Prevalence and Viral Etiology of Community-Acquired Pneumonia in Children in Suzhou, China
Article Information
Ting Shi, Linlin Huang, Weiming Li, Jianmei Tian*
Department of Infectious Diseases, Children’s Hospital of Soochow University, 303 Jingde Road, Suzhou, Jiangsu, China
*Corresponding Author: Dr. Jianmei Tian, Department of Infectious Diseases, Children’s Hospital of Soochow University, 303 Jingde Road, Suzhou, Jiangsu, China
Received: 16 December 2020; Accepted: 22 January 2021; Published: 08 February 2021
Citation:
Ting Shi, Linlin Huang, Weiming Li, Jianmei Tian. Prevalence and Viral Etiology of Community-Acquired Pneumonia in Children in Suzhou, China. Journal of Pediatrics, Perinatology and Child Health 5 (2021): 018-031.
View / Download Pdf Share at FacebookAbstract
Introduction: Children with community-acquired pneumonia (CAP) in Soochow University Affiliated Children's Hospital account for 85% of the annual pediatric CAP cases in Suzhou, China. It is essential to explore viral etiology in children with CAP in the local place. In this study, we aimed to expand our understanding of the viral epidemiology in children with CAP in this area by comparing seasonal and clinical characteristics of respiratory viruses from patients at this hospital.
Methods: Clinical and laboratory data were collected for 22,825 children with radiologically-confirmed CAP who were admitted to our hospital from January 2010 to December 2014. On the day of admission, sputum specimens were cultured as per routine practice and tested for the respiratory syncytial virus (RSV), influenza A and B (Inf-A and Inf-B), parainfluenza virus 1 to 3 (PInf1-3), and adenovirus (ADV) by direct fluorescent antibody tests and for human bocavirus (HBoV), Mycoplasma pneumonia (MP) and Chlamydophila pneumonia (CP) by real-time fluorescence quantitative polymerase chain reaction. Bacterial infection was examined by conventional bacteriological identification methods.
Results: Of the 22,825 nasopharyngeal secretion samples, 6,314 (27.66%) had evidence of viral infection. Respiratory viruses caused CAP at a high rate in children under 1 year of age (66.7%). The peak rate of respiratory virus detection occurs in winter. RSV, HBoV, and PInf3 are the most common viral pathogens of CAP in children in the Soochow area. The epidemic season of RSV was winter and autumn, that of HBoV was summer and autumn, and that of Pinf3 was summer.
Conclusion: In the Soochow area, a substantial proportion of childhood CAP is due to viruses. The viral pathogens vary by season and clinical fe
Keywords
Respiratory tract virus, Community-acquired pneumonia, Epidemic season
Respiratory tract virus articles; Community-acquired pneumonia articles; Epidemic season articles
Article Details
1. Introduction
Childhood community-acquired pneumonia (CAP) is the leading cause of mortality and morbidity of children after neonatal [1]. There is an increasing incidence of CAP, from 0.22% in 2009 to 0.41% in 2012, as reported in China. Over 50% of the cases showed undetected pathogens, identifying the causative agent and proper empirical treatment difficult [2]. The major causes of CAP shifted with antibiotics treatment, and areis complicated with the influenza outbreak [3]. The detailed contribution of viral etiology of CAP remains elusive as infection of the various virus, including respiratory syncytial virus (RSV), influenza A and B (Inf-A and Inf-B), parainfluenza virus 1-3 (Pinf1-3), adenovirus (ADV) or human bocavirus (HBoV) might be the primary causes or predispose children to secondary bacterial infection, including Mycoplasma pneumonia (MP) and Chlamydophila pneumonia (CP). Therefore, there is an urgent need for in-depth characterization of the CAP's viral etiology to foster rational treatment to improve patient response and reduce adverse effects. To our knowledge, there is no clinical study addressing the prevalence and viral etiology of CAP in the Suzhou area. Moreover, appropriate clinical management for viral-induced CAP has not been identified. This study retrospectively analyzed the children with CAP admitted to the Soochow University Affiliated Children's Hospital (SCH) during 2010 and 2014. We aim to provide evidence for early diagnosis and further control of CAP caused by the virus in clinical work.
2. Materials and Methods
2.1 Study population and data collection
Between January 2010 and December 2014, a total of 184,734 children under 15 years old were admitted to the Department of Respiratory Diseases of SCH, and 107,813 (58.4%) were residents of downtown Suzhou. CAP is diagnosed according to the following criteria (A) two of the following symptoms were identified in patients: cough, history of fever, pleuritic pain, crackles, or bronchial breath sounds; (B) focal airspace consolidation and patchy increased interstitial markings were evident in chest radiographic findings, which is consistent with pneumonia; (C) patients were admitted within 48 hours. Children who had experienced vertical infection, chronic disease, immunosuppressed status, history of recurrent attacks of pneumonia, antibiotic intake within the last month, pneumococcal vaccine intake, or healthcare-associated or hospital-acquired pneumonia is excluded. A final 22,825 children (96.4%) were included in this study.
2.2 Sputum Specimen Collection
Individual medical charts of eligible participants were reviewed by trained investigators using a standard questionnaire to obtain retrospective information. Admission and discharge date, clinical symptoms including fever, runny nose, polypnea, dyspnea, asthma, twitch, blood biochemical examination results, including white blood cells counts, neutrophil counts and level of C-reactive protein were extracted from an n experienced lab technician collected nasopharyngeal secretions of study participants within 24 h upon admission. An aseptic plastic sputum catheter was inserted into the nostril to a depth of about 7-8 cm to reach the pharynx. Approximately 2 mL of nasopharyngeal secretions were collected by applying negative pressure. The sample was mixed with 4-8 mL PBS and centrifuged for 10 minutes at 300-500 rpm at room temperature. The supernatant was discarded, and the pellet was resuspended with 4-8 ml PBS again and centrifuged for an additional 10 minutes. The supernatant was removed, and the pellet was stored at -80°C.
2.3 DNA extraction from sputum specimen
The sputum specimen was washed with phosphate-buffered saline (PBS), pH 7.0. specimens were centrifuged, and the supernatant was removed. Lysis buffer was added to the pellet and boiled at 95°C for 10 minutes to release the DNA. After lysis, samples were centrifuged at 12,000 rpm for 5 minutes. The supernatant was collected for DNA.
2.4 Real-time Polymerase chain reaction (RT-qPCR)
Real-time PCR detection system iCycler from Bio-rad was employed. For RT-qPCR of HBoV, MP, and CP genomic DNA, the amplification of genes was performed with a denaturation step at 95°C for 10 minutes, followed by 40 cycles of denaturation at 94°C 30s, primers annealing at 56°C for 30s and primer extension at 72°C for 30s.
2.5 Viral Detection
RSV, Inf-A, Inf-B, PInf-1, PInf-2, PInf-3, and ADV were detected fluorescent assay kit purchased from Chemicon. A small sputum sample was smeared onto a glass slide and heat-fixed, bypassing the slides over the Bunsen burner 3-4 times. Staining procedures were performed according to the manufacturer's instructions. Immuno-stained specimen slides were examined with a fluorescent microscope (Leica 020-518.500, Germany).
HBoV was detected by real-time quantitative PCR (RT-qPCR). The sequence of the primers is listed below:
HBoV-F: 5’-TGACATTCAACTACCAACAACCTG-3’
HBoV-R: 5'-CAGATCCTTTTCCTCCTCCAATAC-3'3'
HBoV-probe: AGCACCACAAAACACCTCAGGGG-TAMRA
2.6 Bacterial Detection
DNA was extracted and examined with real-time PCR for the detection of MP and CP Primers used for RT-PCR were listed below:
MP-F: 5’-CCAACCAAACAACAACGTTCA-3’
MP-R: 5'-ACCTTGACTGGAGGCCGTTA-3'3'
MP-Probe: 5’-TCAACTCGAATAACGGTGACTTCTTACCACTG-3’
CP-F: 5’-TGAAGTCGGAATTGCTAGTAATGG-3’
CP-R: 5’-GTGTGTACAAGGCCCGGGAAC-3’
CP-FB1: 5’-TGTCAGCTATAACGCCGTGAAT-3’
A direct smear of sputum was stained with Gram stain and examined under bright-field microscopy. All sputum smear was screened for interpretability. Three fields were randomly picked on each slice, and only smear with >25 leukocytes and <10 epithelial cells per low power field (10x) were selected. Respiratory specimens were inoculated onto blood agar, heated blood agar, and Mac-Conkey's agar media, respectively. Inoculated blood and MacConkey's agar plates were incubated at 37°C aerobically, while inoculated heated blood agar plates were incubated at 10% CO2 by the candle jar method [4]. Bacterial cultures were incubated overnight at 37°C, and blood agar plates were blindly subcultured. If no growth was observed, the bottles were examined daily for 7 more days. Any sign of growth was followed by subculture. Isolated bacterial clones obtained from agar cultures were identified by a trained lab technician using standard techniques[5].
2.7 Serological diagnosis
Blood samples were collected from the study participant within 2 h after admission. Blood was clotted and was centrifuged at 1000 g for 10 minutes. Sera were separated and stored at −20°C until assayed by indirect immunofluorescent technique for the presence of specific IgM antibodies against Mycoplasma pneumonia. Blood sample Blood samples were examined a week after, and blood samples were tested again.
2.8 Statistical Analysis
Statistical analyses were performed with SPSS software (v19.0, SPSS, Chicago, IL, USA). Chi-squared tests were performed to analyze frequency data. If the p-value was approximately equal to α, ' 'Fisher's exact method was adopted. Values of p < 0.05 were statistically significant.
2.9 Ethics statement
Informed consent was obtained from parents or guardians of each participant enrolled in this study, according to the Declaration of Helsinki [6]. The Ethics Committee has approved the work of Soochow University Affiliated Children's Hospital. (Approval number 2019KS004).
3. Results
3.1 Respiratory virus detection in children with CAP
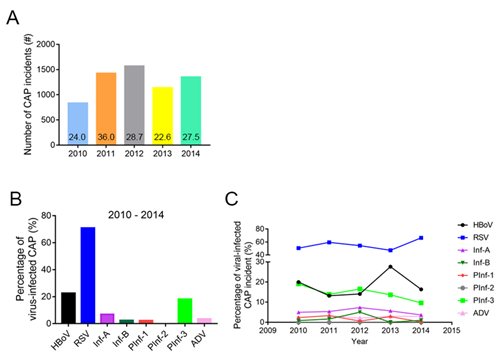
The annual number of viral detections in children diagnosed with CAP for the years 2010 through 2014 were 832 (24.04%), 1424 (35.99%), 1569 (28.75%), 1139 (22.57%), and 1350 (27.54%), respectively (Figure 1A). The respiratory virus detection rate was highest in 2011. Of the 22,825 nasopharyngeal secretion samples, 6,314 (27.66%) showed evidence of virus infection. Among them, 4,437 cases (70.89%) were RSV-positive, and 1,431 cases (22.64%) were HBoV-positive. PInf-1, PInf-2 and PInf-3 contributed to 141 (2.23%), 3 (0.04%) and 1,145 (18.13%) cases while 439 (6.95%) and 151 (2.39%) cases are found to be Inf-A and Inf-B positive. At last, there were 218 (3.45%) ADV-positive cases (Figure 1B). There is no significant difference among the percentage of virus-positive CAP cases of the respective virus from 2010 to 2014 (Two-way ANOVA: Time: F (4,28) =1.661e-13, P > 0.99). RSV-positive cases are significantly higher than all other types of virus-positive cases (Two-way ANOVA: Virus: F (7,28) =108.2, P < 0.0001, ' 'Tukey's post hoc test, RSV vs. all other virus P < 0.0001). While there is no difference between the percentage of HBoV+ and PInf-3+ cases, both are significantly higher than Inf-A, Inf-B, PInf-1, PInf-2, and ADV (Statistical analysis in supplementary information) (Figure 1C).
3.2 Monthly and seasonal respiratory virus detection rates
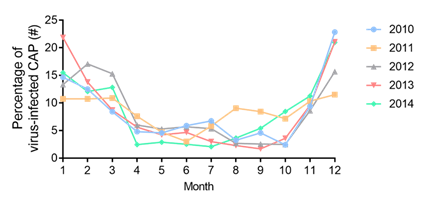
Respiratory virus infection during this study exhibited clear seasonal trends. Based on the climate in the Soochow area, March, April, and May are the spring, June, July, and August are the summer, September, October, and November are the autumn, and January, February, and December are the winter. The respiratory virus detection rates were significantly different between seasons (Two-way ANOVA: Month: F(11,44) = 16.17, P < 0.0001). From January to December, the annual rate of respiratory virus detection was U-shaped, having a higher rate from October to April. The peak detection rates appeared in the winter. Respiratory viruses were observed less frequently from June to October (Figure 2).
3.3 Seasonal distribution of the detection rates of eight respiratory viruses
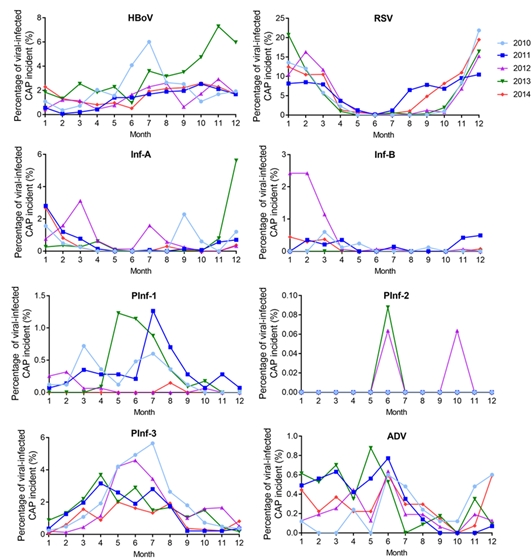
When plotted from January to December, the rate of RSV detection was U-shaped. The peak RSV detection rate occurred in December. The detection rate of HBoV exhibited a gradually increasing trend from January to July, maintaining a high level until December. The detection rate of PInf-1 and PInf-3 had a normal distribution from January to December. The peak detection rate of both was in June. The ADV, Inf-A, and Inf-B detection rates were generally evenly distributed throughout the year, lacking any obvious peak values. Additionally, PInf-2 detection was sporadic throughout the year (Figure 3 and 4).
3.4 Age distribution of patients with a viral infection
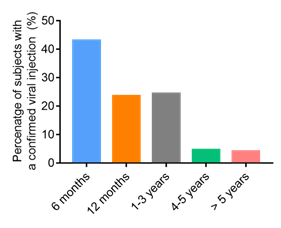
Of the 22,825 nasopharyngeal secretion samples, 6,314 (27.66%) showed evidence of virus infection. The detection rate of viral CAP is the highest by 6 months of age (43.1%). As the age increases, the viral CAP detection rate was lower after 1 year of age (Figure 5). The detection rate is similar from the age of 12 months to 36 months (23.6%), and then the rate drops over the age of four years old (4.7%) (Figure 5).
3.5 Gender and age distributions of patients with a single viral infection.
In this study, patients with single viral infection were excluded from mixed infections with bacteria, including M.P., CP, and other viruses. The difference between males and females showed statistical significance concerning the incidences of the seven kinds of the virus, as shown in table 1. The incidence of the seven types of virus-infected CAP is higher in males than in females (Table 1). RSV infection was more common in young children aged <6months group (58.0%, 886/1527) than in the other four age groups. RSV infection exhibited a gradually decreasing trend with increasing age, as did PInf-3. HBoV, Inf-A, Inf-B, and PInf-1 were more common in 1-3y than in other age groups.
3.6 Clinical characteristics of patients with a single viral infection
In table 2, fever was the most common symptom exhibited by ADV patients (87.3%). Runny nose was documented in 50.6% of patients with Inf-A infection and 50.0% of patients with Inf-B infection, which is higher than that observed in other virus single-infections. Asthma and wheezing were the most common symptom exhibited in patients with RSV infection. In imaging, Bronchial pneumonia was the most common X-ray performance exhibited by patients with a single viral infection. In laboratory examinations, WBC count was higher in patients with HBoV infection (43.6%) and patients with ADV infection (45.6%). The patients with ADV infection (63.3%) had the highest rate of having CRP higher than 8mg/L (Table 2).
Figure 1: Several incidents of CAP cases and virus detection rate from 2010 to 2014. (A) The number of CAP identified from 2010-2014. The percentage of CAP cases was presented on the graph; (B) The Percentage of CAP cases with RSV, HBov, Inf-A, ADV, Inf-B, PInf1, PInf2, and PInf-3 detected in the nasopharyngeal secretion samples from children from 2010-2014; (C) The trend of virus-positive CAP cases detection rate. (Two-way ANOVA - Time: F(4,28)=1.661e-13, P > 0.99; Virus: F(7,28)=108.2, P < 0.0001).
Table 1: Gender and age distribution of patients with a single viral infection.
Table 2: Clinical characteristics of patients with single viral infection.
4. Discussion
CAP is a common disease that is frequently encountered in infants and young children. In this study, 22,825 nasopharyngeal secretion samples were collected from children with CAP hospitalized at 'Children's Hospital Affiliated to Soochow University, and 6,314 (27.66%) of these patients had evidence of virus infection. Respiratory viruses cause CAP at a high rate in children under 1 year of age (66.7%). The peak rate of respiratory virus detection occurs in winter. RSV, HBoV, and Pinf3 are the most common viral pathogens of CAP in children in the Soochow area. The epidemic season of RSV was winter and autumn, that of HBoV was summer and autumn, and that of PInf3 was summer.
According to the World Health Organization, pneumonia is the major cause of death in children, accounting for 18% of all deaths in the under 5 years age group. Among the cases, many of these conditions are caused by virus infection. Recently, Ali et al. reported that respiratory viruses caused a significant percentage of WHO-defined severe pneumonia in young, hospitalized children in Karachi, Pakistan [7]. In China, the overall estimated viral etiology in children is 3.73%, 4.56%, and 16.01% for InF-A, ADV, and influenza virus A[2]. While the etiology of CAP varies in a different province in China, an in-depth understanding of epidemiology in the Suzhou area is required for better treatment for patients.
Viral pathogens have been detected in 30-67% of CAP cases in the studies conducted outside of China [8]. Such high detection rates could not be achieved in our study (22.5-35.9%), partially due to our inability to employ a similarly wide armamentarium of microbiologic diagnostic methods and differences in the local environment and climate.
Our results showed that RSV, HBoV, and PInf-3 are the most common viral pathogens of CAP in children in the Soochow area. Of the eight viruses detected in this study, RSV had the highest detection rate. This finding is in line with worldwide findings that RSV is the leading cause of lower respiratory tract infections in the pediatric population worldwide [9]. In this study, the HBoV detection rate maintained a high level throughout the study period. Other reports also showed similar phenomena that HBoV is a major cause of ' 'doctor's office visits and hospitalization and has a high impact on morbidity and mortality, particularly among children in developing countries [9, 10].
In contrast, the cumulative frequency of Inf-A and Inf-B (2.58%) in this study was much lower than what has been previously reported from developing countries, with detection rates ranging from 28-36% reported for pneumonia in younger children [10, 11]. On the other hand, other research groups have reported influenza infection rates as low as 10% [12]. These suggested that influenza-infected CAP might vary in the different geographical regions. Our results indicate that Inf-A and Inf-B were not the main causative pathogens of pediatric CAP Soochow patients.
When the detection rates of respiratory viruses in the Soochow area were plotted from January to December, they formed a U-shape. RSV-infected CAP mainly contributed to this observation as it has the highest number of incidents. The peak respiratory virus detection rate appeared in the winter, supported by other literature kinds [13-18]. Additionally, the most common viral pathogens differed by season. RSV had the highest detection rates in the winter and autumn and is lowest in summer. This result is in accordance with those reported previously in China and other countries [19, 20]. We observed that HBoV most frequently occurred in summer and autumn. However, in contrast, it contrasts with a report by L M Ghetto et al. [21], claiming that HBoV cases occurred in fall through spring and peaked during the winter. It might be due to geographical factors. Our study found that PInf-3 frequently occurred in summer, which is similar to another report [22], showing that PInf-3 occurred mostly in England and Wales from April through August. Small peaks of detection rate in the Inf-A and Inf-B were observed in winter, that of PInf-1 in the summer, and ADV in spring and summer.
Our results indicated that males are more susceptible to virus infection in children under 1 year and represented the largest proportion of patients with virus infections. Virus infection exhibited a gradual decrement with increasing age. Age a significant predictor in RSV hospitalization, with the youngest age groups (<6 months) more frequently hospitalized for an RSV infection [23]. However, HBoV, Inf-A, Inf-B, and PInf-1 were more common in 1-3y than in other age groups. Clinical manifestations are relied on to differentiate among various viral pneumonia. Asthma and wheezing were the most common symptoms in the RSV group. The most common symptom exhibited by ADV infection was fever. In imaging, chest X-rays of most patients with a single viral infection were manifested as bronchial pneumonia. In this study, simple respiratory virus infection rarely caused severe pneumonia.
In conclusion, CAP is a major cause of mortality and morbidity. There are significant differences in pathogens in different parts of the world, and there is no single global guideline applicable universally. The respiratory virus exists with distinct seasonal characteristics and clinical manifestations in the Suzhou area. Physicians must be familiar with the local epidemiology to provide suitable treatment to patients with minimal adverse effects. This fosters early diagnosis and prevents community-acquired pneumonia in general.
Acknowledgments
The authors are grateful for the patients without whom this study would not have been done.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
This work was supported by the grant from: Soochow University Affiliated Children’s Hospital Research Council (http://www.sdfey.cn/; grant number: 653). Professor Jianmei Tian obtained the grant entitled by “Retrospective study of hospitalization related to community-acquired pneumonia and invasive pneumococcal disease in children in Suzhou, Jiangsu Province, China” at 2016.
References
- Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet 386 (2015): 1097-1108.
- Ying-gang Zhu, Xiao-dan Tang, Yun-tao Lu, et al. Contemporary Situation of Community-acquired Pneumonia in China: A Systematic Review. J Transl Int Med 6 (2018): 26-31.
- Musher DMM and Thorner AR. Community-Acquired Pneumonia. N Engl J Med 371 (2014): 1619-1628.
- Gnarpe H and Svensson L. Evaluation of a candle-jar system in culture diagnosis of gonorrhoea. Acta Derm Venereol 60 (1980): 368-370.
- Versalovic J, Carroll KC, Funke G, et al. Manual of clinical microbiology. 10th ASM Press (2011).
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 310 (2013): 2191-2194.
- Asad Ali, Asif Raza Khowaja, Maaman Zahoor Bashir, et al. Role of human metapneumovirus, influenza A virus and respiratory syncytial virus in causing WHO-defined severe pneumonia in children in a developing country. PLoS One 8 (2013): e74756.
- Manikam L and Lakhanpaul M. Epidemiology of community acquired pneumonia. Paediatrics and Child Health 22 (2012): 299-306.
- Hay AJ, Gregory V, Douglas AR, et al. The evolution of human influenza viruses. Philos Trans R Soc Lond B Biol Sci 356 (2001): 1861-1870.
- Smyth A. Pneumonia due to viral and atypical organisms and their sequelae. Br Med Bull 61 (2002): 247-262.
- Carhan A, Altas AB, Albayrak N, et al. [Influenza surveillance results in 2007-2008 winter season in nine provinces of Turkey]. Mikrobiyol Bul 43 (2009): 235-241.
- Glezen WP, Frank AL, Taber LH, et al. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis 150 (1984): 851-857.
- ’O’Dempsey TJ, McArdle TF, Laurence BE, et al. Overlap in the clinical features of pneumonia and malaria in African children. Trans R Soc Trop Med Hyg 87 (1993): 662-665.
- English M, Punt J, Mwangi I, et al. Clinical overlap between malaria and severe pneumonia in Africa children in hospital. Trans R Soc Trop Med Hyg 90 (1996): 658-662.
- Suwanjutha S, Sunakorn P, Chantarojanasiri T, et al. Respiratory syncytial virus-associated lower respiratory tract infection in under-5-year-old children in a rural community of central Thailand, a population-based study. J Med Assoc Thai 85 (2002): 1111-1119.
- Yuksel H, Yilmaz O, Akçali S, et al. [Common viral etiologies of community acquired lower respiratory tract infections in young children and their relationship with long term complications]. Mikrobiyol Bul 42 (2008): 429-435.
- Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health 3 (1998): 268-280.
- Rudan I, Tomaskovic L, Boschi-Pinto C, et al. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ 82 (2004): 895-903.
- Nolan T, Borja-Tabora C, Lopez P, et al. Prevalence and Incidence of Respiratory Syncytial Virus and Other Respiratory Viral Infections in Children Aged 6 Months to 10 Years With Influenza-like Illness Enrolled in a Randomized Trial. Clin Infect Dis 60 (2015): e80-89.
- Huang G, Yu D, Mao N, et al. Viral etiology of acute respiratory infection in Gansu Province, China, 2011. PLoS One 8 (2013): e64254.
- Ghietto LM, Cámara A, Zhou Y, et al. High prevalence of human bocavirus 1 in infants with lower acute respiratory tract disease in Argentina, 2007-2009. Braz J Infect Dis 16 (2012): 38-44.
- Laurichesse H, Dedman D, Watson JM, et al. Epidemiological Features of Parainfluenza Virus Infections: Laboratory Surveillance in England and Wales, 1975-1997. European Journal of Epidemiology 15 (1999): 475-484.
- Bont L, Checchia PA, Fauroux B, et al. Defining the Epidemiology and Burden of Severe Respiratory Syncytial Virus Infection Among Infants and Children in Western Countries. Infect Dis Ther 5 (2016): 271-298.