Profile of Tissue Selenium Concentration In A Cohort Of Nigerian Men With Prostate Cancer
Article Information
Igbokwe MC*, 1,2, Badmus TA1, Salako AA1, Obiajunwa EI3, Olasehinde O1, Igbokwe CA4, David RA1
1Department of Surgery, Obafemi Awolowo University, Ile-Ife, Nigeria
2Zenith Medical and Kidney Center, Abuja, Nigeria
3Center for Energy Research and Development, Obafemi Awolowo University, Ile-Ife, Nigeria
4Department of Dentistry, Federal Medical Centre, Abuja, Nigeria
*Corresponding author: Igbokwe Martin C, Urology Division, Department of Surgery, Obafemi Awolowo University, Ile-Ife, Nigeria/2Zenith Medical and Kidney Center, Abuja, Nigeria.
Received: 08 November 2021; Accepted: 22 November2021; Published: 20 December 2021
Citation: Igbokwe MC, Badmus TA, Salako AA, Obiajunwa EI, Olasehinde O, Igbokwe CA, David RA. Profile of Tissue Selenium Concentration In A Cohort Of Nigerian Men With Prostate Cancer. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 576-586.
View / Download Pdf Share at FacebookAbstract
Introduction: The global burden of prostate cancer (CaP) has led to increasing research into the aetiological factors and preventive measures for the disease. The role of Selenium (Se) has recently been the focus of several research efforts. Its influence on development of prostate cancer is however still controversial.
Objectives: The aim of this study was to determine the relationship between tissue selenium levels and the occurrence of prostate cancer among men in Ile-Ife, Nigeria.
Methodology: This was a prospective hospital-based comparative study carried out in the Urology unit of the Obafemi Awolowo University Teaching Hospital Complex, Ile Ife, over a period of one year. Consecutive consenting men with histologically confirmed prostate cancer alongside age-matched controls were recruited for this study. These participants had whole blood and toe-nail samples collected for selenium assay using the Particle induced X-ray emission (PIXE) technique. The patients with prostate cancer had same samples collected 1 month after orchidectomy to reassess the selenium levels.
Data Analysis: The data was entered and analysed using the IBM Statistical Package for Social Sciences (SPSS) Statistical Software for Windows, Version 20. Univariate analysis was utilized to determine the sociodemographic data of the subjects, using percentages and means (with standard deviation). The independent T-test was employed to determine if there was any significant difference in the whole blood and toe-nail selenium between the study and control groups while the paired T-test was used to compare the pre and post-orchidectomy tissue selenium concentrations using a significance level of 0.05 at 95% confidence interval.
Results: Fort
Keywords
<p>Prostate cancer; Selenium; Particle-induced Xray emission; Ile-Ife</p>
Prostate cancer articles; Selenium articles; Particle-induced Xray emission articles; Ile-Ife articles
Prostate cancer articles Prostate cancer Research articles Prostate cancer review articles Prostate cancer PubMed articles Prostate cancer PubMed Central articles Prostate cancer 2023 articles Prostate cancer 2024 articles Prostate cancer Scopus articles Prostate cancer impact factor journals Prostate cancer Scopus journals Prostate cancer PubMed journals Prostate cancer medical journals Prostate cancer free journals Prostate cancer best journals Prostate cancer top journals Prostate cancer free medical journals Prostate cancer famous journals Prostate cancer Google Scholar indexed journals Selenium articles Selenium Research articles Selenium review articles Selenium PubMed articles Selenium PubMed Central articles Selenium 2023 articles Selenium 2024 articles Selenium Scopus articles Selenium impact factor journals Selenium Scopus journals Selenium PubMed journals Selenium medical journals Selenium free journals Selenium best journals Selenium top journals Selenium free medical journals Selenium famous journals Selenium Google Scholar indexed journals Particle-induced Xray emission articles Particle-induced Xray emission Research articles Particle-induced Xray emission review articles Particle-induced Xray emission PubMed articles Particle-induced Xray emission PubMed Central articles Particle-induced Xray emission 2023 articles Particle-induced Xray emission 2024 articles Particle-induced Xray emission Scopus articles Particle-induced Xray emission impact factor journals Particle-induced Xray emission Scopus journals Particle-induced Xray emission PubMed journals Particle-induced Xray emission medical journals Particle-induced Xray emission free journals Particle-induced Xray emission best journals Particle-induced Xray emission top journals Particle-induced Xray emission free medical journals Particle-induced Xray emission famous journals Particle-induced Xray emission Google Scholar indexed journals Ile-Ife articles Ile-Ife Research articles Ile-Ife review articles Ile-Ife PubMed articles Ile-Ife PubMed Central articles Ile-Ife 2023 articles Ile-Ife 2024 articles Ile-Ife Scopus articles Ile-Ife impact factor journals Ile-Ife Scopus journals Ile-Ife PubMed journals Ile-Ife medical journals Ile-Ife free journals Ile-Ife best journals Ile-Ife top journals Ile-Ife free medical journals Ile-Ife famous journals Ile-Ife Google Scholar indexed journals urological malignancy articles urological malignancy Research articles urological malignancy review articles urological malignancy PubMed articles urological malignancy PubMed Central articles urological malignancy 2023 articles urological malignancy 2024 articles urological malignancy Scopus articles urological malignancy impact factor journals urological malignancy Scopus journals urological malignancy PubMed journals urological malignancy medical journals urological malignancy free journals urological malignancy best journals urological malignancy top journals urological malignancy free medical journals urological malignancy famous journals urological malignancy Google Scholar indexed journals male malignancy articles male malignancy Research articles male malignancy review articles male malignancy PubMed articles male malignancy PubMed Central articles male malignancy 2023 articles male malignancy 2024 articles male malignancy Scopus articles male malignancy impact factor journals male malignancy Scopus journals male malignancy PubMed journals male malignancy medical journals male malignancy free journals male malignancy best journals male malignancy top journals male malignancy free medical journals male malignancy famous journals male malignancy Google Scholar indexed journals Particle induced X-ray emission articles Particle induced X-ray emission Research articles Particle induced X-ray emission review articles Particle induced X-ray emission PubMed articles Particle induced X-ray emission PubMed Central articles Particle induced X-ray emission 2023 articles Particle induced X-ray emission 2024 articles Particle induced X-ray emission Scopus articles Particle induced X-ray emission impact factor journals Particle induced X-ray emission Scopus journals Particle induced X-ray emission PubMed journals Particle induced X-ray emission medical journals Particle induced X-ray emission free journals Particle induced X-ray emission best journals Particle induced X-ray emission top journals Particle induced X-ray emission free medical journals Particle induced X-ray emission famous journals Particle induced X-ray emission Google Scholar indexed journals post-orchidectomy articles post-orchidectomy Research articles post-orchidectomy review articles post-orchidectomy PubMed articles post-orchidectomy PubMed Central articles post-orchidectomy 2023 articles post-orchidectomy 2024 articles post-orchidectomy Scopus articles post-orchidectomy impact factor journals post-orchidectomy Scopus journals post-orchidectomy PubMed journals post-orchidectomy medical journals post-orchidectomy free journals post-orchidectomy best journals post-orchidectomy top journals post-orchidectomy free medical journals post-orchidectomy famous journals post-orchidectomy Google Scholar indexed journals tissue selenium articles tissue selenium Research articles tissue selenium review articles tissue selenium PubMed articles tissue selenium PubMed Central articles tissue selenium 2023 articles tissue selenium 2024 articles tissue selenium Scopus articles tissue selenium impact factor journals tissue selenium Scopus journals tissue selenium PubMed journals tissue selenium medical journals tissue selenium free journals tissue selenium best journals tissue selenium top journals tissue selenium free medical journals tissue selenium famous journals tissue selenium Google Scholar indexed journals cancer preventative agent articles cancer preventative agent Research articles cancer preventative agent review articles cancer preventative agent PubMed articles cancer preventative agent PubMed Central articles cancer preventative agent 2023 articles cancer preventative agent 2024 articles cancer preventative agent Scopus articles cancer preventative agent impact factor journals cancer preventative agent Scopus journals cancer preventative agent PubMed journals cancer preventative agent medical journals cancer preventative agent free journals cancer preventative agent best journals cancer preventative agent top journals cancer preventative agent free medical journals cancer preventative agent famous journals cancer preventative agent Google Scholar indexed journals
Article Details
1. Introduction
Prostate cancer (CaP) is a common urological malignancy accounting for a huge percentage of work load in urology out-patient clinics worldwide. This has a huge economic and social impact on patients, surgeons, and the society at large [1]. It is considered the commonest male malignancy in African men and a major cause of morbidity and mortality [2-4]. The incidence as well as mortality figures of CaP is known to be higher among men of African descent with a two-fold increase in the deaths from CaP compared to Caucasian men [5, 6]. There is evidence of increasing incidence of this condition among Nigerian men, the reasons for this rise however remain unclear [7].
The impact of CaP worldwide has stimulated more research aimed at identifying modifiable risk factors in order to proffer possible preventive measures. One of such, is the role of trace elements such as selenium in the aetiology and possible prevention of CaP [8]. Interest in the role of Selenium as a possible cancer preventative agent was stimulated with the observation that selenium supplementation induced a significant reduction in the incidence of cancers in the treatment arm of a large Nutritional Prevention of Cancer Trial conducted in a Caucasian population [9]. Some studies have also shown an inverse relationship between environmental selenium levels and cancer incidence as well as cancer related mortality [10, 11].
In Nigeria, a few studies have been conducted on this subject. Famurewa and colleagues compared serum selenium and tocopherol amongst CaP and BPH patients using Atomic Absorption Spectrophotometer Technique (AAST) and High Performance Liquid Chromatography (HPLC) respectively [12]. They found a statistically insignificant lower selenium levelin CaP patients [12]. A similar study in Nnewi by Onyema-Iloh et al compared serum trace elements including selenium and antioxidant vitamins between 50 CaP patients and 50 controls. Their study however showed a statistically significant lower selenium level in CaP patients [13]. These studies therefore suggest the need to further explore the role of Se in CaP in Nigeria. This study therefore set out to compare blood and tissue selenium levels in CaP patient with age-matched controls using the PIXE technique and compare this with yardsticks of disease severity – Prostate Specific Antigen and Gleason Score.
2. Patients and Methods
2.1 Study Design & Participants
This was a prospective hospital-based descriptive study performed in a Nigerian tertiary hospital between October 2016 and September 2017 (a 1-year period). The participants were forty-one (41) consecutive men with histologically confirmed CaP while forty-one (41) age-matched controls were men of similar age group +/- 2 years without CaP following PSA check and DRE. All patients had written informed consent. The study was approved by the local ethical committee.
The exclusion criteria for the disease arm were:
- Participants on Se supplements
- Those with mal-absorptive disorders
- Presence of Nail disease
- Use of anti-androgens or 5 alpha-reductase inhibitors which could affect PSA levels.
- Chronic illnesses and malignancies were excluded among controls.
Participants had 5 milli-litres of blood and big-toe nail clippings obtained following informed consent. The blood was stored in a -80 deep freezer while the nails were stored in a normal freezer. Men with CaP who had Androgen deprivation therapy by Bilateral Orchidectomy following the unit protocol and had repeat sampling of blood and toe-nails after 1-month.
2.2 Laboratory Analysis
The labelled frozen whole blood was subjected to freeze drying in a Manifold Lyophilizer (Model: Christ Beta 1-8 LD Plus) at the Central Laboratory, Obafemi Awolowo University, Ile Ife (Figure 6). The lyophilizer contains a vacuum pump and a condenser to remove the moisture by condensation on a surface cooled to -40 to -80°C. The labelled freeze-dried material is made into pellets (figure 7c) and analysed for Selenium at the Center for Energy Research and Development, Obafemi Awolowo University, Ile Ifeusing the Proton-induced X-ray emission (PIXE) technique. X-ray spectra obtained from PIXE measurements will be analyzed with the computer-coded GUPIX developed by Guelph University (Guelph, Ontario, Canada).
Calibration of the PIXE system was performed by irradiating suitable micrometer thin-film targets [95].
The analysis was performed using a 3.0 MeV proton beam obtained from the Center for Energy Research and Development ion beam analysis facility. A Pelletron accelerator was used to generate ions and hence proton and helium ions were produced.
2.3 Statistical Analyses
The data was entered and analysed using Statistical Package for Social Sciences (SPSS) statistical software version 20. Linear relationship was assessed between numeric data using Spearman correlation while median comparison between two groups were carried out using Mann-Whitney U and non-parametric tests. P-value <0.05 was assumed to be significant at 95% confidence interval. Charts including box plots, scatter plots and bar charts were used for data presentation where appropriate.
3. Results
A total of 41 patients and 41 age-matched controls were used for this study. The age distribution of patients in the study group ranged from 56 to 90 years with mean of 72.83 ± 7.06 years. The peak age incidence in the CaP group was in the 8th decade (71-80 years). Similarly, the age range of patients in the control group was between 55 to 89 years with a mean of 70.73 (±6.40 years) (Table 1).
|
Variables |
CaP Group |
Control Group |
Statistics |
|
Age, years Mean ± SD |
72.83± 7.06 |
70.73 ±6.40 |
p=0.250 |
|
Age distribution (years), n (%) 51-60 61-70 71-80 81-90 |
3 (7.3) 12 (29.3) 20 (48.8) 6 (14.6) |
3 (7.3) 13 (31.7) 19 (46.4) 6 (14.6) |
t=3.471 p>0.13 |
|
Occupation Retired Civil servant Business man Clergy |
21 (51.2) 12 (29.3) 6(14.6) 2 (4.9) |
13 (31.7) 19 (46.4) 9 (21.9) 0 (0) |
Table 1: Socio-demographic Characteristics of Total Study Population.
Among the Prostate cancer patients the PSA values ranged from 12.0 to 177 ng/ml with a mean of 46.10 ± 40.62 while the PSA in the control group ranged from 1.8 to 6.5 ng/ml with a mean of 3.88 ± 1.58 which was significantly lower than in the CaP group (p=0.001). The Gleason score of CaP patients in this study ranged from 6 to 10 with a modal score of 8. Twenty five (62.5%) of these patients had a score of 8 and above. Overall, Se levels were found to be consistently lower in the toenails than whole blood of both CaP patients and controls (p=0.02 and p= 0.03 respectively). Using toenail Se levels, thirty-one (75.6%) of CaP patients in our study population had selenium deficiency (<40ng/ml) while only 2 participants (5%) had selenium deficiency in the control population (p=0.001).
The whole blood selenium concentration among Prostate Cancer group ranged from 2- 160 ng/ml with a median value of 67.00 ng/ml and inter-quartile range of 52.50 while in the control group ranged between 45-245ng/ml with median value of 132.00 ng/ml and inter-quartile range of 83.00. There was a statistically significant lower blood selenium concentration among the Prostate cancer group compared to controls (p = 0.002) (Figure 1).
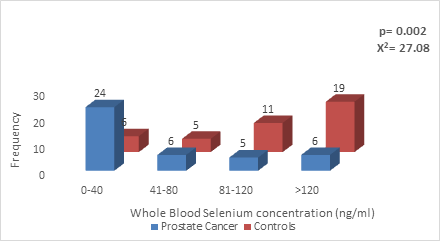
Figure 1: Whole blood Selenium concentration in CaP patients Vs Age-matched controls.
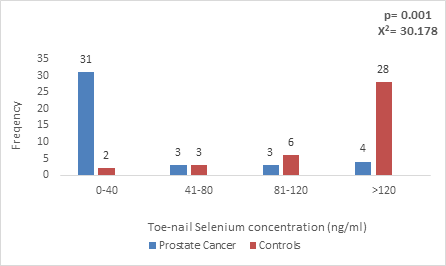
Figure 2: Toe-nail Selenium concentration in CaP patients and Age-matched controls.
The toe-nail selenium concentration in Prostate cancer patients ranged from 0-129ng/ml with a median value of 11.85 ng/ml and inter-quartile range of 51.94 while in controls ranged from 0-215ng/ml with median of 67.00 ng/ml and inter-quartile range of 71.00. There was also a statistically significant lower toe-nail selenium concentration among prostate cancer patients (p=0.001), with the mean value below the expected for individuals in the community (40-197.4ng/ml) in patients with CaP (Figure 2).
The post-orchidectomy blood selenium concentration among CaP patients ranged from 6.5- 186.0ng/ml with mean of 84.40 (±64.00) ng/ml. Compared to the pre-orchidectomy blood Se concentration there was an increase though it was not statistically significant (p= 0.16). Post-orchidectomy toe-nail selenium concentration among CaP patients ranged from 0-37ng/ml with a mean of 28 (±30.5) ng/ml. This appeared to have a marginal increase when compared to the pre-orchidectomy values however was not statistically significant (X2=14.60, p= 0.47) Table 2.
|
Mean (ng/ml) |
Standard deviation |
N |
df |
p |
|
|
Pre-orchidectomy toe-nail Selenium level (ng/ml) |
25.67 |
29.44 |
41 |
39 |
0.47 |
|
Post-orchidectomy toe-nail Selenium level (ng/ml) Pre-orchidectomy blood Selenium level (ng/ml) Post-orchidectomy blood Selenium level (ng/ml) |
28.00 76.95 84.40 |
30.50 61.30 64.00 |
41 41 41 |
39 |
0.16 |
Table 3: Independent t test comparing pre- and post-orchidectomy toe-nail selenium concentration in Prostate cancer patients.
The relationship of the toe-nail selenium level with serum PSA showed a negative correlation with lower selenium levels correlating with a higher PSA value (R2=0.086, P<0.05) (Figure 3).
Similarly, there was a negative correlation between the toe-nail selenium concentration and the Gleason score among men with CaP with lower selenium levels found among men with higher Gleason scores (R2 = 0.067, P<0.05) (Figure 5).
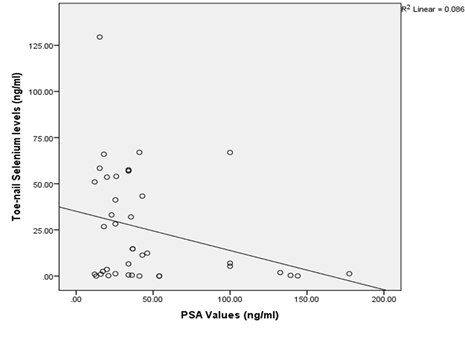
Figure 4: Relationship of Toe-nail Selenium and Prostate Specific Antigen values among Prostate Cancer patients.
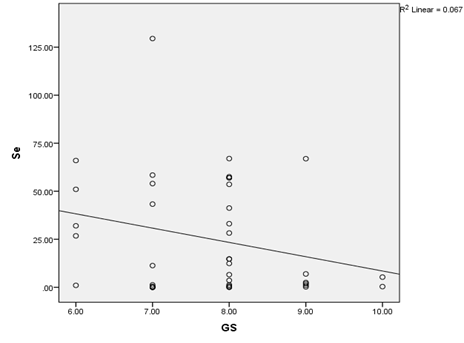
Figure 5: Scatter diagram showing the relationship between toe-nail Selenium concentration and Gleason score among Prostate Cancer patients.
4. Discussion
This study evaluated the possibility of a biochemical underpinning to the aetiology of prostate cancer. Although causality was not established, this study demonstrated a statistically significant association between prostate cancer and selenium levels, with CaP patients having lower selenium levels compared to age-matched controls. It also demonstrated significant selenium deficiency in men with CaP with mean values below the expected in normal adult males [14]. These observations persisted even after treatment, with no differences found between pre-and post orchidectomy selenium levels.
The age distribution of men in this study (55-90 years) is a reflection of the age range established in literature for the occurrence of prostate cancer particularly amongst men of African descent [15, 16]. Findings from this study are therefore applicable to men with CaP in this population. The successful age-matching of patients in the two groups as shown by the near equal mean ages reflects the similarity between the two groups being compared except for the presence of CaP. This similarity in age coupled with the fact that men in both groups were domicile in the same part of the country suggesting similar environmental exposure provides the clinical equipoise needed for comparison. The observed difference in Selenium levels can therefore be attributed to the presence of the disease given the similarities in other respects.
The finding of low selenium levels in CaP patients in this study correlates with findings from several other studies [17, 18]. One of the most elaborate of these was a meta-analysis of 20 case control studies conducted in Europe and North America by Brinkman and his associates which showed significantly lower levels of Se in blood, serum and toe-nail of CaP men as compared with controls [19]. There are however few studies which have demonstrated no association between Se and CaP [20].
This study further showed that lower selenium levels correlated significantly with rising PSA levels. This finding suggests that the association between selenium levels and CaP was not only with the presence of the disease, but also with the extent in terms of PSA levels. This is consistent with findings from Adaramoye’s work in Ibadan, Nigeria who demonstrated a progressive decrease of 30%, 52% and 58% in selenium levels in subjects with serum PSA levels of 5–10 ng/ml, 11–20 ng/ml and > 20 ng/ml, respectively [21]. A similar observation was also made between tissue selenium concentration and the Gleason score which also suggests that lower selenium concentration was associated with more severe forms of prostate cancer. This observation is in tandem with the work of Kenfield et al. who reported worse gleasonscores among Se deficient prostate cancer patients [22]. These associations suggest the possible role of selenium not only in the occurrence of the disease but also in its progression.
The exact patho-mechanistic processes underlying these associations is however still a subject of investigation. It is unknown whether the relationship is that of ‘cause’ or ‘effect’. Some authors have theorized that selenium deficiency predisposes to prostate cancer while others believe that low selenium result from depletion of Se reserves by the tumour. One of the most recent evidences available on this subject is an experimental work by Chun et al which evaluated the direct effect of methyl-selenic acid on human cultured CaP cells from American Type Culture Collection (Manassas, VA) using Northern and western blot ELISA techniques. His findings suggest that selenium deficiency predisposes to CaP by down-regulating androgen receptor (AR) signalling as well as initiation of other molecular pathways which culminate in carcinogenesis [24]. This postulation is however yet to be confirmed by interventional studies. Nicastro et al in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) which entailed supplemental selenium administration did not observe a decrease in the incidence of CaP over a seven year period. They however attributed this to the possibility of inadequate dosing and or formulation of the selenium supplement used in their study [8]. Further research on this may help unravel the possible chemo-preventive effects of Se.
It is interesting that bilateral total orchidectomy does not seem to influence Se levels among CaP patients as demonstrated in this current study. This is similar to findings in a study which assessed Se levels in Wister rats pre and post orchidectomy [24]. Selenium levels might have remained unchanged in this study because hormonal ablation via bilateral total orchidectomy only down-regulates androgen-receptors by drastically reducing circulating androgens but does not remove the malignant prostatic tissue. It is also possible that a longer follow-up period may be needed to detect a change in Se levels. A similar study among men following radical prostatectomy which ablates the entire disease with may however be a better way to assess the temporal profile of Selenium following therapy.
5. Discussion
This study demonstrates significantly lower levels of Se among CaP patients with significant association between the Se and both the occurrence and severity of the disease. No demonstrable change was seen in Se levels one month after orchidectomy.
References
- Patel ND, Parsons JK. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian journal of urology: IJU: journal of the Urological Society of India 30 (2014): 170-176.
- Aligbe JU, Forae GD. Prostatic tumours among Nigerian males: a private practice experience in Benin-City, South-South, Nigeria. The Nigerian postgraduate medical journal 20 (2013): 193-196.
- Opalinska E, Stoma F, Michalak A, et al. Benign prostatic hyperplasia, prostate cancer and other prostate diseases diagnosed as a result of screening procedure among 1,004 men in the Lublin district. Annales Universitatis Mariae Curie-Sklodowska Sectio D: Medicina 57 (2002): 493-501.
- Umezurike BI, Ekanem TB, Eluwa MA, Etta KK, Udo-Affah GA, Aligwekwe AU. The frequency of benign prostate hypertrophy in Calabar. The Nigerian postgraduate medical journal 13 (2006): 236-239.
- Ifere GO, Abebe F, Ananaba GA. Emergent Trends in the reported incidence of Prostate Cancer in Nigeria. Clinical Epidemiology 4 (2012): 19-32.
- Robbins CM, Hernandez W, Ahaghotu C, et al. Association of HPC2/ELAC2 and RNASEL Non-synonymous Variants with Prostate Cancer risk in African American Familial and Sporadic Cases. NHPA Manuscripts 68 (2008): 1790-1797.
- Ogunbiyi JO, Shittu OB. Increased Incidence of Prostate Cancer in Nigeria. Journal of National Medical Association 91 (1999): 159-164.
- Nicastro HL, Dunn BK. Selenium and Prostate Cancer Prevention: Insights from the Selenium and Vitamin E Cancer Prevention Trial (SELECT. Nutrients 5 (2013): 1122-1148.
- Clark LC, Combs GF, Jr., Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama 276 (1996): 1957-1963.
- Shamberger RJ, Willis CE. Selenium distribution and human cancer mortality. CRC Crit Rev Clin Lab Sci 2 (1971): 211-221.
- Clark LC, Cantor KP, Allaway WH. Selenium in forage crops and cancer mortality in U.S. counties. Arch Environ Health 46 (1991): 37-42.
- Famurewa AC, Akinosun OM. Plasma levels of α-tocopherol, γ-tocopherol and selenium in patients with prostate cancer in Nigeria. International Journal of Medicine and Biomedical Research 3 (2014): 161-167.
- Onyema-iloh BO, Meludu SC, Iloh E, Nnodim J, Onyegbule O, Mykembata B. Biochemical Changes In Some Trace Elements, Antioxidant Vitamins And Their Therapeutic Importance In Prostate Cancer Patients. Asian journal of medical sciences 6 (2014): 95-97.
- Adeniyi MJ, Agoreyo FO. Nigeria and the Selenium Micronutrient: A Review. Ann Med Health Sci Res 8 (2018): 5-11.
- Ikuerowo SO, Omisanjo OA, Bioku MJ, Ajala MO, Mordi VPN, Esho JO. Prevalence and Characteristics of Prostate Cancer among Participants of a Community-based Screening in Nigeria using Serum Prostate specific Antigen and Digital Rectal Examination. Pan African Medical Journal 15 (2013): 129.
- Badmus TA, Adesunkanmi AR, Yusuf BM, Oseni GO, Eziyi AK, Bakare TI, et al. Burden of prostate cancer in southwestern Nigeria. Urology 76 (2010): 412-416.
- Brooks JD, Metter EJ, Chan DW, Sokoll LJ, Landis P, Nelson WG. Plasma selenium level before diagnosis and the risk of prostate cancer development. J Urol 166 (2001): 2034-2038.
- Nomura AM, Lee J, Stemmermann GN, Combs GFJ. Serum selenium and subsequent risk of prostate cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 9 (2000): 883-887.
- Brinkman M, Reulen RC, Kellen E, Buntinx F, Zeegers MP. Are men with low selenium levels at increased risk of prostate cancer? European journal of cancer (Oxford, England 42 (2006): 2463-2471.
- Paul Knekt, Arpo Aromaa, Jouni Maatela, Georg Alfthan, Ritva-Kaarina Aaron, Matti Hakama, et al. Serum Selenium and Subsequent Risk of Cancer Among Finnish Menand Women. Journal of the National Cancer Institute 82 (1990): 864-868.
- Adaramoye OA, Akinloye O, Olatunji IK. Trace elements and Vitamin E in Nigerian Patients with Prostate Cancer. African Health Sciences 8 (2010): 2-8.
- Kenfield SA, Van Blarigan EL, DuPre N, et al. Selenium Supplementation and Prostate Cancer Mortality. JNCI Journal of the National Cancer Institute 107 (2015): dju360.
- Jae Yeon Chun, Nagalakshmi Nadiminty, Soo Ok Lee, et al. Mechanisms of selenium down-regulation of androgen receptor signaling in prostate cancer. Mol Cancer Ther 5 (2006): 913-918.
- Cornelia Riese, Marten Michaelis, Birgit Mentrup, Franziska Gotz, Josef Kohrle, Ulrich Schweizer, et al. Selenium-Dependent Pre- and Posttranscriptional Mechanisms Are Responsible for Sexual Dimorphic Expression of Selenoproteins in Murine Tissues. Endocrinology 147 (2006): 5883-5892.
