Prevalence of Hepatitis B and Associated Factors among Pregnant Women in N'djamena, Chad
Article Information
Nalda Debsikréo*, 1, 2, Birwé Léon Mankréo1, Azoukalné Moukenet1,3, Anna Julienne Selbé Ndiaye2, Nafissatou Leye Diouf2, Gora LO2, Merwa Ouangkake4, Madjikoula Jotham5, Ali Mahamat Moussa3, 6, Ndèye Coumba Toure-Kane1, 2, Françoise Lunel-Fabiani7
1Cheikh Anta Diop University, Dakar, Senegal
2Institute for Health Research, Epidemiology Surveillance and Training, Dakar, Senegal
3University of Ndjamena, Chad, Senegal
4Peace Hospital, N'Djamena, Chad, Senegal
5Ardeptiman Health Center in N'Djamena, Chad, Senegal
6University Hospital Center of National Reference, Chad, Senegal
7University Hospital of Angers, SFR 4208-UPRES EA3859 University of Angers, Angers, France
Corresponding author: Nalda Debsikréo. Cheikh Anta Diop University, Department of biology and human pathology, Dakar, Senegal.
Received: 30 June 2023; Accepted: 10 July 2023; Published: 13 July 2023
Citation: Nalda Debsikréo, Birwé Léon Mankréo, Azoukalné Moukenet, Anna Julienne Selbé Ndiaye, Nafissatou Leye Diouf, Gora LO, Merwa Ouangkake, Madjikoula Jotham, Ali Mahamat Moussa, Ndèye Coumba Toure-Kane, Françoise Lunel-Fabiani. Prevalence of Hepatitis B and Associated Factors among Pregnant Women in N'djamena, Chad. Fortune Journal of Health Sciences. 6 (2023): 253-262.
View / Download Pdf Share at FacebookAbstract
Background: Hepatitis B virus (HBV) infection is a major global health problem. It is thus a high priority to develop strategies to reduce HBV transmission, especially mother to child transmission (MTCT), by incorporating birth dose HBV vaccination and if possible additional preventive interventions. However, the prevalence and risk factors of hepatitis B among pregnant women in N'Djamena are not yet documented. The aims of this study were to determine the prevalence of HBV and to identify the risk factors associated with hepatitis B among pregnant women.
Methods: A cross-sectional survey was conducted among pregnant women in eight health facilities in the city of N'Djamena (Chad) from 4 April to 2 August 2021. Pregnant women were included in the study using quota sampling, and participants were recruited consecutively. Blood samples were collected, and serum was tested for hepatitis B surface antigen (HBsAg) using a rapid test, confirmed by a chemiluminescent microparticle immunoassay (CMIA). HBV Viral load was also quantified using real-time PCR. Descriptive analysis, binary and multivariate logistic regressions were used to examine the relationship between dependent variables and socio-demographic factors including other associated factors.
Results: A total of 458 pregnant women were included in the study. The mean age of the participants was 25 years (95% CI: 20 - 30 years) and the prevalence of HBV infection was 7.2% (95% CI, 5.0 - 9.9). HBV DNA was detectable in 51.51% of HBsAg positive serum samples, of which 35.29% had viral DNA titers >200,000 IU/ml. Most participants, 454 (99.12%), were married and 341 (74.45%) were from monogamous households. The majority (274, 59.82%) were housewives. Of the participants, 73.58% were unaware of their HBV status. Having an HBsAg+ mother (AOR: 20.70 95%CI: 3.63-117.05, p=0.001) and a history of HBV screening (AOR: 4.3395%CI: 1.81-10.56, p=0.001) were associated with a higher risk of HBV infection.
Conclusion: We found a prevalence of HBsAg of 7.2% in pregnant women, indicating an intermediate endemicity rate. Having an HBsAg+ mother and a history of HBV screening were factors associated with HBV infection in pregnant women. A policy of free hepatitis B screening of pregnant women and newborn HBV vaccination should be implemented together with training of health personnel on hepatitis B risks and prevention measures.
Keywords
Pregnant women, hepatitis B, HBsAg, prevalence, associated factors, Chad
Article Details
Introduction
Hepatitis B is a systemic infection caused by the hepatotropic virus, hepatitis B virus (HBV) [1]. HBV is a highly oncogenic virus: its specific virological characteristics and the natural history of infection contribute to the development of liver fibrosis, cirrhosis, and Hepatocellular Carcinoma (HCC). In particular, a prolonged duration of infection and a high viral load, which is more frequent among patients infected in childhood, favor chronicity and carcinogenesis [2]. The impact of chronic liver disease is significant worldwide, as liver cancer is the third most common cause of cancer deaths [3]. The prevalence of hepatitis B varies from region to region. An estimated 316 million people, in 2019, were chronic carriers of HBV [4]. The African regions accounted for 70 % [5]. It is really a global public health problem, as underlined by many authors [6, 7].
A systematic review by GBD 2019 Hepatitis B Collaborators estimates that the prevalence in Chad is high, with overall 12.4% all ages [4]. A prevalence of HBsAg of 7.87% among blood donors was reported in the 2022 Health Statistics Yearbook [8]. In 2017, a study conducted in the population of the southwest region reported a prevalence of hepatitis B of 22.9% [9]. Mother-to-child transmission (MTCT) is one of the main routes of HBV transmission worldwide, despite the proven effectiveness of immunoprophylaxis, in particular birth dose of HBV vaccine [10]. The risk of mother-to-child transmission was 38.3 % without prophylaxis for mothers with an HBeAg positive and the risk was 4.8% without prophylaxis for HBeAg-negative women [11], and in a recent study performed in Cameroon the risk of HBsAg positivity in children who had a timely HBV birth-dose vaccine was 32.4% from HBeAg-positive mothers with high viraemia [12].
High maternal HBV DNA concentration (>200 000 IU/ml) and positivity of HBeAg is thus associated with a high risk of transmission, even in infants receiving hepatitis B vaccine [13]. Preventing mother-to-child transmission (PMTCT) by vaccinating newborns is the best strategy for eliminating HBV, but peripartum antiviral prophylaxis might be required in high prevalence regions [12, 14]. In Chad, there are very few data on the seroprevalence of HBV infection in pregnant women and the risks of mother-to-child transmission. HBeAg testing and quantification of HBV DNA in pregnant women are not standard practices, and not reimbursed. In terms of prevention, systematic vaccination of infants against HBV was included in the routine Expanded Programme on Immunization (EPI) in 2008. WHO recommends HBsAg screening during pregnancy, birth dose HBV vaccination for the newborn of seropositive mothers and Hepatitis B Immunoglobulins, if available. However, pregnant women and newborns rarely receive adequate prevention and care because there is no national program to prevent MTCT of HBV in Chad. The current study aims were to estimate the prevalence and identify the risk factors associated with HBV infection among pregnant women in N'Djamena, Chad.
2. Methods
2.1. Study design and setting
A cross-sectional study was carried out from 4 April to 2 August 2021 in the gynaecology and obstetrics departments of the Assiam Vantou Hospital, the mother and Child Hospital, the Guinebor Hospital, the Notre Dame des Apôtres Hospital and the health centres of Ardeptiman, Goudji, N'gueli and Boutalbagar. The 8 healths facilities were chosen because they are representative of the maternities of N'Djamena, Capital of Chad.
2.2. Study population and eligibility
Our study focused on pregnant women who were visited the gynaecology and obstetrics departments of the health facilities mentioned above. Inclusion criteria were women who had antenatal care follow up with prenatal consultation and who gave informed consent.
2.3. Sampling procedure
Quota sampling was used in each health facility, based on the expected annual population of pregnant women in the gynaecology and obstetrics department of each hospital. Participants were recruited consecutively as they presented for antenatal care at the selected health facilities, during the study period. The sample was therefore distributed among the following maternities: Assiam Vantou Hospital Gynaecology and Obstetrics Department (100 pregnant women), Mother and Child Hospital (58 pregnant women), Guinebor Hospital (50 pregnant women), Notre Dame des Apôtres Hospital (50), Ardeptiman Health Centres (50), Goudji Health Centres (50), N'gueli Health Centres (50) and Boutalbagar Health Centres (50).
2.4. Data collection method
A structured questionnaire was used to collect data. The questionnaire was divided into two parts: socio-demographic characteristics (age, employment status, marital status, type of marriage) and risk behaviours (blood transfusion, history of surgery, history of hospitalization, HBsAg+ mother, family history of HBV infection, history of transcutaneous procedure like acupuncture, genital mutilation, scarification, drug use, history of HBV testing, prison stay and unprotected sex).
2.5. Sample collection
After obtaining informed consent and completing the questionnaire, a 5 ml blood sample was drawn. Blood samples were given a unique identification number and centrifuged at 3000 rpm for 15 minutes. Sera were aliquoted into two parts, one for serology and one for molecular analysis. Both aliquots were frozen at -20°C in the biobank laboratory of the National Referral Hospital for one year before being sent to the Institut de Recherche en Santé, de Surveillance Epidémiologique et de Formation (IRESSEF) in Senegal for further serological and molecular testing.
2.6. Laboratory procedure
2.6.1. Serological tests
All serum samples were tested for the presence of hepatitis B surface antigen (HBsAg) using the SD Bioline HBsAg immunochromatographic test (ICT). This is a qualitative test for the rapid detection of HBsAg with very good intrinsic values, i.e., sensitivity and specificity of 100%, according to the manufacturer. The tests were performed according to the manufacturer's instructions (15). HBsAg status was confirmed using the Abbott Architect i1000SR analyzer (Abbott Diagnostics, Abbott Park, IL, USA).
2.6.2. Extraction and quantification of HBV DNA
HBV DNA was extracted from serum using the MagMAXTM Viral / Pathogen II Nucleic Acid Kit on a Thermo Scientific™ KingFisher™ Flex automated system according to the manufacturer's instructions. HBV DNA was eluted from 200 µl of serum into 50 µl of elution buffer. Real-time quantitative polymerase chain reaction (qPCR) was performed on Amplix-NG-48 using the GeneProof Hepatitis B Virus (HBV) PCR Kit with a lower limit of quantification of 13.9 IU/ml (16) according to the manufacturer's instructions.
2.7. Data analysis
Data were entered into an Excel database and analyzed using R version 4.1.2 (2021-11-01) software. Descriptive analysis and logistic regression were used to examine the association between dependent variables, sociodemographic characteristics and risk factors. Quantitative variables were expressed as mean ± standard deviation, and categorical variables were expressed as numbers (percentages). An independent t-test was used to compare the means of continuous variables. When normal distribution or equality of variance could not be assumed, the Mann-Whitney U test was used. The chi-squared test was used to compare proportions. In both cases, the significance level was set at p < 5%.
Logistic regression analysis was used as a multivariate analysis to identify factors associated with HBV serological status in pregnant women. Adjusted odds ratios (AORs) were calculated to measure the degree of dependence between the dependent and independent variables after controlling for all other variables. All variables that were less than 30% significant (i.e. with a p-value < 30%) in the univariate analysis were included in the initial multivariate logistic regression model. The forced variables, i.e. age and sex, were also included in the multivariate analysis even though they did not have a p-value < 30% in the univariate analysis. Logistic regression was then performed to obtain the final multivariate model. Variables were selected according to the statistical selection criteria of the descending stepwise procedure. The final model was selected by excluding the non-significant variables, using Akaike's information criterion as an index of parsimony. Variables with a p-value < 5% were considered statistically significant. A 95% confidence interval was calculated for all odds ratios.
3. Results
3.1 Sociodemographic characteristics
A total of 458 pregnant women were included in the study. The mean age was 25 years (95% CI: 20-30), and more than half of the women (51.09%) were aged between 21 and 30 years. Of the 458 participants, 454 (99.12%) were married, of which 341 (74.45%) were from a monogamous household. Several occupations were represented, the majority being housewives (274, 59.82%), followed by students (52, 11.35%). Regarding their HBV serology, 73.58% of the participants did not know their status beforehand (Table 1).
Table 1: Socio-demographic characteristics of pregnant women
|
Variable |
Categories |
Frequency |
Percent (%) |
|
Age |
15-20 |
134 |
29,25 |
|
21-30 |
234 |
51,09 |
|
|
31-40 |
81 |
17,68 |
|
|
41-50 |
9 |
1,96 |
|
|
Job status |
Employee |
48 |
10,48 |
|
Housewife |
274 |
59,82 |
|
|
Informal |
47 |
10,26 |
|
|
Pupil |
52 |
11,13 |
|
|
Student |
37 |
8,07 |
|
|
Marital status |
Married |
454 |
99,12 |
|
Not married |
4 |
0,87 |
|
|
Type of marriage |
monogamous |
341 |
74,45 |
|
polygamous |
117 |
25,54 |
|
|
Site |
AHC |
50 |
10,91 |
|
BHC |
50 |
10,91 |
|
|
GHC |
50 |
10,91 |
|
|
NHC |
50 |
10,91 |
|
|
AVH |
100 |
21,83 |
|
|
GH |
50 |
10,91 |
|
|
MCH |
58 |
12,66 |
|
|
NDAH |
50 |
10,91 |
|
|
History of HBV screening |
No |
337 |
73,58 |
|
Yes |
121 |
26,41 |
AVH: Assiam Vantou Hospital, MCH: Mother and Child Hospital, GH: Guinebor Hospital, NDAH: Notre Dame des Apôtres Hospital, AHC: Ardeptiman Health Centres, GHC: Goudji Health Centres, NHC: N'gueli Health Centres and BHC: Boutalbagar Health Centres
3.2. Prevalence of HBV among pregnant women
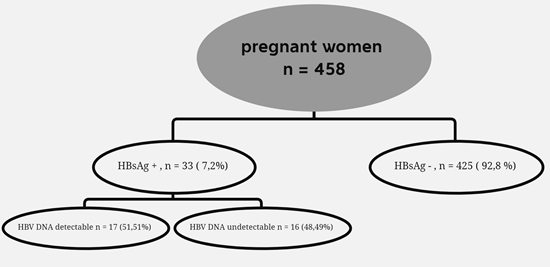
Thirty-three (33) of the 458 participants tested positive for HBsAg, giving an overall prevalence of 7.2% (95% CI 5.0 - 9.9). Results presented in Table 2 show that the prevalence of HBV infection was 44% in women whose mothers were HBsAg positive and 6.1% in women whose mothers were HBsAg negative (p = 0.003). The prevalence of HBV infection was 16% in women with a history of HBV testing versus 4.2% in women without a history of HBV testing (p <0.001) (Table 2).
Table 2: Estimated prevalence of HBsAg
|
Variable |
Category |
HBsAg - n (%) |
HBsAg + n (%) |
Total |
P |
|
Overall prevalence |
425(92.7) |
33(7.2) |
458 (100) |
2.2 10-16 |
|
|
Type of marriage |
Monogamous |
317 (93) |
24 (7.0) |
341 (74. 45) |
0.8 |
|
Polygamous |
108 (92) |
9 (7.7) |
117 (25.54) |
||
|
History of HBV |
No |
323 (96) |
14 (4.2) |
337 (73.58) |
<0.001 |
|
screening |
|||||
|
Yes |
102 (84) |
19 (16) |
121 (26.41) |
||
|
Blood transfusion |
No |
387 (93) |
28 (6.7) |
415 (90.61) |
0.2 |
|
Yes |
38 (88) |
5 (12) |
43 (9.38) |
||
|
History of surgery |
No |
387 (93) |
30 (7.2) |
417 (91.04) |
>0.9 |
|
Yes |
38 (93) |
3 (7.3) |
41 (8.95) |
||
|
History of hospital admission |
No |
358 (93) |
25 (6.5) |
383 (83.62) |
0.2 |
|
Yes |
67 (89) |
8 (11) |
75 (16.37) |
||
|
HBsAg + mother |
No |
306 (94) |
20 (6.1) |
326 (71.17) |
0.003 |
|
Yes |
5 (56) |
4 (44) |
9 (1.96) |
||
|
Family history of |
No |
274 (93) |
21 (7.1) |
295 (64.41) |
0.8 |
|
HBV infection |
|||||
|
Yes |
19 (90) |
2 (9.5) |
21 (4.58) |
||
|
History of |
No |
421 (93) |
33 (7.3) |
454 (99.10) |
>0.9 |
|
Transcutaneous procedure |
|||||
|
Yes |
3 (100) |
0 (0) |
3 (0.65) |
||
|
Acupuncture |
No |
423 (93) |
33 (7.2) |
456 (99.56) |
>0.9 |
|
Yes |
2 (100) |
0 (0) |
2 (0.43) |
||
|
Genital mutilation |
No |
299 (92) |
26 (8.0) |
325 (70.96) |
0.3 |
|
Yes |
126 (95) |
7 (5.3) |
133 (29.03) |
||
|
Scarification |
No |
306 (92) |
27 (8.1) |
333 (72.70) |
0.2 |
|
Yes |
119 (95) |
6 (4.8) |
125 (27.29) |
||
|
Prison stay |
No |
422 (93) |
32 (7.0) |
454 (99.12) |
0.3 |
|
Yes |
3 (75) |
1 (25) |
4 (0.87) |
||
|
Drug use |
No |
423(92.76) |
33(7.2) |
456(99.56) |
|
|
Yes |
2(100) |
0(0) |
2(0.43) |
||
|
Unprotected sex |
No |
28 (97) |
1 (3.4) |
29 (6.33) |
0.7 |
|
Yes |
397 (93) |
32 (7.5) |
429 (93.66) |
3.3. Viral load in HBsAg positive pregnant women
HBV DNA was detectable in 17 of the 33 HBsAg positive cases. The median viral load among those with detectable HBV DNA was 11.375 IU/ml. Of the 17 cases with detectable viral DNA, 64.70% had a viral DNA titre of less than 200.000 IU/ml and 35.29% had a viral DNA titre of >200.000 IU/ml (Table 3).
Table 3: Viral load in HBV-positive pregnant women
|
Viral Load |
Frequency |
Percent (%) |
|
<10.000UI/ml |
8 |
47.07 |
|
10.000–200.000 UI/ml |
3 |
17.64 |
|
>200.000 UI/ml |
6 |
35.29 |
3.4. Factors associated with HBV infection among pregnant women.
In the univariate regression analysis, the following variables: age, blood transfusion, occupational status, genital mutilation, scarring, HBsAg+ mother, prison stay, history of HBV testing and history of hospital admission were identified as candidate variables for multivariate analysis. Multivariate regression showed that having an HBsAg positive mother (AOR: 20.70 95%CI: 3.63-117.05, p=0.001) and a history of HBV testing (AOR: 4.3395%CI: 1.81-10.56, p=0.001) were the risk factors associated with HBsAg positivity (Table 4).
Table 4: Factors associated with HBV status of study participants
|
Variables |
Categories |
Univariate regression |
Multivariate regression |
||
|
COR (95% CI) |
P |
AOR (95% CI) |
P |
||
|
Age |
1.00 (0.95-1.06) |
0.905 |
- |
||
|
15-20 |
- |
- |
|||
|
21-30 |
1.09 (0.48-2.62) |
0.843 |
0.55 (0.20-1.56) |
0.249 |
|
|
31-40 |
0.91 (0.27-2.75) |
0.876 |
0.37 (0.09-1.43) |
0.159 |
|
|
41-50 |
3.97 (0.54-19.59) |
0.114 |
1.58 (0.19-9.46) |
0.637 |
|
|
Job status |
Employee |
- |
- |
||
|
Housewife |
0.34 (0.13-0.93) |
0.027 |
0.49 (0.15-1.64) |
0.239 |
|
|
Informal |
0.54 (0.13-1.94) |
0.36 |
1.23 (0.26-5.22) |
0.784 |
|
|
pupil |
0.36 (0.07-1.38) |
0.155 |
0.43 (0.07-2.29) |
0.336 |
|
|
Student |
0.71 (0.17-2.56) |
0.609 |
0.32 (0.05-1.73) |
0.212 |
|
|
Marital status |
Married |
- |
- |
||
|
Not married |
0.00 (NA-08.00) |
0.991 |
- |
||
|
Matrimonial regime |
Monogamous |
- |
- |
||
|
Polygamous |
1.10 (0.47-2.36) |
0.813 |
- |
||
|
History of HBV screening |
No |
- |
- |
||
|
4.30 (2.09-9.03) |
<0.001 |
4.33 (1.81-10.56) |
0.001* |
||
|
Transfusion |
No |
- |
- |
||
|
Yes |
1.82 (0.59-4.63) |
0.245 |
2.03 (0.61-5.80) |
0.21 |
|
|
Surgery |
No |
- |
- |
||
|
Yes |
1.02 (0.24-3.04) |
0.977 |
- |
||
|
History of hospital admission |
No |
- |
- |
||
|
Yes |
1.71 (0.70-3.80) |
0.21 |
1.42 (0.53-3.46) |
0.461 |
|
|
HBsAg positive mother |
No |
- |
- |
||
|
Yes |
12.24 (2.84-49.87) |
<0.001 |
20.70 (3.63-117.05) |
<0.001* |
|
|
Family history of hepatitis B |
No |
- |
- |
||
|
Yes |
1.37 (0.21-5.18) |
0.683 |
- |
||
|
Acupuncture |
No |
- |
- |
||
|
Yes |
0.00 (NA-.00) |
0.99 |
- |
||
|
Excision |
No |
- |
- |
||
|
Yes |
0.64 (0.25-1.43) |
0.307 |
3.14 (0.14-24.66) |
0.341 |
|
|
scarification |
No |
- |
- |
||
|
Yes |
0.57 (0.21-1.33) |
0.228 |
0.21 (0.02-5.06) |
0.227 |
|
|
Prison stay |
No |
- |
- |
||
|
Yes |
4.40 (0.21-35.45) |
0.205 |
- |
||
|
Unprotected sex |
No |
- |
- |
||
|
Yes |
2.26 (0.46-40.88) |
0.431 |
- |
||
|
History of drug injection |
No |
- |
- |
||
|
Yes |
0.00 (NA-0.00) |
0.99 |
- |
||
4. Discussion
In the present study, the seroprevalence of HBV infection in pregnant women was 7.2% (95% CI, 5.0 - 9.9). According to the WHO classification, this prevalence might be considered as an intermediate rate (2-7%) [17]. Interestingly, among the 33 positives pregnant women, 17 were viremic, but HBV DNA was > 200 000 UI only in 6 (35.29%) of them, indicating probably a rather low risk of MTCT in this population.
The prevalence of HBsAg found in our study is lower than that reported among pregnant women attending the Guelendeng health district in the province of Mayo-Kebbi East in Chad (13%) [18]. This difference could be due to the characteristics of the study population. Women living in urban areas are likely to have fewer risk factors than those living in rural areas, because they might have a higher level of education. It suggests that rural areas should also be the targeted in future studies and should benefit also from HBV awareness, screening and vaccination campaigns. Our results are comparable to recent studies in pregnant women in the Wolaita zone of southern Ethiopia (7.3% (95% CI: 5-9)) [19] and in Yaoundé, Cameroon (7.7%) [20]. In contrast, HBV seroprevalence in our study is lower than that reported in Northern Cameroon (20%) [21] and Southern Sudan (11%) [22], regions of political-military instability and weakened health systems. On the other hand, HBV seroprevalence in our study is higher than that reported in studies conducted in Eritrea [23], and Egypt [24] (3.2% and 5% respectively).These differences may be explained by local/national strategies to prevent [25].
In our study, 73.58% of participants did not know their HBV status. This observation corroborates the data reported by Gebreerkos in Ethiopia, who reported that 85.87% of pregnant women had never been screened for HBV [26]. The lack of financial resources and of national prevention strategies, together with a lack of knowledge about hepatitis B prevention and treatment among health professionals involved in the care of pregnant women may explain these results.
While routine screening for hepatitis B is an important means of preventing mother-to-child transmission of HBV, early detection of infection in women would also encourage vaccination of family members. Currently, there is no policy for routine HBsAg testing of pregnant women unless they pay for it. Thus, there is a need to introduce a policy of systematic and free screening of pregnant women, at the antenatal visit, with follow-up in case of HBsAg positivity.
HBV DNA was detectable in 51.51% of pregnant women with a positive HBsAg test, and 35.29% had a viral load >200 000UI/ml. The higher the viral load, the greater the risk of infants being infected and the more likely the disease becomes chronic. The high viral load increases the risk of maternal-fetal transmission by up to 90% [25]. This result demonstrates the importance of performing HBeAg testing and, if possible, HBV DNA quantification in HBsAg positive mother [13], to assess the risk of HBV MTCT, especially in our country. Testing for HBeAg and quantifying HBV load during pregnancy not only allows, in case of HBeAg positivity or high viral load to perform birth dose HBV vaccine, but also, if possible, maternal antiviral treatment during the third trimester of pregnancy, when viremia is high (>200 000UI/ml) [6, 25].
In our study, having an HBsAg+ mother was associated with HBV infection, confirming the real risk of MTCT in sub-Saharan Africa. Forty to fifty percent of chronic infections worldwide are due to MTCT [7]. As underlined before, the risk of vertical transmission of HBV is much higher if the mother is HBeAg positive or has a high HBV viral load [12, 27]. Eighty five percent of HBV infected children born to HBeAg-positive mothers become chronic carriers [28], compared with 5-10% of those exposed as adults [29]. Unfortunately, in this study, we did not have the opportunity to perform HBeAg.
We also found that prior screening was independently associated with hepatitis B surface antigen carriage (p=0.001). Sixteen per cent of the pre-screened participants tested positive. A similar result was reported by Halatoko in Togo, who reported a statistical association between knowledge of HBV status and hepatitis infection (p<0.001) [30]. This means that pregnant women were informed during the antenatal consultation of their previous HBV status and may have asked for confirmation. However, in Chad, after screening, no further tests (HBeAg, viral load) are performed. Pregnant women with financial means may be able to buy the vaccine for their baby if they are informed. In addition, in the case of HBsAg positivity, they will not be referred to a hepatologist, and will not have an assessment of their liver disease, i.e. liver tests, and, nor of appropriate monitoring and treatment.
In this study, risk factors such as unprotected sex, history of surgery, long hospital stay, excision, scarification, acupuncture were not associated with HBV infection. Our findings are consistent with those of Mudardum and Mohammed in Sudan [31], Bayo et al in Ouangda [32], Umare et al [33] and Roble et al. [34] in Ethiopia , who found not a statistically significant association between surgical procedure and family history of HBV [32, 35]. Population-based studies are needed to determine the number of chronic HBsAg carriers in the population and to identify risk factors for HBV infection on a national scale.
5. Conclusion
Despite the introduction of a vaccination program against hepatitis B in the EPI, this disease remains a public health problem in Chad, like in other countries in Africa (24). In the present study, our results show that HBV seroprevalence (7.2%) is in the intermediate range according to the WHO classification. More than 30% of women with a positive HBsAg status had a high viral load, which implies a high risk of HBV transmission to newborns. This risk is even higher in women who do not know their serological status. However, HBV MTCT can be prevented by simple measures such as developing and implementing a national strategy for systematic, integrated and free HBsAg screening of pregnant women, including additional testing for maternal HBeAg and HBV viral load and ALT levels in pregnant women with chronic hepatitis B infection, as recommended [7]. It is also needed to build the capacity of health workers to assess and manage people with hepatitis B, vaccinating newborns, monitoring EPI (including HBV valence) and treating the mother if possible.
List of abbreviations
CI: confidence interval, CMIA: chemiluminescent microparticle immunoassay, EPI: Expanded Programme on Immunization, HCC: hepatocellular carcinoma, HBeAg: Hepatitis e antigen; HBsAg: Hepatitis B Surface Antigen; HBV: Hepatitis B Virus; mother-to-child transmission (MTCT) Prevention of mother-to-child transmission (PMTCT), WHO: World Health Organization.
Approval and Consent statements
This study was approved by the National Bioethics Committee of Chad on 8 November 2020 (201/PR/MESRI/DG/CNBT/2020), by the Research Ethics Committee of UCAD on 7 December 2021 (CER /UCAD/AD/MSN/050/2020) and by the Ministry of Public Health and National Solidarity on 18 February 2021 (Nº294/PR/MSPSN/SE/DGM/DGTPC /DPERO2021). Informed consent was obtained from all participants in accordance with the approval of the National Bioethics Committee of Chad and the Research Ethics Committee of UCAD. The objectives of the study were explained to the participants. A unique anonymous study identification number was assigned to each participant. Each participant was informed of the results of their serology and advised of the action to be taken.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Funding
No funding was obtained for this study.
Author’s contributions
ND, AMM, NCTK and FLF designed the concept of the study. ND and BLM collected the data. ND, BLM, AJSN, NLD, GL and AM wrote the protocol and wrote the first draft of the manuscript. MO and MJ conducted the laboratory analysis. ND managed the literature searches. Critical revision of the manuscript FLF. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank all the people involved in the study, especially the laboratory technicians at the Hôpital de la paix in N'Djamena, the managers of the health facilities and the pregnant women who participated in the study. We would also like to thank all the data.
References
- Cacoub P, Asselah T. Hepatitis B Virus Infection and Extra-Hepatic Manifestations: A Systemic Disease. Am J Gastroenterol 1 févr 117 (2022): 253-63.
- Rizzo GEM, Cabibbo G, Craxì A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses. Mai 14 (2022): 986.
- Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. Janv 70 (2019): 151-71.
- GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. Sept 7 (2022): 796-829.
- 91 million Africans infected with Hepatitis B or C [Internet]. WHO | Regional Office for Africa (2023).
- Breakwell L, Tevi-Benissan C, Childs L, Mihigo R, Tohme R. The status of hepatitis B control in the African region. Pan Afr Med J 17 (2017).
- Spearman CW, Afihene M, Ally R, Apica B, Awuku Y, Cunha L, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol 2 (2017): 900-9.
- INSEED-TCHAD - INSEED-TCHAD ANNUAIRE STATISTIQUE SANITAIRE.
- Suesstrunk J, Djongali FB. Hepatitis B virus prevalence in rural areas in south-west Chad. Trop Doct 47 (2017): 374-7.
- Yi P, Chen R, Huang Y, Zhou RR, Fan XG. Management of mother-to-child transmission of hepatitis B virus: Propositions and challenges. J Clin Virol Off Publ Pan Am Soc Clin Virol 77 (2016): 32-9.
- Keane E, Funk AL, Shimakawa Y. Systematic review with meta-analysis: the risk of mother-to-child transmission of hepatitis B virus infection in sub-Saharan Africa. Aliment Pharmacol Ther 44 (2016): 1005-17.
- Shimakawa Y, Veillon P, Birguel J, Pivert A, Sauvage V, Guillou-Guillemette HL, et al. Residual risk of mother-to-child transmission of hepatitis B virus infection despite timely birth-dose vaccination in Cameroon (ANRS 12303): a single-centre, longitudinal observational study. Lancet Glob Health 10 (2022): e521-9.
- Boucheron P, Lu Y, Yoshida K, Zhao T, Funk AL, Lunel-Fabiani F, et al. Accuracy of HBeAg to identify pregnant women at risk of transmitting hepatitis B virus to their neonates: a systematic review and meta-analysis. Lancet Infect Dis 21 (2021): 85-96.
- Liu Z, Chen Z, Cui F, Ding Y, Gao Y, Han G, et al. Management Algorithm for Prevention of Mother-to-child Transmission of Hepatitis B Virus (2022). J Clin Transl Hepatol 2022 (10): 1004.
- SD BIOLINE HBsAg. GENTAUR ONLINE.
- Aurea s.r.o VA. GeneProof Hepatitis B Virus (HBV) PCR testing.
- Maddrey WC. Hepatitis B: an important public health issue. J Med Virol 61 (2000): 362-6.
- Nguwoh P, Kamga Wouambo R. Associated risk factors with seroprevalence of HIV and HBV co-infection among Pregnant women attending guelendeng health District in the Mayo-kebbi East Region of Chad. Int J Pregnancy Childbirth 6 (2020): 155-60.
- Bancha B, Kinfe AA, Chanko KP, Workie SB, Tadese T. Prevalence of hepatitis B viruses and associated factors among pregnant women attending antenatal clinics in public hospitals of Wolaita Zone, South Ethiopia. PLOS ONE 15 (2020): e0232653.
- Fomulu NJ, Morfaw FL, Torimiro JN, Nana P, Koh MV, William T. Prevalence, correlates and pattern of Hepatitis B among antenatal clinic attenders in Yaounde-Cameroon: is perinatal transmission of HBV neglected in Cameroon? BMC Pregnancy Childbirth 13 (2013): 158.
- Ducancelle A, Abgueguen P, Birguel J, Mansour W, Pivert A, Le Guillou-Guillemette H, et al. High Endemicity and Low Molecular Diversity of Hepatitis B Virus Infections in Pregnant Women in a Rural District of North Cameroon. PLoS ONE 8 (2013): e80346.
- Kirbak ALS, Ng’ang’a Z, Omolo J, Idris H, Usman A, Mbabazi WB. Sero-prevalence for Hepatitis B virus among pregnant women attending antenatal clinic in Juba Teaching Hospital, Republic of South Sudan. Pan Afr Med J 26 (2017): 72.
- Fessehaye N. Prevalence of Hepatitis B Virus Infection and Associated Seromarkers among Pregnant Women in Eritrea 6 (2018).
- Kishk R, Mandour M, Elprince M, Salem A, Nemr N, Eida M, et al. Pattern and interpretation of hepatitis B virus markers among pregnant women in Northeast Egypt. Braz J Microbiol 51 (2020): 593-600.
- El-Karaksy HM, Mohsen LM, Saleh DA, Hamdy MS, Yassin NA, Farouk M, et al. Applicability and efficacy of a model for prevention of perinatal transmission of hepatitis B virus infection: Single center study in Egypt. World J Gastroenterol WJG 20 (2014): 17075-83.
- Gebrecherkos T, Girmay G, Lemma M, Negash M. Knowledge, Attitude, and Practice towards Hepatitis B Virus among Pregnant Women Attending Antenatal Care at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Int J Hepatol (2020): 5617603.
- Huang P, Zhu LG, Zhu YF, Yue M, Su J, Zhu FC, et al. Seroepidemiology of hepatitis B virus infection and impact of vaccination. World J Gastroenterol 21 (2015): 7842-50.
- Lamberth JR, Reddy SC, Pan JJ, Dasher KJ. Chronic hepatitis B infection in pregnancy. World J Hepatol 7 (2015): 1233-7.
- Xu DZ, Yan YP, Choi BCK, Xu JQ, Men K, Zhang JX, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. mai 67 (2002): 20-6.
- Halatoko WA, Patassi A, Yanogo P, Banla LI, Koba A, Issa Z, et al. Risk factors of hepatitis B virus surface antigen carriage and serological profile of HBsAg carriers in Lomé Togo, 2016. BMC Public Health 19 (2019): 32.
- Mudardum AH, Mohammed AA. Prevalence and Risk Factors for Hepatitis B Infection among Pregnant Women attending Antenatal Clinic in UM Dafog Area, South Darfur State, Sudan. Sudan J Med Sci SJMS.
- Bayo P, Ochola E, Oleo C, Mwaka AD. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care: a cross-sectional study in two hospitals in northern Uganda. BMJ Open 4 (2014): e005889.
- Umare A, Seyoum B, Gobena T, Haile Mariyam T. Hepatitis B Virus Infections and Associated Factors among Pregnant Women Attending Antenatal Care Clinic at Deder Hospital, Eastern Ethiopia. PLoS ONE 11 (2016): e0166936.
- Roble AK, Roba KT, Mengistie B, Abdurke Kure M. Seroprevalence of Hepatitis B Virus and Associated Factors Among Pregnant Women Attending Antenatal Care in Public Health Facilities in Jigjiga Town, Eastern Ethiopia. Int J Womens Health 12 (2021): 1299-310.
- Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep 54 (2005): 1-31.