Prevalence of Antigens/Antibodies Against Hepatitis B and C Viruses in A Cohort of Italian Patients with Pancreatic Adenocarcinoma Admitted to Two Hospital Wards in Italy: A Pivotal Retrospective Study
Article Information
Sirio Fiorinoa*, Maddalena Zippib, Claudia Beninic, Angelo Luca De Quartod, Michele Masettic, Maria Federica Lerroc, Andrea Lazzaric, Raffaele Lombardic, Matteo Zanelloc, Laura Mastrangeloc, Silvia Aldrovandic, Giuseppina De Sarioc, Mario Chisaric, Laura Dovac, Nicosia Simonec, Giulia Ciabattic, Giorgia Acquavivae, Michela Visanie, Adele Fornellif, Andrea Turag, Paolo Emilio Orlandih, Giuseppe Occhigrossib, Paolo Leandria, Francesca Ballarinii,j, Silva Bortolussii, Annalisa Pessionl, Leonardo Rascitim, Enrico Giampierin, Ivan Corazzan, Maria Letizia Bacchi-Reggianin, Dario de Biasel, Elio Jovinec
aInternal Medicine Unit C, Azienda USL-Maggiore Hospital, Bologna, Italy
bUnit of Gastroenterology and Digestive Endoscopy, Sandro Pertini Hospital, Rome, Italy
cSurgery Unit, Azienda USL-Maggiore Hospital, Bologna, Italy
dOncology Unit, Sandro Pertini Hospital, Rome, Italy
eDepartment of Medicine (Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale), University of Bologna, Azienda USL di Bologna, Bologna. Italy
fAnatomic Pathology Unit, Azienda USL-Maggiore Hospital, Bologna, Italy
gCNR Institute of Neuroscience, Padova, Italy
hRadiology Unit, Azienda USL-Maggiore Hospital, Bologna, Italy
iDepartment of Physics, University of Pavia, Pavia, Italy
jINFN (National Institute of Nuclear Physics), Pavia, Italy
lDepartment of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy
mInternal Medicin Unit, Budrio Hospital, Italy
nExperimental, Diagnostic and Specialty Medicine Department, University of Bologna, Italy
*Corresponding Author: Sirio Fiorino, U.O.S.D. di Medicina Interna C, Ospedale Maggiore Largo Nigrisoli (Bologna), 40133 Bologna, Italy
Received: 16 August 2019 Accepted: 01 September 2019 Published: 09 September 2019
Citation: Sirio Fiorino, Maddalena Zippi, Claudia Benini, Angelo Luca De Quarto, Michele Masetti, et al.. Prevalence of Antigens/Antibodies Against Hepatitis B and C Viruses in A Cohort of Italian Patients with Pancreatic Adenocarcinoma Admitted to Two Hospital Wards in Italy: A Pivotal Retrospective Study. Archives of Microbiology & Immunology 3 (2019): 107-132.
View / Download Pdf Share at FacebookAbstract
Background/Objectives: Pancreatic adenocarcinoma (PAC) is a disease with a poor prognosis. Hepatitis B (HBV)/Hepatitis C (HCV) viruses are hepatotropic pathogens with pro-carcinogenic properties able to attack also the pancreas. Although several trials, mainly carried out in the USA and in the Eastern Countries, strongly suggested that HBV/HCV exert a role in PAC development, no study on this topic was still performed in Italy. Through this present work, we aimed to assess HBV antigens/antibodies and anti-HCV antibodies prevalence in a small cohort of Italian patients with PAC, irrespective of the other risk factors for PAC development, like smoking, alcohol drinking, and diabetes.
Methods: This pivotal-retrospective-study was led both at Surgery Unit of Maggiore Hospital, (Bologna) and at Unit of Gastroenterology and Digestive Endoscopy of Sandro Pertini Hospital, (Rome). Data concerning age, sex, pancreatic cancer localization (head, body, tail) and serum HBV/HCV profiles of subjects with a histological/radiological/biochemical diagnosis of PAC were collected from files concerning pancreatectomy and endoscopic-retrograde-cholangiopancreatography (ERCPs).
Results: It was found that 4 patients were HBsAg positive and 28 were HBsAb/HBcAb-positive, with a prevalence equal to 1% and 7.5%, respectively. Sixteen patients were HCV positive, with a prevalence equal to 4.3%.
Conclusions: Our observational study describes, for the first time in our Country, HBsAg, HBsAb/HBcAb and HCV prevalence in a small-sized cohort of patients suffering from PAC. Despite no definitive conclusions on the association between HBV/HCV infection and PAC may be drawn, our research could represent the basis for additional epidemiological/histological nationwide trials in Italy.
Keywords
HBV; HCV; pancreatic adenocarcinoma;
Hepatitis viruses; virus
Article Details
1. Introduction
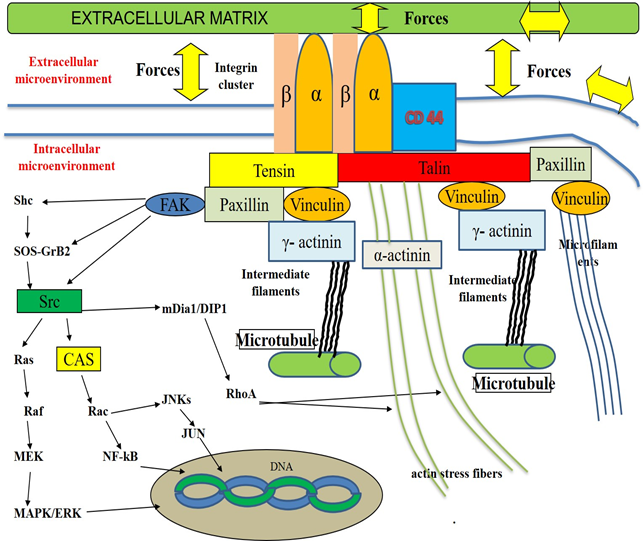
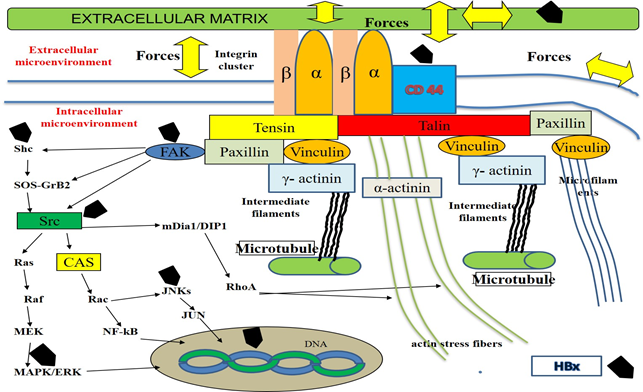
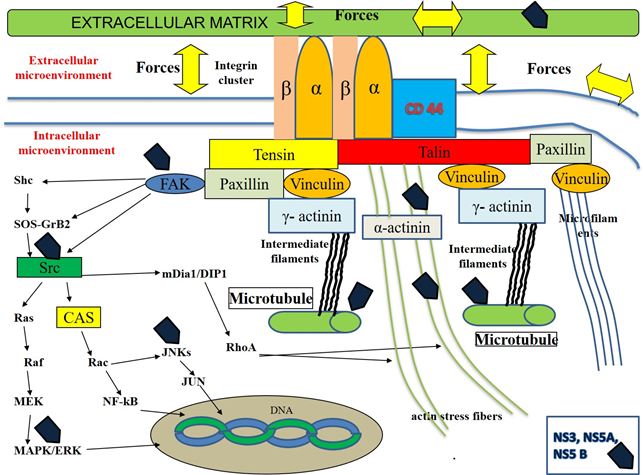
Pancreatic adenocarcinoma (PAC) is one of the most aggressive human malignancy entailing a very dismal prognosis [1], with about 459,000 cases and 432,000 deaths worldwide in 2018, according to the Global cancer statistics estimates [2]. Despite the improvement in the understanding of molecular mechanisms and events involved in the development of this neoplasm, the overall five-year survival is < 5%. Therefore, this neoplasm ranks as the 14th most common malignancy and the 7th highest cause of tumor-related mortality in the world. The poor prognosis of PAC depends on several factors, such as its aggressive biological behavior, its early ability to spread locally as well as to metastasize at distance and its asymptomatic course in the initial stages of carcinogenesis [3]. Despite several efforts in the past and even in the last times, the diagnosis of this cancer is very often performed at an advanced stage, when the available therapeutic options, both surgical and pharmacological ones, are ineffective or useless. At the time of diagnosis, most PACs have already disseminated beyond the pancreas [4, 5] and recurrence rates of this tumor are very high (about 85%), even if a curative resection is performed [6]. Only a few risk factors are known to be associated with its development, including smoking, chronic pancreatitis, familial cancer syndromes, diabetes and alcohol [7]. Since a substantial enhancement in PAC incidence was progressively observed over recent years and it is still expected to increase in the next decades, at least in the most developed Countries for several reasons [8], the identification of modifiable or treatable risk factors of this severe and lethal disease has become a pressing need [9]. An approach intended to the development of screening programs, targeted at high-risk people for prevention or early detection of PAC is strongly required, like this strategy, if adopted, could contribute to increase survival rates and improve the quality of life of people suffering from this pathology. In our clinical and scientific study, we mean to evaluate the possible factors associated with pancreatic carcinogenesis. As multiple pieces of evidence from epidemiological and basic research studies suggest that about 15-20% of all malignancies are somehow linked to a viral infection [10], in the last years we have turned our attention to investigate the potential role of Hepatitis B (HBV) and C (HCV) viruses in PAC development. These hepatotropic pathogens own well-known carcinogenic properties for the liver, but their antigens and genomes may be detected also in extra-hepatic tissues, including the pancreas. HBV antigens and genome, as well as intermediate HCV genome replication forms, have been found in exocrine and endocrine pancreatic cells [11-13]. Taking advantage of these evidence, in the last years, some studies have been carried out with the aim to investigate the possible mechanisms involved in the processes of pancreatic carcinogenesis. Several reports have shown the possible intra- and extra-cellular targets of some viral proteins, including Hepatitis x protein (HBx) for HBV, non-structural 3 (NS3), 4 A (NS4A), 5A (NS5A) and 5B (NS5B) for HCV. Most of these binding sites have been shown in animal and human cell culture systems derived mainly from hepatocytes or hepatoma cell lines, but also from pancreatic cancer cells [14]. HBx, NS3, NS5A and NS5B have been demonstrated to dysregulate the expression profile of some cellular cytoskeletal genes [15] or to interact with distinct elements of cytoskeleton (microfilaments, microtubules, intermediate filaments and actin stress fibers) [16-18] of different elements of intracellular signalling pathways, like Rat-sarcoma/proto-oncogene serine/threonine-protein kinase/mitogen-activated protein kinases (Ras/Raf/MAPK) [19], proto-oncogene tyrosine-protein kinase Src (Src-) [20] and Focal adhesion tyrosine-kinase (FAK)-pathways [21-24] and of cell to cell and cell to extracellular-matrix adhesion molecules [25], such as Integrins [26, 27], E-Cadherin, β-catenin [28] and CD 44 [29, 30]. In addition, it has reported that HBx may directly modulate the activities of a lot of nuclear transcription factors, influencing their function. These events lead to a perturbation of cell and stroma composition as well as of their structure, shape, disposition and of their physical- and chemical-properties [31-34]. This complex series of modification induces deep deregulation of normal cell and extracellular matrix function and promotes the malignant transformation of the cells and of the matrix surrounding them. Some of these events are described in Table 1 and Figure 1, 2 and 3.
Table 1: Targets of HBV and HCV proteins, alteration in properties and in function of cells and extracellular matrix, with possible mechanisms leading to malignant transformation.
The scheme shows the cellular membrane, some intracellular micro-organelles (nucleus and microtubules), the focal adhesion molecules (integrin receptors and signaling proteins like talin, paxillin, vinculin, tensin, α-actinin and γ-actinin), the elements constitutive of intracellular cytoskeleton (actin stress fibers, intermediate filaments and microfilaments) and several components of the intracellular signaling cascades. According to Ingber's hypothesis, in normal conditions, several tightly-regulated mechanisms cooperate to maintain homeostasis within the extracellular and intracellular compartments and contribute to preserve their function. Cells with their microorganelles and cytoskeleton as well as the stroma with its constitutive elements, such as structural- (Collagens, Proteoglycans and Glycosaminoglycans) and non-structural-(Regulatory proteins like Matricellular Proteins) components surronding cells constitute a tensed network of elements. These components self-stabilize their structure, form, spatial disposition and activity throughout the generation of micro-tensional and micro-compressive forces. Cells sense the mechanical properties in the extracellular matrix via Integrins and offset these stimuli, by means of some responses including the transient rearrangement of cytoskeleton and the transitory modification of its tension. Integrins are involved in cell-matrix interactions, providing a bi-directional contact between extracellular- and intracellular microenvironments. A tightly regulated loop exists among the stroma components, the intracellular elements and the tensional/compressive forces generated in this microenvironment. In particular, according to the current knowledge, the direct or indirect action of the above-mentioned forces on the cell membranes and on the extracellular stroma causes the transient activation of focal adhesion structures in the cell membranes and the association of integrin receptor monomers (α and β) with the consequent clustering of the integrin receptors and the recruitment of the signalling proteins. These focal adhesion structures bind to cell actin cytoskeletal filaments, microfilaments, intermediate filaments and microtubules. This event induces the rearrangement of intracellular cytoskeleton, modifying its disposition and structure and, as consequence, modulating the activities of several intracellular microorganelles including microtubule, mitochondria, endoplasmic reticulum and nucleus as well as the function of several enzymatic transduction pathways in the cytoplasm such as c-Jun N-terminal Kinase (JNK), mitogen-activated protein-Kinase (MAPK), mitogen-activated protein kinase kinase kinase (MEK), extracellular signal-regulated kinase (ERK) and non-receptor tyrosine kynases family, known as Src Family Kinases (Src), Focal Adhesion Tyrosine-Kinase (FAK). The elements of these cascades are in direct or in indirect connection with focal adhesion molecules, via additional elements, including the components of the Rho Family of GTPases. Src may directly activate Ras, Raf, MEK, MERK, MAPK or indirectly, throughout the induction of Crk-associated substrate (CAS) and Rac. These events lead to the stimulation of JNK, JUN and several nuclear factors, including nuclear factor kB (NF-kB). In addition, ERK/MAPK pathways activity may be induced by FAK, by means of the involvement of several proteins, such as son-of sevenless (SOS) and growth factor receptor-bound-2 (GRB-2). In normal conditions, this very complex system is able to transfer stimuli from extracellular- to intracellular-compartments and to cause changes in both chemical and physical properties of intracellular microenvironment The stiffness of intracellular microorganelles is modified and this event is associated with changes in the activity of intracellular metabolic machinery, in the genome replication and transcription as well as in the processes of protein translation. Pathological processes, such as inflammation, may induce significant changes in the quali/quantitative composition of extracellular matrix, in its structure and in its chemical and physical properties. One of the most important effects of this event is generally represented by an increase of the extracellular matrix rigidity (stiffness) and of the whole tissue. The consequence of these processes is represented by an unbalanced stimulation of the enzimatic-pathways, by a dysregulated and persistent modification in the disposition and shape of nucleus, mitochondria, microtubules and endoplasmic reticulum with the alteration of cellular and stroma function and a chronic perturbation of homeostasis both at a microscopic- and macroscopic level. On the whole, this model may contribute to explain the process of pancreatic carcinogenesis.
In cells HBx may act both in the nuclear and cytoplasmic compartments. In nucleus HBx may stimulate the function of 7 genes, which encode several proteins with a regulatory role in the organization of distinct cytoskeletal elements. These components include: γ-actin, periplakin, keratin-8, keratin-18, microtubule tubulin-β2, tubulin-β3 and tubulin-β6. In cytoplasm, HBx is able to influence the ativity of: a) some cellular transcription factors, regulating the function of Src family kinase- and Ras-Raf-MAP kinase-paths; b) some adaptor proteins, like GRB-2, SOS and Shc, promoting the association of these elements; c) FAK with the stimulation of some intracellular proteins, including paxillin and α-actinin; d) some cellular membrane adhesion molecules, including CD44. The results of all these events is the disruption of the system of focal adhesion structures, via the Src-mediated phosphorilation of β-catenin and its subsequent detachment from E-cadherin and the down-regulation in the synthesis of wild-type β-catenin and E-cadherin. These changes are associated with the modification of the cytoskeleton architecture and of its tensional activity, with the alteration of cellular morphology, shape, motility, polarization and adhesion ability as well as of processes of energy production by mitochondria. Furthermore, the HBx-induced alteration of cellular nuclear factors causes the perturbation of the processes of cell DNA transcription and duplication. HBx is able to induce paracrine activation of and proliferation of stellate cells in extracellular matrix and promote the expression of genes encoding extra-cellular matrix proteins, like collagen type I.
In cells HCV may act both in the intracellular- (cytoplasm) and in the extracellular microenvironment. HCV-RNA synthesis requires the polimerization of microtubules and actin filaments. In cytoplasm HCV replication complexes directly interact with these components of cytoskeleton. NS3 and NS5A regulate this process. Furthermore, these proteins stimulate cytoplasmic FAK, Src, MAPK/ERK and JNKs pathway. In particular, the up-regulation of FAK modulates the activity of several proteins, which are involved in the regulation of important cellular function. NS3A, NS5A and NS5B may directly or indirectly interact with paxillin and α-actinin, promoting the dysregulation in the form, in the disposition and in the organization of cellular Tigh-Junctions. These events lead to the perturbation of the cellular homeostasis with very important effects on its morphology, shape, motility, polarization, adhesion ability as well as on the processes of energy production in the mitochondria and of genome transcription and duplication in the nucleus.. In addition, NS3A, NS5A and NS5B also promote the paracrine activation and proliferation of stellate cells in extracellular matrix and induce the expression of genes encoding extra-cellular matrix proteins, like collagen type I.
In addition to studies performed in pancreatic tissue, some epidemiological trials and meta-analyses have also suggested that HBV and HCV infections are associated with an increased risk of PAC occurrence. Before carrying out our research in this field, in June 2019, according to the methods used in our previous meta-analysis on the same topic [35], we performed a preliminary systematic review of available evidence concerning the association between HBV/HCV infection and risk of PAC development. We considered the papers published on this subject until the first quarter of 2019.
In particular, based on these criteria, we obtained the following results:
- concerning HBV infection and risk of PAC development, we identified 6 case-controls (in USA [36], Taiwan [37], Korea [38] and China [39-41]), 9 cohort studies (in Taiwan [42, 43], USA [44, 45], Australia [46, 47], Sweden [48], Denmark [49] and Japan [50]) and 7 meta-analysis (in China [51-55], USA [56] and Italy [35]). Most of case-controls, cohort trials and all meta-analyses registered an increased risk of PAC development;
- as regards HCV infection, 4 case-controls (in China [41], USA [36], Korea [38] and Taiwan [37]), 7 cohort studies (in USA [57, 58], Australia [46, 47], Denmark [59] , Sweden [48] and Japan [50]) and 3 meta-analyses (in China [54, 55] and in Italy [35]) were considered. By examining the most of case-control, cohort-studies and all meta-analyses a higher probability of PAC development emerged.
Since 2014 no additional meta-analyses about this topic were published and before most trials or meta-analyses were performed mainly in the USA, in China and in the South-Eastern Countries. Only three studies carried out in Europe are detectable in literature. To our knowledge, no data are available among the Italian population about the possible relationship occurring between HBV/HCV infection and the risk of PAC. Therefore, additional studies in Italy are needed to clarify this point. From an epidemiological point of view, the annual pancreatic cancer incidence in this Country is equal to 9.2/100,000 [60]. Prevalence of serum patterns of HBV markers and anti-HCV antibodies implied important changes in the general population in Italy, with a progressive reduction of their prevalence in the latest times. According to some recent surveys, the HBsAg prevalence in the Italians is estimated to be largely below 2%. This percentage decreased from 3-5% in the 1980s, settling down to about 0.8-1% in 2010 [61], so that, to date, Italy may be considered as a low HBV endemic Country, such as United States of America or Northern Europe [62], where most of the individuals with a previous contact with HBV show a serum pattern characterized by absence of HBsAg and presence of specific antibodies against two antigens of this pathogen agent represented by HBcAb and HBsAb. It is also well-known that coexistent serum HBsAb and HBcAb antibodies in some individuals, HBsAg-/HBcAb+/HBsAb- or HBsAg- /HBcAb+/HBsAb+ in association, are the evidence of a previous exposure to HBV, not necessarily related to an underlying vaccination. In the past, both patterns were considered to be specific markers of a complete recovery from a previous exposure to HBV. However, the progressive improvement of molecular biology techniques for the study of this pathogen afforded to demonstrate, in some circumstances, its persistence and replication at low levels even in subjects with HBsAg-/HBcAb+/HBsAb- or HBsAg- /HBcAb+/HBsAb+ patterns. This condition, characterized by the presence of HBV-DNA in liver tissue and its absence in serum [63], is defined as “occult” HBV infection and its clinical impact in the human pathology, as well as in pancreatic carcinogenesis, is still unknown.
Only a few trials were performed to calculate the percentages of isolated anti-HBs+ or anti-HBc+, as well as anti-HBs+/anti-HBc+, in Italian people. In a survey conducted on the general population in Northern Italy [64] their prevalence resulted to be 23.8%, 4.2% and 8.4%, respectively. In an analogous trial, performed in Southern Italy, HBcAb+ prevalence in general people dropped from 66.9% to 7.6% in a period ranging from 1978 to 2006 [65]. In both studies, the decline of HBsAg+ and anti-HBc+ was even more striking in younger individuals (aged from 15 to 24 years). On the other hand, in a recent study, the overall prevalence of anti-HCV antibodies in the Italian general people is estimated to range between 1% and 2.2-2.7% [66], although an existing wide variability, depending both on distinct geographical areas (about 1.6% in North, 2.6% in Centre and 2.4% in South Italy) and on different age classes, reaching the highest values in individuals born between 1935 and 1944 (until 7%), as well as in subjects born before the year 1935 (about 4.2%), regardless of the geographical areas in which they are settled [67]. Based on all these preliminary results and considerations, we first aimed to perform a retrospective pivotal work to assess HBV antigens/antibodies, as well as anti-HCV antibodies prevalence in a cohort of Italian patients with PAC admitted to two hospital wards in Italy, without taking into account the other well known risk factors for the development of PAC, such as smoking, alcohol drinking, and diabetes. Therefore, these variables have not been collected and included in our analysis. We have organized our data, stratifying our patients both in birth cohorts and in groups of age to assess the HBsAg, HBsAb/HBcAb and anti-HCV prevalence in these different classes, according to Fabris’ and Andriulli’s trials. These two Authors have evaluated the prevalence of HBV and HCV serum markers in two large Italian populations [64, 67].
Material and methods
Study Population
In this pivotal bi-Centre retrospective study we collected data of patients to which a primary diagnosis of PAC was made, consecutively admitted and treated in the following Units of two large-sized Italian Hospitals:
- Surgery Unit A, Azienda USL- Bologna, Maggiore Hospital, Bologna (Bologna group);
- Unit of Gastroenterology and Digestive Endoscopy, Sandro Pertini Hospital, Rome (small center with low case volume) (Rome group).
Sources included records of inpatients extracted from the pancreatectomy files and from the ERCP files available in Hospital Units in Bologna and in Rome, respectively.
This study was carried out in accordance with the ethical principles of the Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/).
In the Surgery Division A, Bologna, Maggiore Hospital, data concerning patients with a diagnosis of PAC were pulled from the pancreatectomy files, whose database includes 839 subject underwent, for several reasons, a surgical partial or total pancreatectomy in this Unit between January 1, 2005, and December 31, 2017. Inclusion criteria for this group were aged 16 years or older and PAC diagnosis, confirmed by histology on pancreatic surgical specimens.
In the Unit of Gastroenterology and Digestive Endoscopy, Sandro Pertini Hospital, Rome, data of individuals with a PAC diagnosis were collected from the ERCP File. This database includes 212 patients to whom was made an endoscopic retrograde cholangiopancreatography (ERCP), between January 1, 2014, and August 31, 2018, owing to various causes. The inclusion criteria for this group were: 16 years of age or older and PAC diagnosis, confirmed either on pancreatic microbiopsy specimens or diagnosed by symptoms, signs, biochemical tumor markers tests (CA 19.9) and at least two types of imaging tools, represented by abdominal Computed Tomography and Endoscopic Ultrasonography (EUS).
The parameters of data recording in the two groups were: age, sex, pancreatic cancer localization (head, body, tail) and serum HBV/HCV profiles (HBsAg, HBsAb/HBcAb, and HCVAb). Data on viral markers were withdrawn by medical records or by the archives of Laboratories in both Hospitals if the first ones were not available. Taking into account that HBV (antigens and antibodies), as well as anti-HCV antibodies prevalence, in Italian population is variable, depending on the different classes of age, we stratified our patients both in a birth cohort and in groups of age to assess the HBsAg, HBsAb/HBcAb and anti-HCV prevalence in them. According to the studies of Fabris and Andriulli, we first considered the following classes of age or birth cohort: 0-14, 15-24, 25-34, 35-44, 45-54. 55-64, 65-74, ≥75, and >1984, 1975-1984, 1965-1974, 1955-1964, 1945-1954, 1935-1944, <1935. Then we summarized our results in some synthetic tables, together with data on the prevalence of HBsAg, HBsAb/HBcAb and anti-HCV.
Continuous variables were expressed as mean ± standard deviation and compared to two sample t-test; categorical data were indicated as numbers (percentages) and p-value less than 0.05 was significant.
Results
The distribution of demographic characteristics and the selected variables of the patients analyzed are reported in Table 2.
|
Variables |
Total of 373 patients |
|
Age (years) |
68.2 ±10.1 |
|
Sex (pts: 373) Male Female |
200 (53.6%) 173 (46.4%) |
|
Cancer localization Head Body/tail Head + Body/Tail |
289 (77.5%) 67 (18.0%) 17 (4.5%) |
|
HBsAg Positive Negative |
4 (1.1%) 369 (98.9%) |
|
Both HBsAb/HBcAb positivity |
28/373 (7.5%) |
|
Anti-HCV Positive Negative |
16/373 (4.3%) 357/373 (95.7%) |
Table 2: Main characteristics of patients included in the study with PAC and HBV/HCV markers status.
In the Bologna group, among 839 patients included in the pancreatectomy files, we collected 311 patients with a proved histological diagnosis of PAC (37.1%). The remaining 528 patients, whose the mean age was 68.3±9.3 years, were not taken into account because suffering from other pathological conditions, including main biliary duct cancers, neuroendocrine pancreatic tumors, Vater’s papilla cancer, metastasis to pancreas from different sites (carcinomas of breast, of kidney and of colorectal, sarcoma in the lower limb).
In the Rome group, among 698 subjects included in the ERCPs File, we found 62 patients with a PAC diagnosis (8.9%). The main indication to perform ERCP in these patients was represented by the endoscopic palliation of jaundice. The remaining 636 patients were left out as not affected by pancreatic cancer, but by other diseases, such as stones in the main biliary duct, cancer of main biliary tract, Vater’s papilla cancer. In this group, the mean age of patients was 67.9±13.2 years.
Merging the two results arising from the two groups, the overall mean age was 68.2 ±10.1 years.
HBV markers prevalence
In our study population, four patients turned out to be HBsAg positive, with a prevalence equal to 1% in 373 patients. In the overall Italian population, the available surveys report similar values. In 28 individuals we observed the coexistence of HBsAb and HBcAb in their serum. The prevalence of HBsAb/HBcAb was equal to 7.5% in 373 subjects (Tables 3 and 4).
|
Birth Cohort/ Number of patients |
HBsAg (373 pts) |
HBsAb/HBcAb (373 pts) |
|
>1984 |
0 |
0 |
|
1975-1984 |
0/1 |
0/1 |
|
1965-1974 |
0/12 |
1/12 (8.3%) |
|
1955-1964 |
1/45 (2.2%) |
4 /45 (8.9%) |
|
1945-1954 |
2/95 (2.1%) |
5/95 (5.2%) |
|
1935-1944 |
1/157 (0.6%) |
10/157 (8.9%) |
|
<1935 |
0/63 |
8/63 (12.6%) |
|
Total |
4/373 (1.1%) |
28/373 (7.5%) |
Table 3: Birth cohort prevalence of HBV markers profile in the series of patients with PAC from Bologna and Rome.
|
Classes of age (years)/ Number of patients |
HBsAg (373 pts) |
HBsAb/HBcAb (373 pts) |
|
0-14 |
0 |
0 |
|
15-24 |
0 |
0 |
|
25-34 |
0 |
0 |
|
35-44 |
0/8 |
1/8 (12.5%) |
|
45-54 |
1/37 (2.7%) |
2/37 (5.4%) |
|
55-64 |
2/70 (2.8%) |
7/70 (10.0%) |
|
65-74 |
1/167 (0.6%) |
8/167 (4.8%) |
|
≥ 75 |
0/91 |
10/91 (11.0%) |
|
Total |
4/373 (1.1%) |
28/373 (7.5%) |
Table 4: Prevalence of HBV markers (HBsAg, HBsAb/HBcAb), according to the age groups in of patients with PAC from Bologna and Rome
In his survey on the general Italian people, Fabris showed that HBsAb/HBcAb overall prevalence was 8.4% and it progressively increases with the age, ranging from 0% to 17.8% in over 65 years subjects.
HCV markers prevalence
Sixteen patients were HCV positive, with a prevalence in our study population equal to 4.3%. The prevalence of anti-HCV positivity in our study population was variable, depending on the different age - as well as birth – classes, as also reported in trials by Andriulli and Fabris (Table 5).
|
Age-groups (year of birth) |
HCV positive patients/ HCV negative patients/Percentage in Andriulli’s study |
HCV positive patients with PAC/ HCV negative patients with PAC /Percentage |
|
>1984 |
1/524 (0.2%) |
0 |
|
1975-1984 |
8/652 (1.2%) |
0/1 |
|
1965-1974 |
14 /868 (1.6%) |
0/12 |
|
1955-1964 |
12/1010 (1.2%) |
2/45 (4.4%) |
|
1945-1954 |
21/972 (2.2%) |
3/95 (3.1%) |
|
1935-1944 |
47/669 (7.0%) |
5/157 (3.2%) |
|
<1935 |
9/212 (4.2%) |
6/63 (9.5%) |
|
Total |
112 /4907 (2.3%) |
16/373 (4.3%) |
Table 5: Birth cohort prevalence of anti-HCV positivity in the general population of five Italian urban areas from Andriulli’s study, in the series of patients with PAC from Bologna and Rome.
In our research, the mean age of HCV positive patients was higher than the one of HCV negative subjects (73.1 ± 12.4 years vs 68.0 ± 9.9 years, p= 0.0471). The prevalence of anti-HCV positivity according to the age groups and birth-classes in the series of patients with PAC from Bologna and Rome is shown in Table 5 and Table 6.
|
Age-groups (years) |
HCV positive patients with PAC/ HCV negative patients with PAC /Percentage |
|
0-14 |
0 |
|
15-24 |
0 |
|
25-34 |
0 |
|
35-44 |
0 |
|
45-54 |
2/37 (5.4%) |
|
55-64 |
2/70 (2.8%) |
|
65-74 |
4/167 (2.4%) |
|
≥75 |
8/91 (8.8%) |
|
Total |
16/373 (4.3%) |
Table 6: Prevalence of anti-HCV positivity according to the age groups in the series of patients with PAC from Bologna and Rome (third column).
Discussion
Our observational study assessed, for the first time in Italy, the prevalence of HBV and HCV antigens/antibody patterns in an Italian cohort of patients with PAC, admitted to two hospital wards in Italy. In our purpose, we just had the opportunity to refer only to a very small number of trials, assessing HBsAg and HBsAb/HBcAb and anti HCVAb prevalence in the general Italian population. In particular, we considered the works of Fabris and Andriulli [64, 67]. In our research HCV prevalence came out to be equal to 4.3% in all patients, whereas this value in the general Italian population-based survey by Andriulli is 2.3%. Similar results have been shown in an additional study by Fabris and colleagues [64], carried out also among the general Italian people. Furthermore, in our research HCV prevalence reached a value of 4.4% in the class of age, ranging from 1955 to 1964, whereas in Andriulli’s research it was fixed to 1.2%. On the other hand, in our study the high HCV prevalence in birth class <1935 seems to be in accordance with the more elevated one in older people, at least in Italy, as reported by the epidemiological studies performed to date. Although our study met several limitations, such as the retrospective design and the small number of the enrolled patients, which are expression of a peculiar case series (patients admitted to two hospital wards) and not of general Italian population, the observed data seem to provide some useful outcomes and stimulate the interest in this research field. Considering that most of the available studies are signed in the USA and in the South-Eastern Asian Countries, their results may differ from the ones emerged in Italy and in Europe, currently lacking consistent trials. In order to fill such this gap, several factors could be usefully evaluated in the future, including the different ethnical characteristics of patients enrolled in the trials, not without focusing on their geographical areas of provenance. However, we succeeded in establishing the prevalence of anti-HCV antibodies in patients with PAC at 4.3%, despite the heavy constraints above reported, whereas the ranges described in the general Italian population are forecasted between 2.2 and 2.7%. This evidence could stimulate the researchers to keep going on since if our data and assumptions will be confirmed in additional trials, it might come out that both the viruses would be able to promote pancreatic carcinogenesis, whose process is mostly sped up in the young.
This hypothesis, if viable at least in patients with HBV infection, would prove to be in line with a previous Editorial by Sherman and with a large-sized USA cohort-study [44, 68]. In these trials, the cohort of patients with PAC and HBV infection was composed of young individuals, predominantly male, black and Asian, in comparison with controls without a history of exposure to this pathogen. Patients aged 26 to 40 years occupied the greater pool of pancreatic cancer–HBV group population. It is difficult assuming the possible mechanisms involved in this process, but some years ago several epidemiological studies highlighted that a persistent inflammation [69, 70] becomes a predisposing factor to carcinogenesis in the tissues, where it occurs [71]. Both HBV and HCV might promote an inflammatory environment in the pancreas, leading to malignant transformation [70]. A previous paper proposed a potential model of HBV/HCV mediated pancreatic carcinogenesis [72]. In synthesis, both these pathogens, by means of their proteins, such as HBx and Hepatitis B surface antigen (HBsAg) and HCV NS3, NS4A, NS5A and NS5B might modulate and modify transcriptional cell genome activities both directly, such as HBx protein, and indirectly, such as HBsAg, HBx, NS3, NS4A, NS5A and NS5B via interaction with components of cytoplasmatic enzymes and cell cytoskeleton. This interaction results in an alteration of the normal balance existing among intracellular and extracellular tensional forces, originated by cytoskeleton and extracellular matrix, respectively, in which it is possible to see a de-regulation of the normal intracellular cytoskeletal architecture and modifications of the disposition of the cell microorganelles, as well of the shape of the nucleus and the cell conformation. These events take so long to determine quali/quantitative alterations of crucial intracellular biochemical activities regarding the transcription, the translation, the transduction of nuclear genes and may also cause chromatin de-arrangement. The macroscopic effects of these microscopic modifications in the cells and in the stroma surrounding them are represented by the development of tissue with biochemical and biophysical differences in comparison with a normal one. In particular, as previously reviewed [73], a modified pattern in the deposition and disposition of several tissue structural (such as collagens or proteoglycans), as well as non-structural proteins (such as matricellular proteins), emerges. One of the most important effects of these complex processes is the increase of the tissue stiffness, a well-known factor promoting carcinogenesis (see Table 6 and Figure 1, 2 and 3).
Therefore, our previous and current studies put our attention once again on the possible role of hepatotropic viruses in pancreatic cancer development and seem to suggest that pancreas is not merely a reservoir of both hepatotropic viruses, but it may support their replication. Taking into account both epidemiological and etiological available studies concerning pancreatic carcinogenesis, it is conceivable that HBV and HCV act more probably in this process as cofactors in cooperation with other actors (smoking, diabetes, alcohol) than as the only cause of this malignancy, although the action of both viruses may accelerate the development and the course of this neoplasm. In addition, as in the USA, also in Italy it is unknown what is the potential role and the clinical impact of "occult" HBV infection in PAC development [74, 75]. In several countries with decreased HBsAg prevalence, such as Italy, owing to long-term vaccination programs a significant number of subjects with markers of past exposure to HBV (serum HBsAb and/or HBcAb positivity) persists and these individuals might present a higher risk of PDAC [76].
Unfortunately, only a small number of trials have been carried out with the aim to understand this important point and the results are not univocal.
In particular, in the past two case-control studies detected an increased risk of PDAC in HBsAg–/HBcAb+/HBsAb– individuals [36, 39], whereas one study did not do it [40]. On the other hand, concerning HBsAg–/HBcAb+/HBsAb+ profile, two case-control studies showed an enhanced probability to develop this malignancy [36, 40] whereas one study did not do it [39]. The recent large sized cohort studies in the USA provided no specific contribution to clarify this important matter of debate. However, two meta-analyses, including these studies, have argued that a significantly increased risk occurs in HBcAb+/HBsAb+ vs HBsAg–/HBcAb– individuals, with RR equals to 1.41 (95% CI: 1.06-1.87) [35, 54] and in HBcAb+/HBsAb– vs HBsAg–/HBcAb– subjects, with OR equals to 1.76 (95% CI: 1.05-2.93). In any case, further studies are necessary.
Conclusion
Even if our observational study describes the prevalence of HBsAg, HBsAb/HBcAb as well as HCV in a small-sized cohort of patients with PAC from two Italian Hospitals, it was not designed to investigate whether HCV or HBV infection is associated with an increased risk of PAC development in Italy. Therefore, this pivotal study represents the basis for additional well-designed and well-sized epidemiological and histological nationwide trials in Italy, with the aim to: i) confirm or deny the potential role in pancreatic carcinogenesis; ii) clarify which is the real impact of HBV and HCV in Italy and in other European Countries and whether it differs from the other nations, where a large body of data are already available in literature, such as China, North America, and Eastern Asia.
Author Contributions
Study concepts: SF
Study design: SF, MZ
Data acquisition: SF, MZ, CB; ALdQ, MM, MFL, AL, RL, MZ, LM, SA, GDS, MC, LD, SN, GA, MV, AF, AT, PEO, GO; GC; PL
Quality control of data and algorithms: MLBR, PA, FB, SB,
Data analysis and interpretation: MLBR, PA, EG, IC, FB, SB
Statistical analysis: MLBR
Manuscript preparation: SF, DdB, PL, PA, FB, EJ
Manuscript editing: DdB, PA, FB, EJ
Manuscript review: SF, PL, PA, FB, EJ, DdB
Final approval of the version: SF, MZ, CB; ALdQ, MM, MFL, AL, RL, MZ, LM, SA, GDS, MC, LD, SN, GA, MV, GC, AF, AT, PEO, GO, PL, PA, FB, EG, LR, IC, SB, MLBR, DdB, EJ
Senior authorship: Ddb and EJ
Acknowledgements
The Authors thank Dr. Simonetta Righi, Biblioteca Centralizzata, Policlinico S. Orsola-Malpighi, Università di Bologna, Bologna, Italy for her support in the search of scientific bibliography and Dr. Saveria De Vito, Italian Medicines Agency, Rome, Italy for English editing.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Conflict of interest
Nothing to declare
References
- Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre and A. Jemal. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018).
- Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre and A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68 (2018): 394-424.
- Kamisawa, L.D. Wood, T. Itoi and K. Takaori. Pancreatic cancer. Lancet 388 (2016): 73-85.
- Diab, A. Azmi, R. Mohammad and P.A. Philip. Pharmacotherapeutic strategies for treating pancreatic cancer: advances and challenges. Expert Opin Pharmacother 20 (2019): 535-546.
- Schnelldorfer, A.L. Ware, M.G. Sarr, T.C. Smyrk, L. Zhang, R. Qin, R.E. Gullerud, J.H. Donohue, D.M. Nagorney and M.B. Farnell. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible?. Ann Surg 247 (2008): 456-62.
- C. Lee, S. Ahn, I.K. Cho, J. Lee, J. Kim and J.H. Hwang, Management of recurrent pancreatic cancer after surgical resection: a protocol for systematic review, evidence mapping and meta-analysis, BMJ Open 8 (2018): e017249.
- Yadav and A.B. Lowenfels. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144 (2013): 1252-61.
- Pilleron, D. Sarfati, M. Janssen-Heijnen, J. Vignat, J. Ferlay, F. Bray and I. Soerjomataram. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int J Cancer (2018).
- Del Chiaro, R. Segersvard, M. Lohr and C. Verbeke. Early detection and prevention of pancreatic cancer: is it really possible today?. World J Gastroenterol 20 (2014): 12118-31.
- Kuper, H.O. Adami and D. Trichopoulos. Infections as a major preventable cause of human cancer. J Intern Med 248 (2000): 171-83.
- Fiorino, M. Visani, G. Acquaviva, A. Fornelli, M. Masetti, A. Cuppini, M.L. Bacchi-Reggiani, E. Jovine, G. Tallini, A. Pession and D. de Biase. Search for HBV and HCV Genome in Cancer Cells of Pancreatic Tumors. Pancreas 45 (2016): e12-4.
- Jin, H. Gao, H. Chen, J. Wang, M. Chen, G. Li, L. Wang, J. Gu and H. Tu. Identification and impact of hepatitis B virus DNA and antigens in pancreatic cancer tissues and adjacent non-cancerous tissues. Cancer Lett 335 (2013): 447-54.
- M. Yan, A.S. Chen, F. Hao, X.P. Zhao, C.H. Gu, L.B. Zhao, D.L. Yang and L.J. Hao. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol 6 (2000): 805-811.
- Chen, X. Bai, Q. Zhang, L. Wen, W. Su, Q. Fu, X. Sun, Y. Lou, J. Yang, J. Zhang, Q. Chen, J. Wang and T. Liang. The hepatitis B virus X protein promotes pancreatic cancer through modulation of the PI3K/AKT signaling pathway. Cancer Lett 380 (2016): 98-105.
- Sun, Y. Wang, Y. Zhang, F. Liu, X. Cheng, N. Hou, X. Zhao and X. Yang. Expression profiling reveals dysregulation of cellular cytoskeletal genes in HBx-induced hepatocarcinogenesis. Cancer Biol Ther 6 (2007): 668-74.
- G. Bost, D. Venable, L. Liu and B.A. Heinz. Cytoskeletal requirements for hepatitis C virus (HCV) RNA synthesis in the HCV replicon cell culture system. J Virol 77 (2003): 4401-8.
- K. Lai, K.S. Jeng, K. Machida and M.M. Lai. Association of hepatitis C virus replication complexes with microtubules and actin filaments is dependent on the interaction of NS3 and NS5A. J Virol 82 (2008): 8838-48.
- M. Ward. The taking of the cytoskeleton one two three: how viruses utilize the cytoskeleton during egress. Virology 411 (2011): 244-50.
- Benn and R.J. Schneider. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc Natl Acad Sci U S A 91 (1994): 10350-4.
- P. Klein and R.J. Schneider. Activation of Src family kinases by hepatitis B virus HBx protein and coupled signaling to Ras. Mol Cell Biol 17 (1997): 6427-36.
- Alisi, M. Arciello, S. Petrini, B. Conti, G. Missale and C. Balsano. Focal adhesion kinase (FAK) mediates the induction of pro-oncogenic and fibrogenic phenotypes in hepatitis C virus (HCV)-infected cells. PLoS One 7 (2012): e44147.
- C. Cross, P. Wen and W.J. Rutter. Transactivation by hepatitis B virus X protein is promiscuous and dependent on mitogen-activated cellular serine/threonine kinases. Proc Natl Acad Sci U S A 90 (1993): 8078-82.
- Nakashima, K. Takeuchi, K. Chihara, T. Horiguchi, X. Sun, L. Deng, I. Shoji, H. Hotta and K. Sada. HCV NS5A protein containing potential ligands for both Src homology 2 and 3 domains enhances autophosphorylation of Src family kinase Fyn in B cells. PLoS One 7 (2012): e46634.
- Zhao and J.L. Guan. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 28 (2009): 35-49.
- Lara-Pezzi, S. Roche, O.M. Andrisani, F. Sanchez-Madrid and M. Lopez-Cabrera. The hepatitis B virus HBx protein induces adherens junction disruption in a src-dependent manner. Oncogene 20 (2001): 3323-31.
- Lara-Pezzi, P.L. Majano, M. Yanez-Mo, M. Gomez-Gonzalo, M. Carretero, R. Moreno-Otero, F. Sanchez-Madrid and M. Lopez-Cabrera. Effect of the hepatitis B virus HBx protein on integrin-mediated adhesion to and migration on extracellular matrix. J Hepatol 34 (2001): 409-15.
- Nejjari, A. Couvelard, J.F. Mosnier, A. Moreau, G. Feldmann, C. Degott, P. Marcellin and J.Y. Scoazec. Integrin up-regulation in chronic liver disease: relationship with inflammation and fibrosis in chronic hepatitis C. J Pathol 195 (2001): 473-81.
- Chen, L. Hu, L. Li, Y. Liu, Q.Q. Tu, Y.X. Chang, H.X. Yan, M.C. Wu and H.Y. Wang. Dysregulation of beta-catenin by hepatitis B virus X protein in HBV-infected human hepatocellular carcinomas. Front Med China 4 (2010). 399-411.
- Lara-Pezzi, J.M. Serrador, M.C. Montoya, D. Zamora, M. Yanez-Mo, M. Carretero, H. Furthmayr, F. Sanchez-Madrid and M. Lopez-Cabrera. The hepatitis B virus X protein (HBx) induces a migratory phenotype in a CD44-dependent manner: possible role of HBx in invasion and metastasis. Hepatology 33 (2001): 1270-81.
- Benedicto, F. Molina-Jimenez, O. Barreiro, A. Maldonado-Rodriguez, J. Prieto, R. Moreno-Otero, R. Aldabe, M. Lopez-Cabrera and P.L. Majano. Hepatitis C virus envelope components alter localization of hepatocyte tight junction-associated proteins and promote occludin retention in the endoplasmic reticulum. Hepatology 48 (2008): 1044-53.
- Watanabe, H. Aizaki, T. Matsuura, S. Kojima, T. Wakita and T. Suzuki. Hepatitis C virus RNA replication in human stellate cells regulates gene expression of extracellular matrix-related molecules. Biochem Biophys Res Commun 407 (2011): 135-40.
- Schulze-Krebs, D. Preimel, Y. Popov, R. Bartenschlager, V. Lohmann, M. Pinzani and D. Schuppan. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology 129 (2005): 246-58.
- Martin-Vilchez, P. Sanz-Cameno, Y. Rodriguez-Munoz, P.L. Majano, F. Molina-Jimenez, M. Lopez-Cabrera, R. Moreno-Otero and E. Lara-Pezzi. The hepatitis B virus X protein induces paracrine activation of human hepatic stellate cells. Hepatology 47 (2008): 1872-83.
- Liu, S.T. Zhu, H. You, M. Cong, T.H. Liu, B.E. Wang and J.D. Jia. Hepatitis B virus infects hepatic stellate cells and affects their proliferation and expression of collagen type I. Chin Med J (Engl) 122 (2009): 1455-61.
- Fiorino, E. Chili, L. Bacchi-Reggiani, M. Masetti, G. Deleonardi, A.G. Grondona, T. Silvestri, E. Magrini, N. Zanini, A. Cuppini, R. Nardi and E. Jovine. Association between hepatitis B or hepatitis C virus infection and risk of pancreatic adenocarcinoma development: a systematic review and meta-analysis. Pancreatology 13 (2013), 147-60.
- M. Hassan, D. Li, A.S. El-Deeb, R.A. Wolff, M.L. Bondy, M. Davila and J.L. Abbruzzese. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol 26 (2008): 4557-62.
- C. Chang, C.H. Chen, J.D. Liang, Y.W. Tien, C. Hsu, J.M. Wong and Y.T. Chang. Hepatitis B and C viruses are not risks for pancreatic adenocarcinoma. World J Gastroenterol 20 (2014): 5060-5.
- M. Woo, J. Joo, W.J. Lee, S.J. Park, S.S. Han, T.H. Kim, Y.H. Koh, H.B. Kim and E.K. Hong. Risk of pancreatic cancer in relation to ABO blood group and hepatitis C virus infection in Korea: a case-control study. J Korean Med Sci 28 (2013): 247-51.
- Ben, Z. Li, C. Liu, Q. Cai, Y. Yuan, K. Wang, L. Xiao, J. Gao and H. Zhang. Hepatitis B virus status and risk of pancreatic ductal adenocarcinoma: a case-control study from China. Pancreas 41 (2012): 435-40.
- S. Wang, D.L. Chen, C. Ren, Z.Q. Wang, M.Z. Qiu, H.Y. Luo, D.S. Zhang, F.H. Wang, Y.H. Li and R.H. Xu. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer 131 (2012): 461-8.
- Zhu, H.R. Li, G.N. Du, J.H. Chen and S.R. Cai. Chronic hepatitis B virus infection and pancreatic cancer: a case-control study in southern China. Asian Pac J Cancer Prev 12 (2011): 1405-8.
- H. Iloeje, H.I. Yang, C.L. Jen, J. Su, L.Y. Wang, S.L. You, S.N. Lu and C.J. Chen. Risk of pancreatic cancer in chronic hepatitis B virus infection: data from the REVEAL-HBV cohort study. Liver Int 30 (2010): 423-9.
- B. Kamiza, F.H. Su, W.C. Wang, F.C. Sung, S.N. Chang and C.C. Yeh. Chronic hepatitis infection is associated with extrahepatic cancer development: a nationwide population-based study in Taiwan. BMC Cancer 16 (2016): 861.
- Desai, U. Patel, S. Sharma, S. Singh, S. Doshi, S. Shaheen, S. Shamim, L.S. Korlapati, S. Balan, C. Bray, R. Williams and N. Shah. Association Between Hepatitis B Infection and Pancreatic Cancer: A Population-Based Analysis in the United States. Pancreas 47 (2018): 849-855.
- Tang, R. Sharma, L. Lamerato, M. Sheehan, R. Krajenta and S.C. Gordon. Is previous exposure to hepatitis B a risk factor for pancreatic cancer or hepatocellular carcinoma?. J Clin Gastroenterol 48 (2014): 729-33.
- Amin, G.J. Dore, D.L. O'Connell, M. Bartlett, E. Tracey, J.M. Kaldor and M.G. Law. Cancer incidence in people with hepatitis B or C infection: a large community-based linkage study. J Hepatol 45 (2006): 197-203.
- Swart, L. Burns, L. Mao, A.E. Grulich, J. Amin, D.L. O'Connell, N.S. Meagher, D.A. Randall, L. Degenhardt and C.M. Vajdic. The importance of blood-borne viruses in elevated cancer risk among opioid-dependent people: a population-based cohort study. BMJ Open 2 (2012).
- Huang, M. Magnusson, A. Torner, W. Ye and A.S. Duberg. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: a nationwide study in Sweden. Br J Cancer 109 (2013): 2917-23.
- S. Andersen, L.H. Omland, P. Jepsen, H. Krarup, P.B. Christensen, N. Obel and N. Weis. Risk of all-type cancer, hepatocellular carcinoma, non-Hodgkin lymphoma and pancreatic cancer in patients infected with hepatitis B virus. J Viral Hepat 22 (2015): 828-34.
- Krull Abe, M. Inoue, N. Sawada, M. Iwasaki, T. Shimazu, T. Yamaji, S. Sasazuki, E. Saito, Y. Tanaka, M. Mizokami and S. Tsugane. Hepatitis B and C Virus Infection and Risk of Pancreatic Cancer: A Population-Based Cohort Study (JPHC Study Cohort II). Cancer Epidemiol Biomarkers Prev 25 (2016): 555-7.
- Li, B. Wu, L.B. Yang, G.C. Yin and J.Y. Liu. Chronic hepatitis B virus infection and risk of pancreatic cancer: a meta-analysis. Asian Pac J Cancer Prev 14 (2013): 275-9.
- Luo, N.B. Hao, C.J. Hu, X. Yong, M.H. Lu, B.J. Cheng, Y. Zhang and S.M. Yang. HBV infection increases the risk of pancreatic cancer: a meta-analysis. Cancer Causes Control 24 (2013): 529-37.
- Wang, S. Yang, F. Song, S. Cao, X. Yin, J. Xie, X. Tu, J. Xu, X. Xu, X. Dong and Z. Lu. Hepatitis B virus status and the risk of pancreatic cancer: a meta-analysis. Eur J Cancer Prev 22 (2013): 328-34.
- Xing, Z.W. Li, Y.F. Tian, L.M. Zhang, M.Q. Li and P. Zhou. Chronic hepatitis virus infection increases the risk of pancreatic cancer: a meta-analysis. Hepatobiliary Pancreat Dis Int 12 (2013): 575-83.
- H. Xu, J.J. Fu, X.L. Wang, J.Y. Zhu, X.H. Ye and S.D. Chen. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol 19 (2013): 4234-41.
- Majumder, B. Bockorny, W.L. Baker and C.A. Dasanu. Association between HBsAg positivity and pancreatic cancer: a meta-analysis. J Gastrointest Cancer 45 (2014): 347-52.
- D. Allison, X. Tong, A.C. Moorman, K.N. Ly, L. Rupp, F. Xu, S.C. Gordon and S.D. Holmberg. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006-2010. J Hepatol 63 (2015): 822-8.
- B. El-Serag, E.A. Engels, O. Landgren, E. Chiao, L. Henderson, H.C. Amaratunge and T.P. Giordano. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology 49 (2009): 116-23.
- H. Omland, D.K. Farkas, P. Jepsen, N. Obel and L. Pedersen. Hepatitis C virus infection and risk of cancer: a population-based cohort study. Clin Epidemiol 2 (2010): 179-86.
- Crocetti and S. Mancini. Pancreatic cancer incidence rises also in Italy. Int J Epidemiol 46 (2017): 2090.
- Sagnelli, C. Sagnelli, M. Pisaturo, M. Macera and N. Coppola. Epidemiology of acute and chronic hepatitis B and delta over the last 5 decades in Italy. World J Gastroenterol 20 (2014): 7635-43.
- Stroffolini. The changing pattern of hepatitis B virus infection over the past three decades in Italy. Dig Liver Dis 37 (2005): 622-7.
- Saitta, G. Tripodi, A. Barbera, A. Bertuccio, A. Smedile, A. Ciancio, G. Raffa, A. Sangiovanni, G. Navarra, G. Raimondo and T. Pollicino, Hepatitis B virus (HBV) DNA integration in patients with occult HBV infection and hepatocellular carcinoma. Liver Int 35 (2015): 2311-7.
- Fabris, V. Baldo, T. Baldovin, E. Bellotto, M. Rassu, R. Trivello, A. Tramarin, G. Tositti and A. Floreani. Changing epidemiology of HCV and HBV infections in Northern Italy: a survey in the general population. J Clin Gastroenterol 42 (2008): 527-32.
- Da Villa, L. Romano, A. Sepe, R. Iorio, N. Paribello, A. Zappa and A.R. Zanetti. Impact of hepatitis B vaccination in a highly endemic area of south Italy and long-term duration of anti-HBs antibody in two cohorts of vaccinated individuals Vaccine 25 (2007): 3133-6.
- Documento di indirizzo dell’Associazione Italiana per lo Studio del Fegato (AISF) per l’uso razionale dei farmaci anti-HCV disponibili in Italia (accessed on september 20, 2018), 2018.
- Andriulli, T. Stroffolini, A. Mariano, M.R. Valvano, I. Grattagliano, A.M. Ippolito, A. Grossi, G. Brancaccio, C. Coco, M. Russello, A. Smedile, E. Petrini, S. Martini, G.B. Gaeta and M. Rizzetto. Declining prevalence and increasing awareness of HCV infection in Italy: A population-based survey in five metropolitan areas. Eur J Intern Med 53 (2018): 79-84.
- Sherman. Pancreatic cancer in chronic hepatitis B. Liver Int 30 (2010): 339-41.
- P. Zambirinis, S. Pushalkar, D. Saxena and G. Miller. Pancreatic cancer, inflammation, and microbiome. Cancer J 20 (2014): 195-202.
- Jackson and B.M. Evers, Chronic inflammation and pathogenesis of GI and pancreatic cancers. Cancer Treat Res 130 (2006): 39-65.
- Schottenfeld and J. Beebe-Dimmer. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin 56 (2006): 69-83.
- Fiorino, L. Bacchi-Reggiani, L. Pontoriero, C. Gallo, E. Chili, M. Masetti, N. Zanini, A. Grondona, T. Silvestri, G. Deleonardi, A. Fornelli, A. Bondi, D. de Biase, P. Baccarini, G. Tallini, A. Tropeano, V. Quartuccio, A. Cuppini, G. Castellani and E. Jovine. Tensegrity model hypothesis: may this paradigm be useful to explain hepatic and pancreatic carcinogenesis in patients with persistent hepatitis B or hepatitis C virus infection?. JOP 15 (2014): 151-64.
- Fiorino, M.L. Bacchi-Reggiani, C. Birtolo, G. Acquaviva, M. Visani, A. Fornelli, M. Masetti, A. Tura, S. Sbrignadello, F. Grizzi, F. Patrinicola, M. Zanello, L. Mastrangelo, R. Lombardi, C. Benini, L. Di Tommaso, A. Bondi, F. Monetti, E. Siopis, P.E. Orlandi, M. Imbriani, C. Fabbri, S. Giovanelli, A. Domanico, E. Accogli, S. Di Saverio, D. Grifoni, V. Cennamo, P. Leandri, E. Jovine and D. de Biase. Matricellular proteins and survival in patients with pancreatic cancer: A systematic review. Pancreatology 18 (2018): 122-132.
- Huang and F.B. Hollinger. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat 21 (2014): 153-62.
- Squadrito, R. Spinella and G. Raimondo. The clinical significance of occult HBV infection. Ann Gastroenterol 27 (2014): 15-19.
- Romero, A. Madejon, C. Fernandez-Rodriguez and J. Garcia-Samaniego. Clinical significance of occult hepatitis B virus infection. World J Gastroenterol 17 (2011): 1549-52.