Prevalence of Methicillin-Resistant Staphylococcus Aureus with Their Susceptibility Pattern and Their Association of Panton-Valentine Leukocidin Gene in a Tertiary Care Hospital in Bangladesh
Article Information
Rehana Khatun*, 1, Dr. Sk. Shehab Hasan2, Nazma Khatun3, Shahida Akter4
1Assistant Professor, Department of Microbiology, Ibrahim Medical College, Dhaka, Bangladesh
2MBBS, MD (Microbiology) Assistant Professor (Microbiology) Department of Pathology, National Heart Foundation Hospital & Research Institute, Mirpur-2, Dhaka, Bangladesh
3Senior Medical officer, Dr.M.R.Khan Shishu hospital, Institute of Child health and Shishu Shasthya Foundation, Dhaka, Bangladesh
4Assistant Professor, Department of Microbiology, Ibrahim Medical College, Dhaka, Bangladesh
*Corresponding author: Rehana Khatun. Assistant Professor, Department of Microbiology, Ibrahim Medical College, Dhaka, Bangladesh
Received: 06 April 2023; Accepted: 11 April 2023; Published: 17 April 2023
Citation: Rehana Khatun, Sk. Shehab Hasan, Nazma Khatun, Shahida Akter. Prevalence of Methicillin-Resistant Staphylococcus Aureus with Their Susceptibility Pattern and Their Association of Panton-Valentine Leukocidin Gene in a Tertiary Care Hospital in Bangladesh. Fortune Journal of Health Sciences. 6 (2023): 160-166.
View / Download Pdf Share at FacebookAbstract
Background: Methicillin-resistant Staphylococcus aureus (MRSA) is a substantial public health problem worldwide, causing significant morbidity and mortality. The emergence and spread of MRSA has made infection control intervention and treatment challenging.
Objective: The study aimed to observe the Prevalence of methicillin-resistant Staphylococcus aureus with their susceptibility pattern and also their association of panton-valentine leukocidin (PVL) gene, in BIRDEM (Bangladesh Institute of Research and Rehabilitations in Diabetes, Endocrine and Metabolic Disorders) hospital in Bangladesh.
Methods: This Study was conducted in the Department of Microbiology, Ibrahim Medical College from September 2018 to February 2019. Total 198 S. aureus were isolated from the pus, wound swab, blood and urine samples of the hospitalized patients, which were collected from the Department of Microbiology, BIRDEM Hospital.Antibiotic susceptibility testing was performed by Kirby-Bauer disk diffusion method using Muller Hinton agar plates. Detection of MRSA by Cefoxitin disc diffusion test. Isolated MRSA were genotypically confirmed by detection of mecA gene and association of PVL gene was also detected by PCR. Statistical evaluation of the results used to be got via the use of a window-based computer software program devised with Statistical Packages for Social Sciences (SPSS-24).
Result: A total 198 S.aureus was collected from pus, wound swab, blood and urine samples. Most of the MRSA were isolated from pus and wound swab 92.04% (81/88) and most of the mecA and PVL positive samples were isolated from wound swab 85.71% (6/7). In case of seven PVL positive MRSA showed all are resistance to cefoxitin, cephalexin, amoxiclav, and erythromycin followed by six were resistance to clindamycin, ciprofloxacin, five were resistance to cotrimoxazole. All S.aureus were 100% sensitive to vancomycin and showed higher sensitivity pattern in fusidic acid, amikacin and netilmicin.
Conclusions: Prevalence of MRSA, their antibiotic resistance pattern as well as molecular characteristics significantly changing. Constant surveillance should be maintained to prevent transmissions and may allow development of regional strategies for rational antibiotic use
Keywords
Methicillin-Resistant, Staphylococcus Aureus, Panton-Valentine, Leukocidin Gene
Methicillin-Resistant articles, Staphylococcus Aureus articles, Panton-Valentine articles, Leukocidin Gene articles
Article Details
1. Introduction
Increasing antibiotic resistance is a worrisome trend being observed worldwide. Among Gram-positive cocci, Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most tenacious anti-microbial resistant pathogens reported in a range of infections, including the skin and soft tissue infections, pneumonia, and bloodstream infections [1, 2]. According to previous reports in Bangladesh, the prevalence rate of MRSA in clinical samples is 32-72% [3-7]. MRSA infections have been traditionally classified as either community-associated (CA-MRSA) or healthcare-associated MRSA (HA-MRSA).
Healthcare-associated risk factors for HA-MRSA infection including recent hospitalization or surgery, residence in a long-term–care facility, dialysis, and indwelling percutaneous medical devices and catheters, community-onset MRSA infections have been observed with increasing frequency among patients in community settings. Similarly, highly successful community-based clones have invaded the healthcare setting and have become successful nosocomial pathogens [8–10]. β-lactam antibiotic resistance in MRSA is attributed to the acquisition of mecA gene encoding the transpeptidase penicillin-binding protein 2a [11], and is a molecular hallmark for MRSA strains. The mecA gene is located on a mobile genetic element, the staphylococcal cassette chromosome mec (SCCmec). In genetic background of MRSA strains associated with HA-MRSA typically harbour SCCmec types I, II, or III, whereas CA-MRSA isolates carry SCCmec types IV, V and VI [12, 13].
PVL is a pore-forming cytotoxin assembled by lukS-PV and lukF-PV, has been demonstrated to have a significant role in the pathogenesis of MRSA. Epidemiological and clinical data provide substantial evidence that the high virulence potential of CA-MRSA isolates is associated with the expression of PVL [14, 15], which was also shown to be an important contributing factor in CA-MSSA infections, particularly in severe SSTIs and most notably in necrotizing pneumonia [16]. PVL genes carried by HA-MRSA strains have also been recently described in many studies [10, 17, 18]. In Bangladesh, a very few studies have yet done to see the association of PVL with MRSA, Parvez et al., showed 58% association of in clinical samples, S. Roy et al., showed 15% association in clinical samples, Islam et al., showed, 26.67% association in hospitalized samples.
The epidemiology of MRSA strains is constantly changing and their prevalence as well as molecular characteristics are known to vary between hospitals in different countries, districts within a country, or among wards of a hospital. Continuous vigilance of the changing epidemiology of MRSA in local healthcare facilities with unique patient population is imperative for obtaining data that may aid empirical therapy and patient management. This study is aimed to investigate the prevalence of MRSA, their antibiotic resistance patterns as well as their association with PVL gene of hospitalized patients.
2. Material and Methods
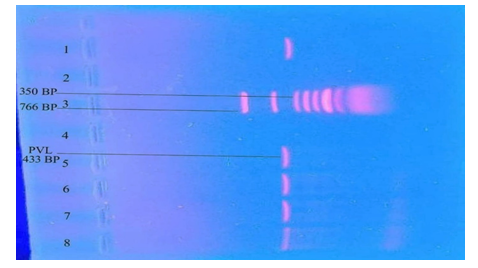
This cross-sectional study was conducted in the Department of Microbiology, Ibrahim Medical College from September 2018 to February 2019. Total 198 S. aureus were isolated from the pus, wound swab, blood and urine samples of the hospitalized patients, which were collected from the department of Microbiology, BIRDEM Hospital. Antibiotic susceptibility testing was performed by Kirby-Bauer disk diffusion method using Muller Hinton agar plates and zone of inhibition were interpreted according to CLSI guideline (CLSI, 2017). Amoxiclav (amoxicillin 20 µg and clavulanic acid 10 µg), Cefalexin (30 µg), Ciprofloxacin (5 µg), Cotrimoxazole (25 µg), Cefoxitin (30 µg), Amikacin (30 µg), Clindamycin (2 µg), Erythromycin (15 µg), Fusidic acid (10 µg), Gentamicin (10 µg), Vancomycin (30 µg), Netilmicin (30 µg), discs were used. Cefoxitin disc diffusion test was performed on Muller-Hinton agar plates using a bacterial suspension with the turbidity adjusted to a 0.5 McFarland standard. Disc containing 30 µg of Cefoxitin were placed. Plates were incubated at 37°C for 24 hour. Staphylococcus aureus strains were screened for MRSA by detection of resistance (zone of inhibition ≤21 mm) following the CLSI guidelines (CLSI, 2017). Moleculer analyses of isolated MRSA strains were carried out for detection of mecA and Panton valentine leucocidin (PVL) by polymerase chain reaction. DNA was extracted using the boiling method. Bacterial colonies were suspended in 300 mL of distilled water and boiled for 10 minutes in a heat block, then placed on ice for 5 minutes. After centrifugation at 13,000 rpm at 4°C for 5 minutes, the supernatant was placed in a microtube and kept at 4°C until used as a DNA template. Extracted DNA was stored at -20°C until PCR amplification. Amplification was carried out in an automated DNA thermal cycler. Using primers for mecA gene - F: 5′TCCAGATTACAACTTCACCAGG-3′ and R: 5′CCACTTCATATCTTGTAACG-3′. The DNA of the S. aureus ATCC 43300 and ATCC 25923 strains were used as positive and negative controls, respectively, for this polymerase chain reaction (PCR) assay of mecA. DNA amplification was carried out using the following thermal cycling profile: initial denaturation at 94°C for 5 minutes, 32 cycles of amplification (denaturation at 94°C for 50 seconds, annealing at 58°C for 50 seconds, and extension at 72°C for 50 seconds), and a final extension at 72°C for 10 minutes in a thermal cycler. Using primers for PVL gene – F: 5′ATCATTAGGTAAAATGTCTGGACATGATCCA-3′ and R: 5′GCATCAAGTGTATTGGATAGCAAAAGC-3′. The The DNA of S. aureus ATCC 25923 strains was used as the negative control for the PVL gene assay. DNA amplification was carried out using the following thermal cycling profile: initial denaturation at 94°C for 4 minutes, 30 cycles of amplification (denaturation at 94°C for 45 seconds, annealing at 56°C for 45 seconds, and extension at 72°C for 30 seconds), and a final extension at 72°C for 2 minutes in a thermal cycler. PCR products were analyzed on 1.5% agarose gel was prepared by melting 0.45gm agarose in 30 ml of diluted 1x TBE buffer (Tris Boric acid EDTA). A 25-766 bp DNA ladder was used for mecA and PVL gene. The gels were stained with 1% ethidium bromide and visualized under UV light. Statistical evaluation of the results used to be got via the use of a window-based computer software program devised with Statistical Packages for Social Sciences (SPSS-24).
3. Results
Table 1: Distribution of S. aureus in various samples
|
Sample |
MSSA(%) N=110 |
MRSA(%) (PVL Negative)N=81 |
MRSA(%) (PVL Positive)N=7 |
Tota (%) N=198 |
|
Pus |
35 (31.82) |
25 (30.86) |
1 (14.29) |
61 ((30.81) |
|
Wound swab |
44 (40.00) |
49 (60.49) |
6 (85.71) |
99 (50.00) |
|
Blood |
14 (12.73) |
2 (2.47) |
0 (0.00) |
16 (8.08) |
|
Urine |
17 (15.45) |
5 (6.17) |
0 (0.00) |
22 (11.11) |
A total 198 S.aureus was collected from pus, wound swab, blood and urine samples. Out of 198 S.aureus, 88 (44.44%) were detected as MRSA by cefoxitin disc diffusion test. Those 88 isolates were positive for mecA gene by PCR. Out of 88 MRSA 7.95% (7/88) were also positive for PVL gene. Most of the MRSA were isolated from pus and wound swab 92.05% (81/88) and most of the mecA and PVL positive samples were isolated from wound swab 85.71% (6/7). From blood and urine samples no PVL gene was isolated (table 1).
Table 2: Antibiotic resistance pattern of methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA)
|
Antibiotics |
MSSA N=110 (%) |
MRSA N=88 (%) |
|
Cefoxitin |
0 (0.0) |
88 (100.0) |
|
Cefalexin |
3 (2.7) |
86 (97.7) |
|
Amoxiclav |
2 (1.8) |
86 (97.7) |
|
Vancomycin |
0 (0.0) |
0 (0.0) |
|
Ciprofloxacin |
61 (55.6) |
58 (65.9) |
|
Cotrimoxazole |
32 (29.1) |
49 (55.7) |
|
Amikacin |
2 (1.8) |
13 (14.8) |
|
Netilmicin |
3 (2.7) |
11 (12.5) |
|
Gentamicin |
7 (6.4) |
32 (36.4) |
|
Erythromycin |
72 (65.5) |
76 (86.4) |
|
Clindamycin |
68 (61.8) |
72 (81.1) |
|
Fusidic acid |
4 (3.6) |
5 (5.7) |
Antibiotic resistance patterns of S. aureus
The antimicrobial resistance patterns of MSSA and MRSA isolates against antimicrobial agents are summarized in table 2. In case of MSSA, showing more resistance to erythromycin (65.5%), clindamycin (61.8%), ciprofloxacin (55.6%), and cotrimoxazole (29.1%). In case of MRSA, showed 100% resistance to cefoxitin followed by cephalexin and amoxiclav (97.7%), erythromycin (86.4%), clindamycin (81.1%), ciprofloxacin (65.9%), cotrimoxazole (55.7%) and gentamicin (36.4%). In case of seven PVL positive MRSA showed all are resistance to cefoxitin, cephalexin, amoxiclav, and erythromycin followed by six were resistance to clindamycin, ciprofloxacin, five were resistance to cotrimoxazole. All S.aureus were 100% sensitive to vancomycin and showed higher sensitivity pattern in fusidic acid, amikacin and netilmicin.
4. Discussion
Global emergence of MRSA is serious public health trouble and challenge to clinicians. A variety of factors make contributions to the pathogenicity and drug resistance of S.aureus. The first PVL positive MRSA used to be determined in the late 1990 and these strains have become globally disbursed in the current years [19]. The feature of PVL in enhancing virulence of S. aureus and their pathogenicity is being debated. Panton Valentine leukocidin will increase the pathogenicity of S. aureus with the aid of necrosis, accelerating apoptosis and destruction of polymorphonuclear and mononuclear cells thereby contributing to morbidity and mortality [20]. However, some researches have shown no affiliation of PVL with the virulence of the organism by using demonstrating higher medical consequence of skin and smooth tissue infections [21]. Therefore, the position of PVL in medical consequence is nevertheless debated. The cause for the consequences in medical effects in this research should be influenced through the effectiveness of antibiotic treatment applied.
Reports from a variety of countries exhibit the growing occurrence of PVL amongst MRSA isolates. Subarna Roy et al. from India, have suggested usual 62.85 % of PVL incidence amongst MRSA and MSSA (MRSA: 85.1 % and MSSA: 48.8 %) which suggests a greater occurrence amongst findings MRSA [22]. Similar find out about through D’Souza et al. from Mumbai, India, stated occurrence of 64% PVL effective isolates amongst MRSA [23]. A decrease incidence of PVL has been stated from different components of world (5 p% in France, 4.9 % in UK, 8.1 % in Saudi Arabia and 14.3 percent in Bangladesh), reflecting that the incidence of PVL varies significantly between geographical places and populations [24].
Skin and soft tissue infections are predominantly caused by PVL producing organisms as the leucocidal activity of PVL provides survival advantage to the bacteria [25]. The association of PVL with isolates from other specimens was less. Presence of PVL in deep seated infections like blood was found less common in our study indicating poor association of PVL with invasiveness of MRSA. In our study, the percentage of MRSA among PVL negative (81 %) isolates was found significantly higher than in PVL positive (7 %) isolates. 88 out of 198 MRSA isolates were found positive predictive value of wound swab was 60.49% and negative predictive value of wound swab was 85.71%.
Most of the studies including our study categorized MRSA based upon the history of the patient or by getting information from medical record. However, information obtained from patient or from medical record may not be reliable all the time. Nepal tested seven isolates of MRSA obtained from the environment of various units of the hospital including wards and intensive care units. As these isolates are not known to be related to the specimen from patients and isolated from hospital environment, we considered them as presumptive hospital strains. These seven isolates were found negative for PVL genes which could indicate that PVL is not normally found in the isolates of hospital environment. However, these isolates are not necessarily representative of hospital environment in general. In contrast, another study from Nepal reported higher prevalence of PVL among nosocomial MRSA isolates [26]. In this study, 88 cefoxitin resistances S. aureus were phenotypically detected as MRSA, which all of them showed positive for mecA gene by PCR . So results of cefoxitin disk diffusion test are in concordance with the PCR for mecA gene. Cefoxitin is a potent inducer of the mecA regulatory system and is being widely used as a marker for mecA gene mediated methicillin resistance (4).
MRSA is well recognized to physicians as a motive of hospital acquired infections and is now rising as a necessary pathogen in the community. First reported in 1961, MRSA accounts for 30%–40% of current nosocomial staphylococcal infections [27]. The manufacturing of PVL may also make a contribution to the special pathogenicity of MRSA. PVL belongs to the synergohymenotropic classification of toxins. It is assembled from two elements that are secretly separated however combined to create lytic pores in cell membranes of neutrophils. The binding of PVL aspects to neutrophils induces release of the neutrophil chemotactic factors IL-8 and leukotriene B4, as properly as a variety of inflammatory mediators, earlier than bringing about cell dying [25]. The aggregate of neutrophil chemotaxis, release of inflammatory mediators, and karyorrhexis promotes tissue necrosis and abscess formation. Purified PVL protein injected intradermally into rabbits leads to extreme irritation and necrosis [28]. Gillet et al. confirmed that hemoptysis was once located in 6 (38%) of sixteen patients with extreme pneumonia related with S. aureus strains carrying PVL genes, in contrast with 1 (3%) of 33 PVL-negative patients. Interestingly, the action of PVL on neutrophils in vitro may additionally be blocked by means of precise anti-PVL antibodies located in industrial preparations of intravenous immunoglobulin [29]. In this study, the PVL gene was detected in 7 (%) isolates out of 88 MRSA.in another study, Islam et all., showed that the prevalence of PVL gene was 26.67% where the samples were only pus and wound swab. In present study all PVL gene were isolated from pus and wound samples, no PVL gene were detected from blood and urine samples. In Nepal, PVL positive HA-MRSA isolates were 7.1% (Nepal)
In this present study, the antimicrobial resistance patterns of MSSA and MRSA isolates against antimicrobial agents are summarized. In case of MSSA, showing more resistance to erythromycin (65.5%), clindamycin (61.8%), ciprofloxacin (55.6%), and cotrimoxazole (29.1%). In case of MRSA, showed 100% resistance to cefoxitin followed by cephalexin and amoxiclav (97.7%), erythromycin (86.4%), clindamycin (81.1%), ciprofloxacin (65.9%), cotrimoxazole (55.7%) and gentamicin (36.4%). In case of seven PVL positive MRSA showed all are resistance to cefoxitin, cephalexin, amoxiclav, and erythromycin followed by six were resistance to clindamycin, ciprofloxacin, five were resistance to cotrimoxazole. In the present study the prevalence of MRSA was 44.44%, which correlates with previous report of other neighbor countries such as India, Pakistan. In Bangladesh some study reported the MRSA prevalence rates of 32-72% . Parvez et all., showed in his study, among 53 isolates 38 (72%) showed positive amplification for mecA (162 bp) gene. Among 38 MRSA isolates 22 (57.89%) confirmed as CA-MRSA and 16 (42.10%) as HA-MRSA. The cause of variation of prevalence is not exactly known, but variation of sample could have variation of prevalence. The prevalence of MRSA varies significantly in different regions, which suggests a need for periodic evaluation of MRSA (23)
The occurrence of the PVL amongst the MRSA isolates in this learn about used to be discovered relatively excessive in particular amongst pus samples which indicate a feasible key role of PVL in pathogenesis of pyogenic infections in particular skin and soft tissue infections in community setting [30]. The PVL positive MRSA isolates confirmed greater sensitivity towards antibiotics as in contrast to PVL negative isolates indicating that PVL is not related with drug resistance mechanisms. The presence of PVL amongst multi drug resistant bacteria like MRSA may also be concerned in virulence and increase the challenges for clinicians [31]. As expected, the majority of PVL positive MRSA have been community-associated isolates, whereas only four MRSA from hospital associated instances have been found positive for PVL. No PVL was detected in MRSA isolated from the medical environment [32]. The presence of PVL can be used as a dependable marker for MRSA in these resource limited settings in Nepal. Some other countries reported that 68-85% of MRSA strains were positive for the PVL gene, which is much higher than in the present study. (21,29 ). In this study, the prevalence of PVL gene with MRSA in hospitalized patient is low. In the present study, however, MSSA strains were not screened for the PVL gene and also SCC mec typing was not done. Therefore, a surveillance mechanism should be set up to detect PVL genes in both MRSA and MSSA in Bangladesh. Usually, PVL positive strains are found in community acquired MRSA but the PVL gene is not restricted to community acquired strains [28].
Limitations:
The present study was conducted in a very short period due to time constraints and funding limitations. The small sample size was also a limitation of the present study.
Conclusion
Prevalence of MRSA, their antibiotic resistance pattern as well as molecular characteristics significantly changing. Constant surveillance should be maintained to prevent transmissions and may allow development of regional strategies for rational antibiotic use.
Recommendation
This study can serve as a pilot to much larger research involving multiple centers that can provide a nationwide picture, validate regression models proposed in this study for future use and emphasize points to ensure better management and adherence.
Acknowledgements
The wide range of disciplines involved in the Prevalence of Methicillin-Resistant Staphylococcus Aureus with their susceptibility pattern and their association of panton-valentine leukocidin gene research means that editors needs much assistance from referees in the evaluation of papers submitted for publication. I am very grateful to many colleagues for their thorough, helpful and usually prompt response to requests for their opinion and advice.
Declaration
Funding:
This study fund source was provided by Ibrahim Medical College, Dhaka, Bangladesh.
Conflict of interest:
None declared.
Ethical approval:
The study was approved by the ethical committee of BIRDEM.
References
- Arjyal C, Kc J, Neupane S. Prevalence of methicillin-resistant Staphylococcus aureus in shrines. Int J Microbiol (2020): 7981648.
- Zhen X, Lundborg CS, Zhang M, et al. Clinical and economic impact of methicillin-resistant Staphylococcus aureus: a multicentre study in China. Sci Rep 10 (2020): 3900.
- Haq JA, Rahman MM, Asna SM, Hossain MA, Ahmed I, Haq T, et al. Methicillin-resistant Staphylococcus aureus in Bangladesh--a multicentre study. Int J Antimicrob Agents 25 (2005): 276-7.
- Tashmin Afroz Binte Islam, SM Shamsuzzaman. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant, vancomycin-resistant, and Panton-Valentine leukocidin positive Staphylococcus aureus in a tertiary care hospital Dhaka, Bangladesh. Tzu Chi Medical Journal 27 (2015): 10-14.
- Roy S, Barman TK, Hossain MA, Paul SK, Haque N, Ahmed S, et al. Molecular-Characterization of Methicillin-Resistance Staphylococcus aureus (MRSA) from Different Tertiary Care Hospitals in Bangladesh. 2017. Mymensingh Med J 26 (2017): 37-44.
- Parvez MAK, Ferdous RN, Rahman MS, Islam S. Healthcare-associated (HA) and community-associated (CA) methicillin resistant Staphylococcus aureus (MRSA) in Bangladesh - Source, diagnosis and treatment J Genet Eng Biotechnol 16 (2018): 473-478.
- Islam T, Kubra K, Hassan Chowdhury MM. Prevalence of methicillin-resistant Staphylococcus aureus in hospitals in Chittagong, Bangladesh: A threat of nosocomial infection. J Microsc Ultrastruct 6 (2018): 188-91.
- Uhlemann AC, Otto M, Lowy FD, et al. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus 21 (2014): 563–74.
- Choo EJ. Community-associated methicillin-resistant Staphylococcus aureus in nosocomial infections. 2017. Infect Chemother 49 (2017): 158–9.
- Amin DHM, Guler E & Baddal B. Prevalence of Panton-Valentine leukocidin in methicillin-resistant Staphylococcus aureus clinical isolates at a university hospital in Northern Cyprus: a pilot study. 2020. BMC Res Notes 13 (2020): 490.
- Hartman BJ, Tomasz A. Low-afnity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J Bacteriol 158 (1984): 513–6.
- David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23 (2010): 616–87.
- Kong EF, Johnson JK, Jabra-Rizk MA. Community-associated Methicillin-resistant Staphylococcus aureus: an enemy amidst us. PLoS Pathog 12 (2016): e1005837.
- Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359 (2002):753–9.
- Chambers HF. Community-associated MRSA - Resistance and virulence converge. N Engl J Med 352 (2005): 1485–7.
- Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of PantonValentine Leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 29 (1999): 1128–32.
- Bhatta DR, Cavaco LM, Nath G. et al. Association of Panton Valentine Leukocidin (PVL) genes with methicillin resistant Staphylococcus aureus (MRSA) in Western Nepal: a matter of concern for community infections (a hospital based prospective study). BMC Infect Dis 16 (2016): 199.
- Klein S, Hannesen J, Zanger P, Heeg K, Boutin S, Nurjadi D. Entry of Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus into the hospital: prevalence and population structure in Heidelberg, Germany 2015-2018. Sci Rep 10 (2020): 13243.
- Gravet A, Rondeau M, Harf-Monteil C, Grunenberger F, Monteil H, Scheftel JM, et al. Predominant Staphylococcus aureus isolated from antibiotic-associated diarrhea is clinically relevant and produces enterotoxin A and the bicomponent toxin LukE-LukD. Journal of Clinical Microbiology 37 (1999): 4012-9.
- Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin—producing Staphylococcus aureus in primary skin infections and pneumonia. Clinical infectious diseases 29 (1999): 1128-32.
- Bae IG, Tonthat GT, Stryjewski ME, Rude TH, Reilly LF, Barriere SL, et al. Presence of genes encoding the Panton-Valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: results of a multinational trial. Journal of clinical microbiology 47 (2009): 3952-3957.
- Kaur H, Purwar S, Saini A, Kaur H, Karadesai SG, Kholkute SD, et al. Status of methicillin-resistant Staphylococcus aureus infections and evaluation of PVL producing strains in Belgaum, South India. JKIMSU 1 (2012): 43-51.
- D'Souza N, Rodrigues C, Mehta A. Molecular characterization of methicillin-resistant Staphylococcus aureus with emergence of epidemic clones of sequence type (ST) 22 and ST 772 in Mumbai, India. Journal of clinical microbiology 48 (2010): 1806-11.
- Diekema DJ, BootsMiller BJ, Vaughn TE, Woolson RF, Yankey JW, Ernst EJ, et al. Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clinical infectious diseases 38 (2004): 78-85.
- Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, et al. Comparison of community-and health care–associated methicillin-resistant Staphylococcus aureus infection. 290 (2003): 2976-84.
- Shrestha B, Singh W, Raj VS, Pokhrel BM, Mohapatra TM. High prevalence of Panton-Valentine leukocidin (PVL) genes in nosocomial-acquired Staphylococcus aureus isolated from tertiary care hospitals in Nepal. BioMed Research International 18 (2014).
- Saiman L, Keefe MO, Graham III PL, Wu F, Salim BS, Kreiswirth B, et al. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clinical Infectious Diseases 37 (2003): 1313-9.
- König B, Prevost G, Piemont Y, König W. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. Journal of Infectious Diseases 171 (1995): 607-13.
- Ward PD, Turner WH. Identification of staphylococcal Panton-Valentine leukocidin as a potent dermonecrotic toxin. Infection and immunity 28 (1980): 393-7.
- Gauduchon V, Cozon G, Vandenesch F, Genestier AL, Eyssade N, Peyrol S, et al. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. The Journal of infectious diseases 189 (2004): 346-53.
- Bhatta DR, Cavaco LM, Nath G, Kumar K, Gaur A, Gokhale S. and et al. Association of Panton Valentine Leukocidin (PVL) genes with methicillin resistant Staphylococcus aureus (MRSA) in Western Nepal: a matter of concern for community infections (a hospital based prospective study). BMC infectious diseases 16 (2016): 1-6.
- Munckhof WJ, Nimmo GR, Carney J, Schooneveldt JM, Huygens F, Inman-Bamber J, et al. Methicillin-susceptible, non-multiresistant methicillin-resistant and multiresistant methicillin-resistant Staphylococcus aureus infections: a clinical, epidemiological and microbiological comparative study. European Journal of Clinical Microbiology & Infectious Diseases 27 (2008): 355-64.