Proenkephalin is an Early Biomarker to Predict Septic Acute Kidney Injury among Patients in Intensive Care Unit
Article Information
Md. Mahmudul Hassan1*, Ahmed Showki Arnob2, A H Hamid Ahmed3, A.K.M Shahidur Rahman4, Abu Ahmed Golam Akbar5, Prianka Jabin6, Saiful Bahar Khan7, Anirban Kishor Singha8, Sharaban Tahora9, A N M Ehsanul Karim10
1Assistant Professor, Department of Nephrology, Monno Medical College and Hospital, Manikgonj, Bangladesh
2Lecturer, Department of Anatomy, Delta Medical College, Dhaka, Bangladesh
3Professor, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
4Medical Officer, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
5Medical Officer, Department of Nephrology, Sylhet MAG Osmani Medical College Hospital, Sylhet, Bangladesh
6Resident (Nuclear Medicine), National Institute of Nuclear Medicine & Allied Sciences (NINMAS), Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
7Assistant Professor, Department of Nephrology, Ad-din Women’s Medical College Hospital, Dhaka, Bangladesh
8Registrar, Department of Nephrology, Kurmitola General Hospital, Dhaka, Bangladesh
9Resident (Nephrology), Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
10Associate Professor, Department of Nephrology, Shaheed Ziaur Rahman Medical College Hospital (SZMCH), Bogura, Bangladesh
*Corresponding Author: Md. Mahmudul Hassan, Assistant Professor, Department of Nephrology, Monno Medical College and Hospital, Monno City, Manikgonj, Bangladesh
Received: 08 April 2021; Accepted: 21 April 2021; Published: 29 April 2021
Citation: Hassan MM, Arnob AS, Ahmed AHH, Rahman AKMS, Akbar AAG, Jabin P, Khan SB, Singha AK, Tahora S, Karim ANME. Proenkephalin is an Early Biomarker to Predict Septic Acute Kidney Injury among Patients in Intensive Care Unit. Archives of Nephrology and Urology 4 (2021): 071-083.
View / Download Pdf Share at FacebookAbstract
Background: Sepsis is the leading cause of acute kidney injury (AKI) in critically ill patients. Plasma Proenkephalin found to be highly specific for early kidney dysfunction and is not influenced by non renal related variables.
Objective: The aim of this study was to evaluate the plasma proenkephalin as a predictor of septic AKI in patients with sepsis or septic shock.
Methods: This prospective observational study was conducted at Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, from January 2019 to December 2019. A total of 88 patients admitted in Intensive Care Unit (ICU), with sepsis or septic shock were enrolled. Their relevant data regarding different physiological parameters, markers of sepsis and vital organ functions were assessed daily. The patients’ serum creatinine and plasma Proenkephalin levels were measured on day 0, day 2 and on day 7 of admission in ICU. Their serum creatinine and plasma Proenkephalin levels were subsequently followed up on day 2 and day 7 to identify the development of AKI.
Results: The mean age of the study patients was 56.3±10.55 year. Among total 88 study patients; AKI was developed in 28 (31.8%) patients. It was observed that plasma Proenkephalin levels on day 0, day 2 and day 7 of admission were significantly increased among patients who developed AKI (p< 0.001).
Conclusion: The patients with AKI had a significant higher plasma Proenkephalin level than the patients without AKI. Therefore plasma Proenkephalin is a good early biomarker for the diagnosis of septic AKI.
Keywords
Acute Kidney Injury (AKI); Biomarker; Proenkephalin; Sepsis
Acute Kidney Injury (AKI) articles; Biomarker articles; Proenkephalin articles; Sepsis articles
Acute Kidney Injury articles Acute Kidney Injury Research articles Acute Kidney Injury review articles Acute Kidney Injury PubMed articles Acute Kidney Injury PubMed Central articles Acute Kidney Injury 2023 articles Acute Kidney Injury 2024 articles Acute Kidney Injury Scopus articles Acute Kidney Injury impact factor journals Acute Kidney Injury Scopus journals Acute Kidney Injury PubMed journals Acute Kidney Injury medical journals Acute Kidney Injury free journals Acute Kidney Injury best journals Acute Kidney Injury top journals Acute Kidney Injury free medical journals Acute Kidney Injury famous journals Acute Kidney Injury Google Scholar indexed journals Biomarker articles Biomarker Research articles Biomarker review articles Biomarker PubMed articles Biomarker PubMed Central articles Biomarker 2023 articles Biomarker 2024 articles Biomarker Scopus articles Biomarker impact factor journals Biomarker Scopus journals Biomarker PubMed journals Biomarker medical journals Biomarker free journals Biomarker best journals Biomarker top journals Biomarker free medical journals Biomarker famous journals Biomarker Google Scholar indexed journals Proenkephalin; Sepsis articles Proenkephalin; Sepsis Research articles Proenkephalin; Sepsis review articles Proenkephalin; Sepsis PubMed articles Proenkephalin; Sepsis PubMed Central articles Proenkephalin; Sepsis 2023 articles Proenkephalin; Sepsis 2024 articles Proenkephalin; Sepsis Scopus articles Proenkephalin; Sepsis impact factor journals Proenkephalin; Sepsis Scopus journals Proenkephalin; Sepsis PubMed journals Proenkephalin; Sepsis medical journals Proenkephalin; Sepsis free journals Proenkephalin; Sepsis best journals Proenkephalin; Sepsis top journals Proenkephalin; Sepsis free medical journals Proenkephalin; Sepsis famous journals Proenkephalin; Sepsis Google Scholar indexed journals Intensive Care Unit articles Intensive Care Unit Research articles Intensive Care Unit review articles Intensive Care Unit PubMed articles Intensive Care Unit PubMed Central articles Intensive Care Unit 2023 articles Intensive Care Unit 2024 articles Intensive Care Unit Scopus articles Intensive Care Unit impact factor journals Intensive Care Unit Scopus journals Intensive Care Unit PubMed journals Intensive Care Unit medical journals Intensive Care Unit free journals Intensive Care Unit best journals Intensive Care Unit top journals Intensive Care Unit free medical journals Intensive Care Unit famous journals Intensive Care Unit Google Scholar indexed journals glomerular filtration rate articles glomerular filtration rate Research articles glomerular filtration rate review articles glomerular filtration rate PubMed articles glomerular filtration rate PubMed Central articles glomerular filtration rate 2023 articles glomerular filtration rate 2024 articles glomerular filtration rate Scopus articles glomerular filtration rate impact factor journals glomerular filtration rate Scopus journals glomerular filtration rate PubMed journals glomerular filtration rate medical journals glomerular filtration rate free journals glomerular filtration rate best journals glomerular filtration rate top journals glomerular filtration rate free medical journals glomerular filtration rate famous journals glomerular filtration rate Google Scholar indexed journals receiver-operating characteristics articles receiver-operating characteristics Research articles receiver-operating characteristics review articles receiver-operating characteristics PubMed articles receiver-operating characteristics PubMed Central articles receiver-operating characteristics 2023 articles receiver-operating characteristics 2024 articles receiver-operating characteristics Scopus articles receiver-operating characteristics impact factor journals receiver-operating characteristics Scopus journals receiver-operating characteristics PubMed journals receiver-operating characteristics medical journals receiver-operating characteristics free journals receiver-operating characteristics best journals receiver-operating characteristics top journals receiver-operating characteristics free medical journals receiver-operating characteristics famous journals receiver-operating characteristics Google Scholar indexed journals area under the curve articles area under the curve Research articles area under the curve review articles area under the curve PubMed articles area under the curve PubMed Central articles area under the curve 2023 articles area under the curve 2024 articles area under the curve Scopus articles area under the curve impact factor journals area under the curve Scopus journals area under the curve PubMed journals area under the curve medical journals area under the curve free journals area under the curve best journals area under the curve top journals area under the curve free medical journals area under the curve famous journals area under the curve Google Scholar indexed journals
Article Details
1. Introduction
Sepsisis a potentially life-threatening condition occurred due to a dysregulated host response to infection. It may progress to septic shock which usually associated with a dramatic drop in blood pressure that can lead to severe organ dysfunction [1]. The incidence of sepsis is increasing which indicate that sepsis is a leading cause of critical illness and mortality worldwide [2, 3]. It was reported that patients who survive sepsis often have long-term physical, psychological, and cognitive disabilities [4]. Sepsis is a multifaceted host response to an infecting pathogen that could be amplified by endogenous factors [5]. It activate both inflammatory and anti-inflammatory responses, along with major modifications in nonimmunologic pathways such as cardiovascular, neuronal, autonomic, hormonal, bioenergetic, metabolic and coagulation [5, 6]. The inflammatory reaction is mediated by the release of cytokines from neutrophils and macrophages these are; tumor necrosis factor-alpha (TNF-α), interleukins (IL), and prostaglandins (PG) [7]. The cytokines activate the extrinsic coagulation cascade that ultimately inhibits fibrinolysis. Activation of the coagulation system leads to consumption of endogenous anticoagulants such as protein C and antithrombin; which play an important role in the development of microvascular coagulation [6, 7]. These overlapping processes trigger microvascular thrombosis; thrombosis is one of the potential factors producing organ dysfunction [7].
Acute kidney injury (AKI) is a common consequence in critically ill patients that occurs up to 35% of hospitalized patients and is associated with high morbidity and mortality [8]. The incidence of AKI among critically ill patients has been reported to be as high as 70%, with an in-hospital mortality of 50% when AKI is associated with multiple organ dysfunction [9, 10]. The most common cause of developing AKI in critically ill patients is sepsis. Recent evidence suggests that the pathogenic mechanisms of AKI in sepsis are different from those seen in other etiologies of AKI. The mechanisms of AKI in sepsis include; endothelial dysfunction, inflammation, coagulation disturbance and adaptive cell responses to injury [11]. Proenkephalin, previously known asproenkephalin A, is the precursor molecule of the enkephalin family is an endogenous opioid polypeptide hormone,which viaproteolyic cleavage produces the enkephalinpeptides met-enkephalin and to a lesser extentleu-enkephalin [12]. Enkephalins are widely expressed and act primarily on delta (δ) opioid receptors, the highest density of these receptors second to the central nervous system is in the kidney [13, 14]. In vivo Proenkephalin has a long half-life, it is stable after collection and its levels are not influenced by age or sex; that makes it a suitable surrogate measure of its biologically active counterparts [15]. Moreover, Proenkephalin is not protein bound in plasma and is exclusively filtrated in the glomerulus, making it a promising biomarker for kidney dysfunction in critically ill patients [15].
The assessment of kidney function and detection of acute kidney injury (AKI) in critically ill patients with sepsis remains challenging due to poor sensitivity and/or specificity of current standard biomarkers (i.e. serum creatinine and urine output). The most widely used methods are creatinine based; which lack in sensitivity, as creatinine is not purely filtrated by the kidney and rises relatively later after onset of AKI [16]. The use of current biomarkers of kidney injury or damage has shown conflicting results to predict AKI, mostly due to specificity issues and uncoupling between kidney damage and loss of renal function [17].
In recent years, several novel biomarkers have been suggested to assess the renal function but clinical implementations of these are limited till now. Proenkephalin represents a new candidate to determine kidney function. Plasma concentrations of Proenkephalin appears to accurately represent glomerular filtration rate (GFR) in patients diagnosed with sepsis or septic shock and thus Proenkephalin is considered a marker of kidney function (not kidney damage) [17]. Upon acute kidney dysfunction, Proenkephalin levels increase more quickly than creatinine [17, 18]. Increase of plasma Proenkephalin found to be highly specific for kidney dysfunction in contrast to other biomarkers; moreover it is not influenced by non-renal related variables such as systemic inflammation [16-18]. In this background this study was aimed to evaluate Proenkephalin as a biomarker of septic AKI among patients admitted in ICU.
2. Methodology
This prospective observational study was conducted at Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh from January 2019 to December 2019. A total of eighty-eight (88) patients, who admitted in the Department of Intensive Care Unit (ICU), BSMMU, Dhaka, Bangladesh during the study period were selected by convenient purposive sampling technique following selection criteria. Adult patients (age ≥ 18 years) of both sexes with sepsis having normal baseline serum creatinine, with or without shock was included in this study. Patient with pre-existing kidney disease, patient with heart failure, patient in vegetative coma and pregnant subjects were excluded from the study. The study was approved by the Ethical Review Committee, BSMMU, Dhaka, Bangladesh.
Informed written consent from each patient or their legal representatives was obtained prior to enrollment in the study. The initial information of the patients was collected from hospital records. Then their demographic profile and clinical examination findings were recorded in a data collection sheet. The presence of sepsis or septic shock based on the definitions for sepsis [1], organ dysfunction scores [Sequential Organ Failure Assessment (SOFA)] [19] as well as organ support (if any), the source of sepsis, pre-existing comorbidities and all relevant laboratory values were recorded accordingly. During the first week of admission in ICU, each patient’s relevant data regarding different physiological parameters, markers of sepsis and vital organ functions were assessed daily. The patients’ serum creatinine and plasma Proenkephalin levels were analyzed on day 0, day 2 and on day 7 of admission. Their serum creatinine and plasma Proenkephalin levels were subsequently followed up on day 2 and day 7 to identify the development of AKI. Each patient’s AKI was defined by KDIGO*2012 guideline. The study population was divided into two groups- group A and group B; group A consists of the patients admitted in the ICU with sepsis developing AKI and group B includes the patients admitted in the ICU with sepsis but did not develop AKI.
2.1 Analysis of blood samples
Following standard procedure 3 ml of venous blood was collected in a plane test tube from each patient. Then centrifugation was done at 2000-3000 rpm/minute in room temperature (22°C - 24°C) for 15 minutes, then plasma was separated by micro-pipette and collected to appendrope and preserved at −80°C until further analysis. Frozen samples were thawed at room temperature and gently mixed just prior to measurement. Plasma Proenkephalin was measured by enzyme-linked immunosorbent assay (ELISA) method and serum creatinine was measured by colorimetric kinetic method.
2.2 Acute kidney injury (AKI)
Acute kidney injury (AKI) is defined by KDIGO* 2012 Clinical Practice Guidelines as any of the following:
- Increase in serum creatinine by ≥ 3 mg/dl (≥ 26.5 μmol/l ) within 48 hours; or
- Increase in serum creatinine to ≥ 5 times baseline, which is known or presumed to have occurred within the prior 7 days; or
- Urine volume <5 ml/kg/h for 6 hours
[*KDIGO = Kidney Disease Improving Global Outcomes]
2.3. Statistical analysis
Data analysis was performed using the Statistical Package for Social Science (SPSS) software version- 26. Quantitative data were expressed as mean with standard deviation (±SD), while qualitative data were expressed as frequency and percentage. The statistics used to analyze the data was descriptive statistics. Associations between categorical variables were examined by chi-square test and continuous variables by t-test. Correlation between variables was observed by Pearson’s correlation test. A p value <0.05 was considered statistically significant.
3. Results
This study was intended to evaluate the plasma Proenkephalin as a biomarker of septic AKI among patients admitted in ICU. A total of 88 patients with sepsis were recruited, of them acute kidney injury (AKI) was developed in 28(31.8%) patients (Group A) and AKI was not observed in 60(68.2%) patients (Group B) (Table 1). The mean (± SD) age of the study patients was 56.3±10.55 year that was 59.1±8.71 year in group A and 55.0±11.1 year in group B respectively. No significant difference was observed between the groups (p= 0.093) (Table 2).
Table 3 shows the gender distribution of the study subjects. It was observed that, among 28 patients of group A, 18(64.3%) were male and 10(35.7%) were female; while in group B, 37(61.7%) patients out of 60 patients were male and rest 23(38.3%) patients were female. Male patients were predominant (62.5%) in both groups but the difference was not statistically significant (p= 0.813). The data analysis reveals that, 22(78.6%) patients out of 28 patients in group A and 25(41.7%) patients out of 60 patients in group B were diabetic, which was statistically significant (p= 0.001). On the other hand, 20(71.4%) patients in group A and 22(36.7%) patients in group B were hypertensive that was also statistically significant (p= 0.002). Rest of the pre-existing conditions did not show any statistically significant difference (p> 0.05) (Table 4).
Among total study patients, 22(78.6%) patients in group A and 47(78.3%) patients in group B were with mechanical ventilation; 20(71.4%) patients in group A and 32(53.3%) patients in group B were with inotrope support; while 2(7.1%) patients in group A needed renal replacement therapy (RRT). There was no significant difference on organ support between the groups (p> 0.05) (Table 5).
In this study, 22(78.6%) patients in group A and 37(61.7%)
patients in group B were in septic shock. No statistically significant differences were observed in different physiological parameters of the study patients between the groups (p> 0.05) (Table 6). Regarding the primary site of infection it was found that; 41(46.6%) patients had infection in the lungs, 36(40.9%) patients had infection in the abdomen, 10(11.4) patients had infection over skin and 1(1.1) patient’s primary infection site was heart out of total 88(100%) study patient (Table 7).
Total Sequential [Sepsis-Related] Organ Failure Assessment (SOFA) score among the study patients was 11.5±4.1; that was 13.5±4.6 in group A and 10.6±3.6 in group B, the difference was statistically significant (p= 0.002) (Table 8). On day 0 of admission, the study patients who were in group A had significant low PH 7.00±0.15 versus 7.10±0.12, p= 0.011) and significant high serum lactate level (4.07±1.44 mmol/L versus 2.53±0.75 mmol/L, p<0.001) compared to those in group B. Other laboratory parameters on day 0 of admission were not statistically significant between the groups (p> 0.05) (Table 9).
It was observed that serum creatinine on day 0 of admission was almost similar and did not demonstrate any statistically significant difference between the groups (p= 0.103). In group A it was found that the serum creatinine levels were significantly increased (p< 0.001) on day 2 and on day 7 of admission, while in the group B there was no significant rise in serum creatinine levels on day 2 and on day 7 of admission. On the other hand plasma Proenkephalin levels on day 0, day 2 and day 7 of admission were significantly increased in group A in comparison to group B (p< 0.001) (Table 10).
Figure 1 showed that the best cut off point of plasma Proenkephalin (blue color) was 145.0 pmol/l for septic AKI. It showed the highest sensitivity (67.9%) and specificity (98.3%) with an area under curve (AUC) of 0.796 (95% confidence interval 0.667-0.924), (p < 0.001). Table 11 shows ROC curve analysis. It was found that, the best cut off point of plasma Proenkephalin was 145.0 pmol/l for septic AKI. That was the highest sensitivity (67.9%) and specificity (98.3%) with an area under curve (AUC) of 0.796 (95% confidence interval 0.667-0.924), (p < 0.001).
|
Study group |
Frequency (n) |
Percentage (%) |
|
Group A (AKI) |
28 |
31.8 |
|
Group B (Non-AKI) |
60 |
68.2 |
|
Total |
88 |
100.0 |
Table 1: Distribution of the study patients on the basis of developing AKI (N=88).
|
Age (years) |
Total (N=88) No. (%) |
Group A (n=28) No. (%) |
Group B (n=60) No. (%) |
p-value |
|
30-40 |
10(11.4%) |
1(3.6%) |
9(15.0%) |
0.093ns |
|
41-50 |
18(20.5% |
5(17.9%) |
13(21.7%) |
|
|
51-60 |
29(33.0% |
11(39.3%) |
18(30.0%) |
|
|
61-70 |
28(31.8%) |
11(39.3%) |
17(28.3%) |
|
|
71-80 |
3(3.4%) |
0(0.0%) |
3(5.0%) |
|
|
Total |
88(100.0%) |
28(100.0%) |
60(100.0%) |
|
|
Mean±SD |
56.3±10.55 |
59.1±8.71 |
55.0±11.1 |
Unpaired t-test was performed to measure the level of significance, ns= not significant
Table 2: Distribution of study patients according to age (N=88).
|
Gender |
Group A (n=28) No. (%) |
Group B (n=60) No. (%) |
p-value |
|
Male |
18(64.3%) |
37(61.7%) |
0.813ns |
|
Female |
10(35.7%) |
23(38.3%) |
|
|
Total |
28(100.0%) |
60(100.0%) |
Chi-square test was performed to measure the level of significance, ns= not significant
Table 3: Distribution of the patients according to gender (N=88).
|
Pre-existing condition |
Total (n=88) |
AKI (n=28) |
No AKI (n=60) |
p-value |
|
Diabetes mellitus |
47(53.4%) |
22(78.6%) |
25(41.7%) |
0.001s |
|
Hypertension |
42(47.7%) |
20(71.4%) |
22(36.7%) |
0.002s |
|
Cerebrovascular disease |
36(40.9%) |
12(42.9%) |
24(40.0%) |
0.800 ns |
|
Heart disease |
7(8.0%) |
1(3.6%) |
6(10.0%) |
0.299 ns |
Chi-square test was performed to measure the level of significance, s= significant, ns= not significant
Table 4: Distribution of the study patients by pre-existing conditions (n=88).
|
Organ support status |
Total (N=88) |
Group A (n=28) |
Group B (n=60) |
p-value |
|
Respiratory support |
0.980 ns |
|||
|
With mechanical ventilation |
69(78.4%) |
22(78.6%) |
47(78.3%) |
|
|
Non-invasive ventilation |
19(21.6%) |
6(21.4%) |
13(21.7%) |
|
|
Blood pressure (inotrope support) |
0.108 ns |
|||
|
With inotrope support |
52(59.1%) |
20(71.4%) |
32(53.3%) |
|
|
Without inotrope support |
36(40.9%) |
8(28.6%) |
28(46.7%) |
|
|
Renal replacement therapy (RRT) |
2(2.3%) |
2(7.1%) |
0(0.0%) |
0.206 ns |
Chi-square test was done to measure the level of significance, s= significant, ns= not significant
Table 5: Organ support of the study patients (N=88).
|
Physiological variables |
Total (N=88) |
Group A (n=28) |
Group B (n=60) |
p-value |
|
Temperature |
||||
|
Normal |
9(10.2%) |
1(3.6%) |
8(13.3%) |
0.159 ns |
|
Raised |
79(89.8%) |
27(96.4%) |
52(86.7%) |
|
|
Pulse (beats/min) (mean±SD) |
107.9±11.9 |
105.3±11.2 |
109.3±12.1 |
0.141 ns |
|
Mean arterial pressure (mmHg) (mean±SD) |
67.1±6.1 |
66.6±6.7 |
67.4±5.96 |
0.616 ns |
|
Septic shock |
59(67.0%) |
22(78.6%) |
37(61.7%) |
0.116ns |
Unpaired t-test was done for quantitative variables and Chi-square test was performed for qualitative variables to measure the level of significance, ns= not significant
Table 6: Physiological variables of the patients on day 0 (N=88).
|
Site of infection |
Frequency (n) |
Percentage (%) |
|
Lungs |
41 |
46.6 |
|
Abdomen |
36 |
40.9 |
|
Skin |
10 |
11.4 |
|
Heart |
1 |
1.1 |
|
Total |
88 |
100.0 |
Table 7: Distribution of patients according to the site of infection (N=88).
|
Organ dysfunction score |
Total (N=88) Mean±SD |
Group A (n=28) Mean±SD |
Group B (n=60) Mean±SD |
p-value |
|
SOFA score |
11.5±4.1 |
13.5±4.6 |
10.6±3.6 |
0.002s |
Unpaired t-test was performed to measure the level of significance, s= significant
Table 8: Organ dysfunction scores of the study patients (N=88).
|
Laboratory findings |
Total (N=88) |
Group A (n=28) |
Group B (n=60) |
p-value |
|
Total WBC count (/cumm) |
0.141 ns |
|||
|
<4000 |
1(1.1%) |
1(3.6%) |
0(0.0%) |
|
|
>11000 |
87(98.9%) |
27(96.4%) |
60(100.0%) |
|
|
Platelet (/cumm) |
79522.7±46920.6 |
72857.1±59245.7 |
82633.3±40113.8 |
0.366 ns |
|
C- reactive protein |
0.060ns |
|||
|
Normal |
7(8.0%) |
0(0.0%) |
7(11.7%) |
|
|
High |
81(92.0%) |
28(100.0%) |
53(88.3%) |
|
|
Blood urea nitrozen (BUN) (mmol/L) |
9.1±3.5 |
9.6±3.0 |
8.9±3.7 |
0.322 ns |
|
Arterial PH |
7.05±0.13 |
7.00±0.15 |
7.10±0.12 |
0.011s |
|
PaO2/FiO2 |
232.9±50.9 |
232.7±58.0 |
233.0±47.7 |
0.978 ns |
|
Serum lactate (mmol/L) |
3.02±1.25 |
4.07±1.44 |
2.53±0.75 |
<0.001s |
|
Total bilirubin (mg/dl) |
4.06±1.33 |
3.85±1.27 |
4.15±1.36 |
0.322 ns |
Unpaired t-test was done for quantitative variables and Chi-square test was done for qualitative variable to measure the level of significance, s= significant, ns= not significant
Table 9: Different laboratory findings of the patients on day 0 of admission (N=88).
|
S. creatinine (mg/dl)
|
Total (N=88) Mean±SD |
Group A (n=28) Mean±SD |
Group B (n=60) Mean±SD |
p-value |
|
Day 0 |
1.12±0.14 |
1.14±0.14 |
1.08±0.17 |
0.103ns |
|
Day 2 |
1.45±0.86 |
2.46±0.91 |
0.98±0.11 |
<0.001s |
|
Day 7 |
1.73±1.47 |
3.26±1.84 |
1.01±0.13 |
<0.001s |
|
Plasma Proenkephalin (pmol/l) |
||||
|
Day 0 |
98.0±66.6 |
159.4±78.7 |
69.4±32.9 |
<0.001s |
|
Day 2 |
199.3±59.7 |
332.6±89.2 |
65.9±30.2 |
<0.001s |
|
Day 7 |
276.6±67.6 |
478.2±98.4 |
75.3±36.7 |
<0.001s |
Unpaired t-test was performed to measure the level of significance, ns= not significant, s= significant
Table 10: Comparison of serum creatinine and plasma Proenkephalin on Day 0, Day 2 and Day 7 of admission between the groups (N=88).
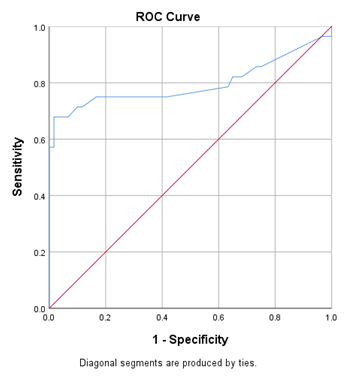
Figure 1: Receiver operating characteristic (ROC) curve of plasma Proenkephalin for the best prediction of septic AKI.
|
Variable
|
Cut off point pmol/l
|
Sensitivity (%)
|
Specificity (%)
|
AUC
|
p-value
|
95% confidence interval (CI) |
|
|
Lower |
Upper |
||||||
|
Plasma Proenkephalin (pmol/L) |
145 |
67.9 |
98.3 |
0.796 |
<.001 |
0.667 |
0.924 |
Table 11: Sensitivity and specificity of plasma Proenkephalin to predict septic AKI.
4. Discussion
In the intensive care unit (ICU), renal failure occurs more frequently and is associated with high mortality among the patients with sepsis. Conventionally, assessment of renal function is primarily based on individual’s urine output and serum creatinine based methods to estimate glomerular filtration rate (GFR). The estimation of GFR is somewhat confounded as creatinine is not exclusively filtered by the kidney and rises later after the onset of acute kidney injury (AKI). This may leads to delays in detection of impaired kidney function and early diagnosis of AKI, particularly in patients with sepsis, where kidney function can change very rapidly. Recently Proenkephalin has been described as an accurate biomarker of kidney function and sensitive in recognition of AKI in various clinical settings including sepsis [15]. Therefore, this study was conducted to evaluate plasma Proenkephalin as a biomarker of septic AKI among patients admitted in ICU. A total of 88 patients with sepsis were enrolled in this study and out of which 31.8% had developed AKI, which was consistent with similar previous studies [16, 20-22].
In this study, the mean age of the study patients was 59.1±8.71 years and most of the study subjects (62.5%) were male. This finding was almost similar with related previous studies [16, 20, 22]. The possible explanation could be; older patients have structural and functional changes in kidney that is decreased number of nephrons and decreased auto-regulation capacity, along with there is accumulation of various co-morbidities which eventually leads to increased susceptibility of AKI [16]. It was observed that 22(78.6%) out of 28 patients who developed AKI were diabetic. On the other hand 20(71.4%) patients in AKI group were hypertensive. These results were comparable with a previous study [16]. This might be explained by the fact that patient with diabetes mellitus and/or hypertension had not only intra-renal atherosclerosis and arteriosclerosis but also non-inflammatory glomerular damage (nephropathy) [16]. Most cases of AKI in diabetes mellitus ensue from transient renal hypo-perfusion [16]. The hallmark in ischemic AKI is tubular cell dysfunction and damage [17]. Ischemia also induces significant interstitial inflammation and functional and structural damage of small peritubular and glomerular blood vessels [17]. Every ischemic insult diminishes the intra-renal total vascular surface area, subsequently followed by endothelial-to-mesenchymal trans differentiation and the ultimate consequence is aggravated fibrosis and an increased risk for renal dysfunction [17]. Patients enrolled in this study who developed AKI were relatively hypotensive. Their mean arterial pressure was 66.6±6.7 mm of Hg, 22(78.6%) out of 28 AKI patients were in septic shock and 20(71.4%) out of 28 AKI patients were on inotrope support. Similar findings were demonstrated in a couple of previous studies [16, 20]. The possible reason could be, when the blood pressure of the patients declined below the level to sustain normal renal auto-regulation function, there would be hypo-perfusion of the kidney leading to the development of AKI [16]. GFR is normally auto-regulated within an optimal mean arterial pressure but as the mean arterial pressure falls below 80 mm of Hg there would be a rapid decline of the renal function leading to development of AKI [20].
In this study 41(46.6%) patients out of 88 study patients had sepsis due to lung infection; this finding was supported by a previous study [22]. It may be due to most of our study patients in ICU were on endotracheal intubation. In this current study, it was observed that SOFA (Sequential [Sepsis-Related] Organ Failure Assessment) score was significantly more in patient with septic AKI. This finding was in concert with similar previous studies [19-22]. This indicates that patient who had severe sepsis been more vulnerable to develop AKI. This study showed that, the study subjects who developed septic AKI had significant low PH and high serum lactate level compared to the patients without AKI. These findings were supported by a couple of previous study [16, 20]. It was observed that 28 patients who had developed AKI, their mean value of plasma Proenkephalin was significantly higher as compared to the patients (60 patients) who did not develop AKI (p< 0.001) on the day 0, day 2 and day 7 of admission. Similar studies also showed plasma Proenkephalin levels were higher in patients who were subsequently developed AKI [18, 20-22]. Therefore plasma Proenkephalin was found to be a good early biomarker for the diagnosis of septic AKI.
In this study, serum creatinine levels among the patients in group A were within normal physiological limit on day 0 of admission in ICU who subsequently developed AKI, but was found to increase to a significant value for the diagnosis of AKI from the second day of admission. Plasma Proenkephalin levels of these patients were found raised on day 0, day 2 and day 7 of admission in ICU that was seems to rise much earlier in comparison to serum creatinine. Although, conventionally used biomarker serum creatinine can detect the development of AKI, there is a significant delay in the diagnosis of AKI, which could be improved by measuring serum plasma Proenkephalin. Moreover, in a previous study it was revealed that rise of serum creatinine occurs only when there is a decline of more than 50% in the glomerular filtration and would detect AKI about 1-2 days after the insult has occurred [23]. Previously published data from patients with sepsis admitted in ICU have provided evidence that, elevated blood levels of plasma Proenkephalin could predict AKI independently from inflammation and other co-morbidities [20, 21, 24]. The receiver-operating characteristics (ROC) curve of plasma Proenkephalin for the diagnosis of septic AKI was depicted in our study. The area under the curve (AUC) for predicting the patients with septic AKI was 0.796 (95% confidence interval 0.667-0.924). Kim H et al. found the AUC of plasma Proenkephalin was 0.725 (0.651–0.791) for diagnosis of septic AKI [21]. The finding of the present study was supported by the findings of this previous study. Using the receiver-operating characteristics (ROC) curve we calculate the potential cut-off value to diagnosis septic AKI for plasma Proenkephalin. An optimal cut-off value of plasma Proenkephalin for septic AKI patient was found 145.0 pmol/l. In this study, sensitivity and specificity of plasma Proenkephalin were found 67.9% and 98.3% respectively. In accordance Kim H et al. found cut-off point of plasma Proenkephalin for diagnosing septic AKI was 154.5 pmol/l (with 65.9% sensitivity and 79.4% specificity) [21].
This study demonstrated a strong and positive association
between plasma Proenkephalin and risk of AKI among patients with sepsis. Individual’s urine output and serum creatinine based methods to estimate glomerular filtration rate (GFR) are not convenient for diagnosis of AKI due to delays in recognition of impaired kidney function and detection of AKI, hence early biomarkers are preferred. Therefore the plasma Proenkephalin may be used as an early biomarker for identification of individuals with septic AKI.
5. Conclusions
This study concluded that acute kidney injury (AKI) is frequently occur in patients with sepsis and septic shock. The patients with sepsis who subsequently develop AKI have a significant higher level of plasma Proenkephalin. Plasma Proenkephalin could be an early biomarker to predict septic AKI.
Limitations
It was a single centre study with a relatively small sample size.
Recommendation
A population based prospective study with large sample size should be done to establish the plasma Proenkephalin as an early biomarker of AKI.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
References
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315 (2016): 801-810.
- Iwashyna TJ, Cooke CR, Wunsch H, et al. Population burden of long-term survivorship after severe sepsis in older Americans. Journal of the American Geriatrics Society 60 (2012): 1070-1077.
- Vincent JL, Marshall JC, Ñamendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. The lancet Respiratory medicine 2 (2014): 380-386.
- Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. Jama 304 (2010): 1787-1794.
- Angus DC, Van der Poll T. Severe sepsis and septic shock. N Engl J Med 369 (2013): 840-851.
- Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity 40 (2014): 463-475.
- Jacobi J. Pathophysiology of sepsis. American Journal of Health-System Pharmacy 59 (2002): S3-S8.
- Bellomo R. Acute Dialysis Quality Initiative workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit care 8 (2004): R204-R212.
- Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Critical care medicine 35 (2007): 1837-1843.
- Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney international 81 (2012): 819-825.
- Le Dorze M, Legrand M, Payen D, et al. The role of the microcirculation in acute kidney injury. Current opinion in critical care 15 (2009): 503-508.
- Pfaff DW. Hormones, brain and behavior. Elsevier 18 (2002): 173.
- Grossman A, Clement-Jones V. 3 Opiate receptors: Enkephalins and endorphins. Clinics in endocrinology and metabolism 12 (1983): 31-56.
- Denning GM, Ackermann LW, Barna TJ, et al. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides 29 (2008): 83-92.
- Donato LJ, Meeusen JW, Lieske JC, et al. Analytical performance of an immunoassay to measure proenkephalin. Clinical biochemistry 58 (2018): 72-77.
- Hollinger A, Wittebole X, François B, et al. Proenkephalin A 119-159 (Penkid) is an early biomarker of septic acute kidney injury: the kidney in sepsis and septic shock (Kid-SSS) study. Kidney international reports 3 (2018): 1424-1433.
- Legrand M, De Berardinis B, Gaggin HK, et al. Evidence of uncoupling between renal dysfunction and injury in cardiorenal syndrome: insights from the BIONICS study. PLoS One 9 (2014): e112313.
- Shah KS, Taub P, Patel M, et al. Proenkephalin predicts acute kidney injury in cardiac surgery patients. Clin Nephrol 83 (2015): 29-35.
- Oda S, Hirasawa H, Sugai T, et al. Comparison of Sepsis-related Organ Failure Assessment (SOFA) score and CIS (cellular injury score) for scoring of severity for patients with multiple organ dysfunction syndrome (MODS). Intensive care medicine 26 (2000): 1786-1793.
- Caironi P, Latini R, Struck J, et al. Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clinical chemistry 64 (2018): 1361-1369.
- Kim H, Hur M, Lee S, et al. Proenkephalin, neutrophil gelatinase-associated lipocalin, and estimated glomerular filtration rates in patients with sepsis. Annals of laboratory medicine 37 (2017): 388.
- Marino R, Struck J, Hartmann O, et al. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. Journal of nephrology 28 (2015): 717-724.
- Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Critical care 12 (2008): 1-9.
- Rosenqvist M, Bronton K, Hartmann O, et al. Proenkephalin a 119–159 (penKid)–a novel biomarker for acute kidney injury in sepsis: an observational study. BMC emergency medicine 19 (2019): 1.
