Pre-Operative Predicting Model of Early Tumor Recurrence outside the Milan Criteria for Hepatocellular Carcinoma
Article Information
Hao-Chien Hung1, Jin-Chiao Lee1, Yu-Chao Wang1, Chih-Hsien Cheng1, Tsung-Han Wu1, Chen-Fang Lee1, Ting-Jung Wu1, Hong-Shiue Chou1, Kun-Ming Chan1, Wei-Chen Lee1*
1Division of Liver and Transplantation Surgery, Department of General Surgery, Chang-Gung Memorial Hospital, Chang-Gung University College of Medicine, Taoyuan, Taiwan
*Corresponding author: Wei-Chen Lee, Division of Liver and Transplantation Surgery, Department of General Surgery, Chang-Gung Memorial Hospital, Chang-Gung University College of Medicine, Taoyuan, Taiwan.
Received: 01 August 2022; Accepted: 08 August 2022; Published: 19 June 2023
Citation: Hao-Chien Hung, Jin-Chiao Lee, Yu-Chao Wang, Chih-Hsien Cheng, Tsung-Han Wu, Chen- Fang Lee, Ting-Jung Wu, Hong-Shiue Chou, Kun- Ming Chan, Wei-Chen Lee. Pre-Operative Predicting Model of Early Tumor Recurrence outside the Milan Criteria for Hepatocellular Carcinoma. Archives of Clinical and Biomedical Research 7 (2023): 408-417.
View / Download Pdf Share at FacebookAbstract
Purpose: Liver resection is a primary curative treatment for early stage hepatocellular carcinoma (HCC), but the outcomes are impeded by early tumor recurrence. This study aimed to establish a prediction model which could predict tumor recurrence outside the Milan criteria prior to liver resection.
Methods: A cohort of 891 HCC patients who had curative liver resections were reviewed. HCC recurrence was divided into 4 groups: early recurrence inside the Milan criteria (ER-MI), early recurrence outside the Milan criteria (ER-MO), late recurrence inside the Milan criteria (LR-MI), and late recurrence outside the Milan criteria (LR-MO). The risk factors of ER-MO were analyzed to establish a prediction model.
Results: During a median follow-up of 83.4 months, 589 (66.1%) patients developed recurrent HCC. Among 589 patients, 276 (31.0%), 86 (9.6%), 46 (5.2%) and 181(23.3%) patients were in ER-MO, ER-MI, LR-MO and LR-MI groups, respectively. In multivariate analysis of pre-operative factors, large tumors, non-single tumors, high α-fetoprotein level, high neutrophil-to-lymphocyte ratio and high indocyanine green retention were the independent predictors of ER-MO. By adjusting the weight of independent risk factors into scores and stratifying patients into class I, II, III and IV according to score 0, 2-6, 7-10 and ≥11, ER-MO events at 24 months were 12.5%, 22.5%, 45.4% and 66.3% for class I, II, III and IV, respectively (P< 0.05).
Conclusions: A risk scoring model was built and could predict ER-MO based on pre-operative factors. This scoring model would be helpful for sharing the decision marking of liver resection or primary liver transplantation with the patients.
Keywords
Hepatocellular Carcinoma; Liver Resection; Liver Transplantation; Milan Criteria; Tumor Recurrence
Hepatocellular Carcinoma articles; Liver Resection articles; Liver Transplantation articles; Milan Criteria articles; Tumor Recurrence articles.
Article Details
1. Introduction
Hepatocellular carcinoma (HCC) is an aggressive malignancy resulting in the third leading cause of cancer-related death worldwide. The treatment modalities for HCC include liver transplantation, liver resection, local ablation, transcatheter hepatic artery chemoembolization (TACE), molecular targetting therapy, immune checkpoint inhibitor, radiotherapy, etc. According to Barcelona Clinic Liver Cancer (BCLC) treatment strategies, liver resection remains a primary curative treatment for early stage HCC [1]. However, high tumor recurrent rate after liver resection is the major obstacle in treating early stage HCC [2,3]. Regarding the high tumor recurrent rate of liver resection for HCC, primary or salvage liver transplantation should be an option of treatments to achieve optimal outcomes. HCC may recur early or late after liver resection. Most of early recurrence is intrahepatic metastasis from primary tumors within 2 years, while late recurrence may develop from multicentric lesions [4]. Early recurrent HCC is interrelated with aggressive tumor biology and microenvironment, and leads to difficulty in treatment. Clinically, early recurrent tumors are frequently beyond the Milan criteria [5] once upon tumor recurrence is found. When the recurrent tumors are beyond the Milan criteria, the options of treatments are declined and the outcomes are less satisfactory than the tumors within the Milan criteria. Therefore, if the pattern of tumor recurrence can be predicted prior to liver resection, the treatment option may be altered to primary liver transplantation rather than liver resection if the primary tumors meet the Milan criteria. When recurrent tumors are beyond the Milan criteria, only non-curative treatments can be offered to the patients. Salvage liver transplantation is curative, but is not indicated until the recurrent tumors can be down-staged successfully. Obviously, it is important to plan the therapeutic strategies precisely at the beginning of HCC diagnosed. The aim of this study was to investigate the prognostic factors which could predict the pattern of tumor recurrence prior to liver resection, and try to build up a pre-operative risk scoring model in predicting recurrent tumors within or beyond the Milan criteria.
2. Materials and Methods
2.1 Patients
1005 HCC patients who had curative liver resection at Lin-Kou Chang Gung Memorial Hospital from 2003 to 2009 were reviewed. All patients’ tumor status was at BCLC stage A and B. Curative liver resection was defined as removal of all macroscopic tumors in pre-operative images and without any residual tumor at surgical margin microscopically. The patients who received other HCC treatments prior to liver resection (n=67) and who had other synchronous or metachronous malignancies (n= 41) were excluded. The patients with surgical mortalities (n=6) were also excluded since the present study was purposed to assess outcomes of tumor recurrence. The remained 891 patients were included in this study. The study was approved by local Ethic Committee of Chang-Gung Memorial Hospital (IBR No. 104-3900B).
2.2 Diagnosis of HCC Recurrence
All patients were followed up regularly after liver resection. Liver function and a-fetoprotein (AFP) were measured every 3 months. Liver ultrasonography were performed every 3 months, too. When liver ultrasonography revealed a suspicious liver nodule or an elevated AFP level was noted, tri-phasic computed tomography (CT) or magnetic resonance imaging (MRI) would be performed to look for any evidence of tumor recurrence. HCC recurrence was defined as typical imaging features of HCC (contrast uptake in arterial phase followed by washout in venous phase) in tri-phasic images of CT or MRI.
2.3 Stratification of HCC Recurrence
HCC recurrence was divided into early and late recurrence. Early recurrence was defined as the tumor recurrence within two years after liver resection, and late recurrence was defined as the tumor recurrence beyond two years after liver resection [6]. The recurrent tumors were further stratified into inside or outside the Milan criteria: solitary tumor < 5 centimeters in maximum diameter, 2 or 3 tumors without any one > 3 centimeters or more than 3 nodules in total tumor number, no major portal/hepatic vein branches invasion by tumor, and no evidence of extrahepatic metastasis [5]. Consequently, the patients with tumor recurrence were stratified into 4 groups: early recurrence inside the Milan criteria (ER-MI), early recurrence outside the Milan criteria (ER-MO), late recurrence inside the Milan criteria (LR-MI), and late recurrence outside the Milan criteria (LR-MO).
2.4 Data Extraction and Clinical Outcome Measurement
Demographic variables, routine hematology/biochemistry, clinicopathological parameters and tumor associated pathologic/histologic factors were documented. Disease-free survival was measured from the date of liver resection to tumor recurrence. Overall survival was measured from the date of liver resection to last follow-up or death which was registered in Bureau of Health, Taiwan.
2.5 Prediction Model for Early Recurrence outside the Milan Criteria
To establish the prediction model for tumor recurrence, the weight of each independent risk factor was determined and referenced to ‘’The Framingham Study risk score’’ as Sullivan et al. described [7].
2.6 Statistical Analysis
Pearson’s chi-square test was used to compare clinical parameters and categorical variables between recurrent patterns. Kaplan–Meier methods were used to assess survival and the differences between subgroups were analyzed by the log-rank test. All potential risk factors of ER-MO recognized in the univariate analysis (p < 0.1) were recruited into multivariate analysis. The discrimination-ability of ER-MO was examined using the area under curve (AUC) under the receiver operating characteristic (ROC). P < 0.05 was considered to be statistically significant. Analyses were done using IBM SPSS version 24.0 (SPSS Incorporation).
3. Results
3.1 Characteristics of Patients and Recurrent Patterns
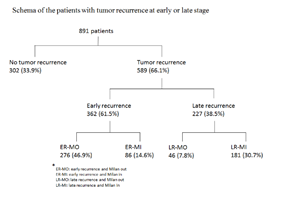
A total of 891 patients who underwent curative liver resection for HCC were enrolled in this study. Male predominance (n=706, 79.2%) and high prevalence of viral hepatitis (n=713, 80.0%) were observed. The mean age was 57.0 ± 13.1 with a range from 11.1 to 89.4 years. The majority (n=656, 73.6%) of the patients had single tumor. Near half of the patients (n=411, 46.1%) had cirrhosis, but most of them (n=880, 98.8%) were classified into Child-Pugh class A (Table 1). After a median follow-up of 83.4 months, 589 (66.1%) patients developed recurrent HCC and 362 (40.6%) of them had early tumor recurrence. Among these 362 patients with early tumor recurrence, 276 (31.0%) patients had tumor recurrence beyond the Milan criteria and 86 (9.6%) patients’ tumors were within the Milan criteria. The other 227 (25.5%) patients had late recurrence. 181 (23.3%) patients’ tumors were inside the Milan criteria and 46 (5.2%) patients’ tumors were outside Milan criteria (Figure 1).
Figure 1: Schema of the patients with tumor recurrence at early or late stage. 589 (66.1%) patients developed recurrent HCC and 362 (40.6%) of them had early tumor recurrence. Among these 362 patients with early tumor recurrence, 276 (31.0%) patients had tumor recurrence beyond the Milan criteria and 86 (9.6%) patients’ tumors were within the Milan criteria. The other 227 (25.5%) patients had late recurrence. 181 (23.3%) patients’ tumors were inside the Milan criteria and 46 (5.2%) patients’ tumors were outside Milan criteria.
|
n = 891 |
|
|
Preoperative conditions |
|
|
Age (≤ 65/ > 65 years old) |
618 (69.4%)/ 273 (30.6%) |
|
Gender (Male/ Female) |
706 (79.2%)/ 185 (20.8%) |
|
CTP score (Class A/ Class B) |
880 (98.8%)/ 11 (1.2%) |
|
Hepatitis status (HBV/ HCV/ both HBV and HCV infection/ none) |
465 (52.2%)/ 199 (22.3%)/ |
|
49 (5.5%)/ 178 (20.0%) |
|
|
Total bilirubin (≤ 1.3/ > 1.3 mg/dL) |
816 (91.6%)/ 75 (8.4%) |
|
Albumin (≤ 3.5/ > 3.5 g/dL) |
92 (10.3%)/ 799 (89.7%) |
|
ICG R15 minutes (≤ 10/ > 10%) |
610 (68.5%)/ 281 (31.5%) |
|
INR (≤ 1.2/ > 1.2) |
839 (94.2%)/ 52 (5.8%) |
|
Plateleta (≤ 100/ > 100 /103μl) |
96 (11.4%)/ 746 (88.6%) |
|
NLR (≤ 2.5/ > 2.5) |
682 (76.5%)/ 209 (23.5%) |
|
Perioperative factors |
|
|
Blood loss, mL (≤ 500/ > 500) |
724 (81.3%)/ 167 (18.7%) |
|
Resection margin, mm (≤ 10/ > 10) |
699 (78.5%)/ 192 (21.5%) |
|
Tumor-associated factors |
|
|
Tumor size (≤ 3/ 3-5/ > 5 cm) |
371 (41.6%)/ 238 (26.7%)/ 282 (31.7%) |
|
Multiplicity (Solitary/ Multiple) |
656 (73.6 %)/ 235 (26.4%) |
|
Alpha-fetoprotein (≤ 800/ > 800 ng/mL) |
741 (83.2%)/ 150 (16.8%) |
|
Capsule (Absence/ Presence) |
164 (18.4%)/ 767 (71.6%) |
|
Tumor rupture (No/ Yes) |
854 (95.8%)/ 37 (4.2%) |
|
Micro-vascular invasion (No/ Yes) |
631 (70.8%)/ 260 (29.2%) |
|
Histology grade (1-2/ 3-4)a |
527 (59.1%)/ 362 (40.9%) |
|
Necrotic change (No/ Yes) |
436 (48.9%)/ 455 (50.1%) |
|
Cirrhosis (No/ Yes) |
480 (53.9%)/ 411 (46.1%) |
|
HAI score (≤ 4 /> 4) |
547 (61.4%)/ 344 (38.6%) |
Table 1: Demographics of 891 HCC patients underwent curative liver resection
a: Not all data were available
CTP- Child-Turcotte-Pugh classification; HBV- Hepatitis B Virus; HCV- Hepatitis C Virus; ICG R15- Indocyanine Green Serum 15-Minute Retention Rate; INR- International Normalized Ratio; HAI- Histological Activity Index.
3.2 Survival in HCC Patients According to Recurrent Patterns
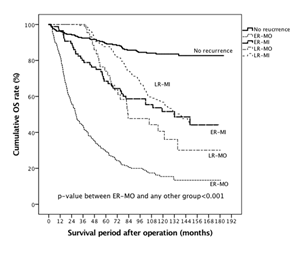
After a median follow-up of 83.4 months, 413 patients (46.4%) died. The cumulative overall survival rates for all enrolled patients were 67.7% and 51.8% at 5 and 10 years, respectively. When the patients with tumor recurrence were stratified into 4 groups according to recurrent patterns, cumulative overall survival rates at 5 and 10 years were 89.5% and 83.6% for the patients without tumor recurrence, 87.7% and 56.8% for LR-MI group, 74.0 and 40.6% for LR-MO group, 68.4% and 51.6% for ER-MI group, and 29.4 and 15.5% for ER-MO group, respectively. ER-MO group had an inferior outcome to other recurrent patterns (Figure. 2, P < 0.001). The long-term survival between ER-MI and LR-MI was comparable. Therefore, ER-MO had the worst outcome and seemed different in natures from other recurrent patterns.
Figure 2: The Kaplan-Meier survival curves for different patterns of tumor recurrence. The patients with tumor recurrence were stratified into 4 groups according to recurrent patterns. The cumulative overall survival rates at 5 and 10 years were 89.5% and 83.6% for the patients without tumor recurrence, 87.7% and 56.8% for LR-MI group, 74.0 and 40.6% for LR-MO group, 68.4% and 51.6% for ER-MI group, and 29.4 and 15.5% for ER-MO group, respectively.
3.3 Clinical Characteristics According to Different Recurrent Patterns
According to 4 recurrent patterns, the clinicopathological features of the patients with tumor recurrence were presented in table 2. For patients in ER-MO and ER-MI groups, there were no significant difference between age, gender, hepatitis status, hematology/biochemistry data, indocyanine green 15-minute retention test (ICG R15) and serum bilirubin. But, the patients in ER-MO group were highly associated with large tumors, multiple tumors, and micro-vascular invasion (P<0.05) and also had the tendency toward high serum AFP level and absence of tumor capsule. When the patients in ER-MO group were compared to LR-MI group patients who had a better outcomes, the patients in ER-MO group had higher population with high level of AFP and worse tumor characteristics including large-sized tumors, multiple tumors, poor histological differentiation, and microvascular invasion (Table 2).
Table 2: The demographic comparative study of early recurrent HCC outside the Milan criteria to other patterns of tumor recurrence.
- Not all data were available
- ER-MO- Early Recurrence Outside the Milan Criteria; ER-MI- Early Recurrence Inside the Milan Criteria; LR-MO- Late Recurrence outside the Milan Criteria; LR-MI- Late Recurrence Inside the Milan Criteria; HBV- Hepatitis B Virus; HCV- Hepatitis C Virus; ICG R15- Indocyanine Green Retention Test; AFP- Alpha-Fetoprotein; HAI- Histological Activity Index.
- ER-MO versus ER-MI; d. ER-MO versus LR-MO; e. ER-MO versus LR-MI
3.4 Development of ER-MO Prediction Model
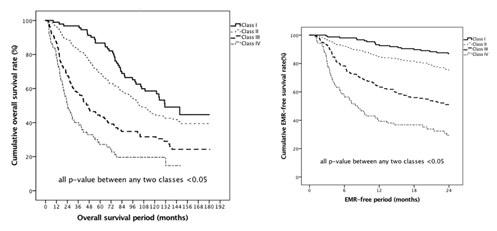
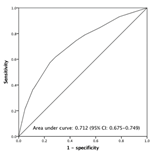
The ER-MO group had the worst survival, compared to other groups. In this study, we tried to identify the risk factors of ER-MO and establish a predicting model of ER-MO prior to liver resection. The results of univariate and multivariate logistic regression analyses of prognostic factors for EM-MO were shown in table 3. In univariate analysis, seven pre-operative and three peri-/post-operative risk factors were identified including large tumor size, non-single tumor, hypoalbuminemia, high AFP level, high neutrophil-to-lymphocyte ratio (NLR), high ICG R15, history of tumor rupture, high intraoperative blood loss, presence of micro-vascular invasion and high histology grade. In the further multivariate analysis focusing only on pre-operative factors, large tumor size (for 3-5cm, HR = 1.90; for > 5cm, HR = 3.39), non-single tumor (HR = 1.76), high AFP (HR = 5.56), high NLR (HR = 1.60) and high ICG R15 (HR = 1.58) were the independent predictors of ER-MO. The relative points were transformed from multiplying the coefficients (β) by five and then run into integer to construct a scoring system. Different score allocations were given 2 points for AFP > 800ng/dL, 2 points for ICG R15 >10%, 2 points for preoperative NLR > 2.5, 4 points for multiple tumors, 3 points for tumor size between 3 to 5 centimeters and 5 points for tumor size > 5 centimeters. Full score is 15 points. As expected, there was no patient situated in a sum score of 1, 12, and 14 by possible permutation, and we also did not have any patient with a full score of 15. To simplify the calculation, we grouped every two score and got 8 groups in sequence (0, 2, 3-4, 5-6, 7-8, 9-10, 11, 13). By comparing the Kaplan-Meier survival curves for the 8 groups, the risk of ER-MO was re-stratified into 4 classes with no survival difference between intra-class groups. Class I, II, III and IV were corresponded to score 0, 2-6, 7-10 and ≥11, respectively (Table 4). Subsequently, this scoring system was used for analyses. The ER-MO event at 24 months after surgery was 12.5% for class I, 22.5% for class II, 45.4% for class III and 66.3% for class IV, respectively (Figure 3a, P< 0.05 between any two groups). Our scoring model also deliberate long-term overall survival difference between each class. The median survival was 104.4, 88.8, 35.2 and 16.9 months for class I to IV, respectively. The 5- and 10-year overall survival were 86.7% and 58.6% for class I, 69.1% and 44.7% for class II, 43.8% and 31.6% for class III, and 27.2% and 19.6% for class IV, respectively (Fig. 3b, P< 0.05 between any two groups). The ROC curve of our scoring system to predict ER-MO had an AUC of 0.712 (95% CI: 0.675-0.749) with a best cut-off at 6. (Figure 4).
Figure 3: The Kaplan-Meier survival curves for the scoring-stratified groups. According to predicting model of scores, the risk of ER-MO was re-stratified into 4 classes. (a) The ER-MO event at 24 months after surgery was 12.5% for class I, 22.5% for class II, 45.4% for class III and 66.3% for class IV, respectively (P< 0.05 between any two groups). (b) The 5- and 10-year overall survival were 86.7% and 58.6% for class I, 69.1% and 44.7% for class II, 43.8% and 31.6% for class III, and 27.2% and 19.6% for class IV, respectively (P< 0.05 between any two groups).
|
Univariate |
Multivariate* |
|||||
|
HR |
95%CI |
p-value |
HR |
95%CI |
p-value |
|
|
Pre-operative factors |
||||||
|
Tumor size, cm |
|
|||||
|
≤ 3 |
1 |
|
||||
|
3-5 |
1.54 |
1.04-2.27 |
0.03 |
1.9 |
1.15-3.14 |
0.012 |
|
> 5 |
4.39 |
3.10-6.23 |
<0.001 |
3.39 |
2.10-5.50 |
<0.001 |
|
Tumor multiplicity |
|
|||||
|
Single |
1 |
|
||||
|
Multiple |
2.26 |
1.32-3.86 |
0.003 |
2.66 |
1.05-4.04 |
<0.001 |
|
Albumin, g/dL |
|
|||||
|
≤ 3.5 |
1.51 |
.97-2.36 |
0.071 |
|
||
|
> 3.5 |
1 |
|
||||
|
Alpha-fetoprotein, ng/mL |
|
|||||
|
≤ 800 |
1 |
|
||||
|
> 800 |
2.56 |
1.78-3.67 |
<0.001 |
1.76 |
1.07-2.90 |
0.027 |
|
NLR |
|
|||||
|
≤ 2.5 |
1 |
|
||||
|
> 2.5 |
1.74 |
1.22-2.49 |
0.002 |
1.6 |
1.15-2.45 |
0.03 |
|
ICG R15, % |
|
|||||
|
≤ 10 |
1 |
1 |
|
|||
|
> 10 |
1.39 |
1.02-1.88 |
0.038 |
1.58 |
1.05-2.37 |
0.027 |
|
Tumor rupture |
|
|||||
|
No |
1 |
|
||||
|
Yes |
3.89 |
1.97-7.68 |
<0.001 |
|
||
|
Peri- and post-operative factors |
||||||
|
Intra-operative blood loss, mL |
||||||
|
≤ 500 |
1 |
|||||
|
> 500 |
2.32 |
1.64-3.28 |
<0.001 |
|||
|
Micro-vascular invasion |
||||||
|
No |
1 |
|||||
|
Yes |
4.92 |
1.31-3.10 |
<0.001 |
|||
|
Histology, grade |
||||||
|
I-II |
1 |
|||||
|
III-IV |
1.29 |
1.84-1.87 |
0.087 |
Table 3: Univariate and multivariate analyses of preoperative factors for early tumor recurrence outside the Milan criteria.
NLR, neutrophil-to-lymphocyte ratio; ICG R15, Indocyanine green 15-minute retention test;
*: Multivariate analysis was only applied for pre-operative factors
|
Variables |
Score allocationa |
Total score |
No. of patients |
ER-MO event (%) |
|
IGG R15 ≤ 10% |
0 |
0 |
152 |
19 (12.5) |
|
>10% |
2 |
|||
|
NLR ≤ 2.5 |
0 |
1-2 |
120 |
24 (20.0) |
|
> 2.5 |
2 |
|||
|
Tumor sizeb |
3-4 |
137 |
27 (19.7) |
|
|
< 3cm |
0 |
|||
|
3-5cm |
3 |
|||
|
>5cm |
5 |
5-6 |
175 |
48 (27.4) |
|
7-8 |
138 |
57 (41.3) |
||
|
AFP> 800 ng/mL |
2 |
9-10 |
80) |
42 (52.5) |
|
Multiple tumors |
4 |
11-12 |
67 |
44 (65.7) |
|
13-15 |
22 |
15 (68.2) |
||
|
Class I |
0 |
152 (17.1) |
19 (12.5) |
|
|
ER-MOc assessment |
Class II |
1-6 |
432 (48.5) |
99 (22.9) |
|
Class III |
7-10 |
218 (24.5) |
99 (45.4) |
|
|
Class IV |
≥ 11 |
89 (10.0) |
59 (66.3) |
Table 4: Model for ER-MO after curative hepatectomy based on pre-operative risks.
ER-MO, early recurrence outside the Milan criteria; NLR, neutrophil-to-lymphocyte ratio; ICG R15, Indocyanine green 15-minute retention test.
- The regression coefficients (β) were multiplied by five and rounded to integer in order to calculate given score
- The maximum diameter of presented tumor
- Area under curve=0.712 (95% CI: 0.675-0.749) for risk-scoring model in predicting EM-MO
3.5 Treatments for Recurrent HCC
The principle of treatments for recurrent HCC is the same as primary diseases by considering disease extension, liver function and ECOG-Performance Status (PS). In the present study, TACE accounted for the most common treatment (369/589 patients, 62.6%) for recurrent HCC. Among the patients with tumor recurrence, 114 (19.4%) patients had curative treatments, including 80 re-hepatectomyies, 14 RFA and 20 liver transplantation. For the 80 patients received re-hepatectomy, 15 patients were in ER-MO group, 24 patients in ER-MI group, and 41 patients in LR-MO/LR-MI groups. For the 20 patients (9 in ER-MO group and 11 in other groups) undergoing liver transplantation, 15 (75.0%) of them had TACE for disease-control or down-staging prior to salvage liver transplantation. The median waiting time between recurrence and liver transplantation were 15.2 months for all transplant recipients, but 30.0 months for the 9 patients in ER-MO group, who were able to have liver transplantation after successful down-staging of tumors. Six of 9 (66.7%) patients in ER-MO group achieved long-term post-transplant disease-free survival and 3 died of HCC recurrence after liver transplantation. Therefore, for the patients in ER-MO group, the curative chance was only 8.7% (24 in 276 patients) including 15 patients having re-hepatectomies and 9 patients having liver transplantation after successful down-staging of recurrent HCC.
4. Discussion
The prognosis of liver resection for HCC is impeded by high tumor recurrent rate. When HCC recurs, curative treatments such as re-hepatectomy or salvage liver transplantation still can be applied to rescue patients. However, the recurrent tumors may be beyond the Milan criteria once upon recurrent HCC is found and curative treatments are declined. According to multivariate analysis, large tumors, multiple tumors, NLR > 2.5, ICG R15 > 10%, and AFP > 800 ng/mL were the significant pre-operative factors of ER-MO for the patients receiving curative hepatectomy for HCC. When transforming these risk factors to scores, four prognostic classes could be stratified as score 0 for class I, score 1-6 for class II, score 7-10 for class III, and score ≥11 for class IV. This scoring system was well-correlated with long-term survival; and Class III and IV counted for 45.4% and 66.3% to develop worst prognostic ER-MO, respectively. Therefore, this pre-operative risk-scoring model for ER-MO prediction fits well for clinical needs. It provides predicting effects of surgery by five easily available pre-operative factors and can serves as a reference for making clinical decisions before surgery. ER-MO presents the worst outcomes for HCC patients with tumor recurrence after liver resection. HCC recurrence can be defined as early and late while early recurrence was reappearance of tumor within 2 years after liver resection [4,8]. Early recurrence was commonly regarded as a consequence from intrahepatic metastases, which originated from the primary tumors [9,10] and highly associated with vascular invasion for tumor spreading [10]. Early recurrence was also highly linked with ER-MO as 76.2% of patients with early tumor recurrence had tumors beyond Milan criteria once upon the recurrent HCC was found. Thus, early recurrence indicates an inferior survival [11]. Fortunately, a minor portion of patients with early recurrence were within Milan criteria (ER-MI) and had compatible survival to the patient with LR-MI. Obviously, HCC with ER-MO were different from other recurrent patterns in nature. It could be the different intrinsic nature of ER-MO, which contributed to poor outcomes [4,12]. Several unfavorable factors were mentioned by Kim et al for recurrent HCC beyond Milan criteria after hepatectomy for solitary HCC [13]. However, there is still few studies specifically discussing ER-MO after hepatecotmy. As ER-MO presented with aggressive tumor biology and was rarely curable in current practice, it is essential to identify high risk patients with ER-MO and to make an effort to detect disease recurrence early in order to arrange proper treatments. In spite of absence of definite adjuvant therapy after hepatectomy, therapeutic approach to achieve a sustained viral suppression and even eradication of the virus via antiviral agents are important to prevent cirrhosis progression, HCC recurrence, and liver failure [14]. Moreover, primary liver transplantation may be considered if the patients are high risk to develop ER-MO after hepatectomy. AFP level and tumor sizes reflected tumor biology. High serum AFP level not only reflects biological tumor burden but also causes tumor progression because it takes part in promoting metastasis of HCC by over-expressing certain oncogenes such as K19, EpCAM, MMP2/9, and CXCR4 [15]. Large-sized tumors are related to presence of micro-vascular invasion and micro-satellite nodules [16]. Both micro-vascular invasion and micro-satellite nodules often contribute to an aggressive tumor biology and poor clinical outcomes. Microscopic hidden lesions may be responsible for ER-MO after liver resections. A retrospective study based on prospective database emphasized the impact of pathologic profile of the first resected tumors, and demonstrated that the presence of either satellite nodules or microvascular invasion on the primary resected tumors declined the survival benefit of curative-intent therapy for recurrent diseases [17]. Pre-operative serum NLR has been considered as a reliable and easily-available information in evaluating cancer-related inflammatory microenvironment [18]. Evidences were shown that a higher level also indicated a negative result after liver resection [19,20]. Pro-tumor inflammatory microenvironment initiated by tumor-associated macrophages (TAMs) in HCC accelerates cancer progression [19,21]. A correlation between TAMs and serum NLR through upregulated inflammation-related cytokines was demonstrated in previous studies [22]. The degree of compactness of TAMs near micro-vascular regions may also explain the development of micro-metastases and subsequent eventful ER-MO [23]. Repeated hepatectomy and salvage liver transplantation are the curative treatments for recurrent HCC. Re-hepatectomy rate for recurrent HCC was between 15.8% to 25.1% [24,25], and long-term survival benefits were compatible to primary liver resection with 31.8 to 50.9% of 5-year overall survival [26,27]. Salvage liver transplantation not only achieves tumor eradication but also cures liver diseases. The outcomes of salvage liver transplantation is comparable to primary liver transplantation [28,29]. When HCC recurs after liver resection, liver transplantation might be the first choice of treatment with an optimal outcome. Previous retrospective studies [30] indicated salvage liver transplantation for HCC recurrence after primary hepatetomy is feasible, and it provided similar overall survival rate as the patients receiving primary liver transplantation. However, the possibility of salvage liver transplantation was only about 40% if tumor recurred after liver resection, and outcomes of salvage liver transplantation was probably impeded by extrahepatic spreading and aggressive tumor behavior of recurrent HCC. In the present study, we’ve conducted salvage liver transplantation for ER-MO HCC after a successful down-staging and 2-3 month waiting time to ensure tumor aggressiveness and obtained an acceptable outcomes. A pre-operative risk scoring model could be established in this study to predict the possibility of ER-MO if tumor recurred after liver resection. This scoring model is supposedly applied to choose treatment options and will favor primary liver transplantation rather than liver resection if the preoperative risk score is more than 6. However, this is a retrospective study and no patients undergo primary liver transplantation according to this scoring model in this study. It is still unknown whether primary liver transplantation can avoid tumor recurrence and improve outcomes for the HCC with high possibility of ER-MO after liver resection. In conclusion, early recurrence (ER-MO) is a clinical challenge in treating HCC after primary curative resection. In this study, we have built up a risk scoring model to predict ER-MO based on pre-operative factors. This scoring model can be helpful for choosing of treatment options. However, further prospective study is needed to verify this risk scoring model.
Author Contributions
Conception and design- Hao-Chien Hung, Jin-Chiao, Lee Wei-Chen Lee: Acquisition of data- Ting-Jung Wu, Hong-Shiue Chou, Kun-Ming Chan: Analysis and interpretation of data- Chen-Fang Lee, Tsung-Han Wu, Yu-Chao Wang, Chih-Hsien Cheng: Drafting the Article- Hao-Chien Hung and Wei-Chen Lee.
Funding
The authors declare no funding was received for this study
Availability of Data and Material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Conflicts of Interest
There is no conflict of interest among authors.
References
- Yamashita Y, Tsuijita E, Takeishi K, et al. Trends in surgical results of hepatic resection for hepatocellular carcinoma: 1,000 consecutive cases over 20 years in a single institution. Am J Surg 207 (2014): 890-896.
- Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int 7 (2008): 237-257.
- Kamiyama T, Nakanishi K, Yokoo H, et al. Recurrence patterns after hepatectomy of hepatocellular carcinoma: implication of Milan criteria utilization. Annals of surgical oncology 16 (2009): 1560-1571.
- Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 89 (2000): 500-507.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334 (1996): 693-699.
- Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med 359 (2008): 2045-2047.
- Sullivan LM, Massaro JM, D'Agostino RB, Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 23 (2004): 1631-1660.
- Du ZG, Wei YG, Chen KF, et al. Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution's experience with 398 consecutive patients. Hepatobiliary Pancreat Dis Int 13 (2014): 153-161.
- Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. Journal of clinical oncology 28 (2010): 3994-4005.
- de Lope CR, Tremosini S, Forner A, et al. Management of HCC. Journal of hepatology 56 (2012): S75-87.
- Kobayashi T, Aikata H, Kobayashi T, et al. Patients with early recurrence of hepatocellular carcinoma have poor prognosis. Hepatobiliary Pancreat Dis Int 16 (2017): 279-288.
- Regimbeau JM, Abdalla EK, Vauthey JN, et al. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. Journal of surgical oncology 85 (2004): 36-41.
- Kim JM, Joh JW, et al. Predicting Hepatocellular Carcinoma Recurrence Beyond Milan Criteria After Liver Resection for Solitary Hepatocellular Carcinoma. Journal of gastrointestinal surgery (2019).
- Kobayashi T, Ishiyama K, Ohdan H. Prevention of recurrence after curative treatment for hepatocellular carcinoma. Surgery today 43 (2013): 1347-1354.
- Lu Y, Zhu M, Li W, et al. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med 20 (2016): 549-558.
- Lim C, Mise Y, Sakamoto Y, et al. Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World journal of surgery 38 (2014): 2910-2918.
- Meniconi RL, Komatsu S, Perdigao F, et al. Recurrent hepatocellular carcinoma: A Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery 157 (2015): 454-462.
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 102 (2001): 5-14.
- Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Annals of surgery 258 (2013): 301-305.
- Hung HC, Lee JC, Cheng CH, et al. Impact of neutrophil to lymphocyte ratio on survival for hepatocellular carcinoma after curative resection. Journal of hepato-biliary-pancreatic sciences 24 (2017): 559-569.
- Capece D, Fischietti M, Verzella D, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. BioMed research international 2013 (2013): 187204.
- Zhao Q, Xiao X, Wu Y, et al. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. European journal of immunology 41 (2011): 2314-2322.
- Peng SH, Deng H, Yang JF, et al. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol 11 (2005): 6521-6524.
- Kubo S, Takemura S, Uenishi T, et al. Second hepatic resection for recurrent hepatocellular carcinoma in patients with chronic hepatitis C. World journal of surgery 32 (2008): 632-638.
- Itamoto T, Nakahara H, Amano H, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery 141 (2007): 589-597.
- Nagano Y, Shimada H, Ueda M, et al. Efficacy of repeat hepatic resection for recurrent hepatocellular carcinomas. ANZ J Surg 79 (2009): 729-733.
- Wu CC, Cheng SB, Yeh DC, et al. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. The British journal of surgery 96 (2009): 1049-1057.
- Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 55 (2012): 132-140.
- Bhangui P, Allard MA, Vibert E, et al. Salvage Versus Primary Liver Transplantation for Early Hepatocellular Carcinoma: Do Both Strategies Yield Similar Outcomes? Annals of surgery 264 (2016): 155-163.
- Liu F, Wei Y, Wang W, et al. Salvage liver transplantation for recurrent hepatocellular carcinoma within UCSF criteria after liver resection. PloS one 7 (11): e48932.