “probable PSP-RS” based on the current MDS criteria (O1+P1, lack of A2 or A3) [13]. Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by rapidly progressive muscular paralysis caused by degeneration of motor neurons leads to muscle wasting (atrophy), muscle spasticity, muscle weakness. Both upper motor neuron and lower motor neuron gets affected. The clinical presentation of amyotrophic lateral sclerosis is variable. Typically, there are both upper motor neuron and lower motor neuron signs along with electrodiagnostic studies which are indicative of ALS [14].
Lack of muscle atrophy, fasciculations, presence of sensory deficits, and normal electromyography in this patient rules out amyotrophic lateral sclerosis. In addition, our patient has had symptoms for 5 years; the median survival time from onset to death ranges from 20 to 48 months in ALS, which is considerably lower than that of PSP. The absence of neurocognitive dysfunction and rigidity rules out the Parkinson-like variant of PSP. The patient also had normal nerve conduction studies indicating an absence of peripheral neuropathy as a cause of diabetes mellitus.
4. Conclusion
Progressive Supranuclear Palsy (PSP) is a disease of unknown etiology, but many researchers now believe that various genetic and environmental factors interact, leading to the destruction of brain stem cells. PSP is underdiagnosed, recent autopsy studies found PSP pathology in 2-6% of elderly people that had no diagnosis of PSP before death. Corticobasal degeneration resembles PSP as both are tauopathies and some experts believe that CBD and PSP are variations of the same disease. Multiple System Atrophy (MSA), a combination of Parkinsonism and cerebellar ataxia, resembles PSP in that many patients with MSA also develop the impaired function of the autonomic nervous system. Another differential is Parkinson’s disease, which progresses more slowly than PSP and usually is not incapacitating for a decade or more. As there is no cure at the present time for PSP, treatment is symptomatic and supportive.
References
- Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 354 (1999):1771-5.
- Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8 (2009): 270-279.
- Bower JH, Maraganore DM, McDonnell SK, et al. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology 49 (1997): 1284-1288.
- Chiu WZ, Kaat LD, Seelaar H, et al. Survival in progressive supranuclear palsy and frontotemporal dementia. J Neurol Neurosurg Psychiatry 81 (2010): 441-445.
- Cosseddu M, Benussi A, Gazzina S, et al. Natural history and predictors of survival in progressive supranuclear palsy. J Neurol Sci 382 (2017): 105-107.
- Bancher C, Lassmann H, Budka H, et al. Neurofibrillary tangles in Alzheimer’s disease and progressive supranuclear palsy: antigenic similarities and differences. Microtubule-associated protein tau antigenicity is prominent in all types of tangles. Acta Neuropathol 74 (1987): 39-46.
- Kato N, Arai K, Hattori T. Study of the rostral midbrain atrophy in progressive supranuclear palsy. J Neurol Sci 210 (2003): 57-60.
- Burn DJ, Lees AJ. Progressive supranuclear palsy: Where are we now? Lancet Neurol 1 (2002): 359-369.
- Duvoisin RC. Clinical diagnosis. In: Litvan I, Agid Y, editors. Progressive Supranuclear Palsy: Clinical and Research
- New York: Oxford University Press (1992): 15-33.
- Conditions that Mimic Parkinson’s. Parkinson’s Foundation. https://www.parkinson.org/Understanding-Parkinsons/Diagnosis/Conditions-that-MimicParkinsons?gclid=EAIaIQobChMI5beC0syC5QIViZ6fCh2cPAv9EAAYASAAEgL6WfD_BwE (2019).
- Progressive Supranuclear Palsy Fact Sheet. National Institutes of Health: National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/disorders/patient-caregiver-education/fact-sheets/progressive-supranuclear-palsy-fact-sheet. Published August 13, 2019. Accessed October 15, 2019.
- Progressive Supranuclear Palsy. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/progressive-supranuclear-palsy/symptoms-causes/syc-20355659 (2019).
- Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Movement Disorders 32 (2017): 853-864.
- Venizelos A, Park Y, Fisher MA. A patient with amyotrophic lateral sclerosis and atypical clinical and electrodiagnostic features: a case report. Journal of medical case reports 5 (2011): 538.
