Predictive value of Procalcitonin Clearance for Prognosis in Intensive Care Unit Patients with Sepsis: A Cohort Study
Article Information
Xuan Song1*, Gangbing Song1, Xinyan Liu1, Huairong Wang2, Xiuyan Guo2, Maopeng Yang1, Daqiang Yang1, Yahu Bai1, Ziwei Liu3, Nana Zhang1
1ICU, DongE Hospital Affiliated to Shandong First Medical University, Liaocheng, China
2Education Department, DongE Hospital Affiliated to ShandongFirst Medical University, Liaocheng, China
3Qingdao University, Qingdao, China
*Corresponding Author: Xuan Song, ICU, DongE Hospital Affiliated to Shandong First Medical University, Liaocheng, China
Received: 02 March 2020; Accepted: 11 March 2020; Published: 20 March 2020
Citation: Xuan Song, Gangbing Song, Xinyan Liu, Huairong Wang, Xiuyan Guo, Maopeng Yang, Daqiang Yang, Yahu Bai, Ziwei Liu, Nana Zhang. Predictive value of Procalcitonin Clearance for Prognosis in Intensive Care Unit Patients with Sepsis: A Cohort Study. Archives of Microbiology & Immunology 4 (2020): 026-037.
View / Download Pdf Share at FacebookAbstract
Objective: Early diagnosis, accurate assessment of the prognosis are essential for the effective treatment of sepsis patients. To investigate the predictive value of procalcitonin clearance (PCTc) for prognostic evaluation of the patients with sepsis.
Material and Methods: Clinical data of 138 patients with sepsis and septic shockwere retrospectively collected. Procalcitonin (PCT) was collected for 4 consecutive days after sepsis diagnosis and PCTc was calculated.
Results: The overall 28-day mortality rate was 23.2% (32/138). The PCTc in the survival group was significantly higher than that in the death group after 48 hours [57.51 (36.06 to 70.73)% vs. 6.6 (-35.33 to 43.93)%, P < 0.001], as well as after 72 hours of treatment [77.47 (59.84 to 86.31)% vs. 22.30 (-62.38 to 63.44)%, P < 0.001]. ROC curve analysis indicated that the area under the curve of 48-hour PCTc (PCTc-48) was 0.811, 95% CI (0.724, 0.898), P < 0.001, while 72-hour PCTc (PCTc-72) was 0.818, 95% CI (0.734, 0.902), P < 0.001. In addition, we found that the difference between the prognosis of sepsis caused by different infection sites was statistically significant (P < 0.001), Sepsis patients with pulmonary infection showed the worst prognosis compared with urinary tract infection and abdominal infection.
Conclusion: PCTc can be an important indicator to evaluate the prognosis in patients with sepsis. PCTc and infection site are risk factors for the prognosis in patients with sepsis.
Keywords
Sepsis; Septic shock; Procalcitonin clearance (PCTc); Procalcitonin (PCT); APACHE II score; Prognosis
Article Details
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, which has emerged as a huge therapeutic challenge for clinicians[1]. The incidence of sepsis has significantly increased over the past 30 years due to aging, increased use of immunosuppressive drugs, and the wide spread multi-drug resistant bacterial strains causing infections[2]. However, diagnosis of sepsis is challenging due to similar manifestations observed between systemic inflammatory response syndrome (SIRS) and non-infectious diseases[3, 4].
Severe sepsis and septic shock are the most leading causes of death in critically ill ICU patients, with a hospital mortality rate ranging from 30% to 50%[5, 6]. In addition, due to the long term treatment and expensive hospitalization in such patients, an accurate and effective evaluation of their prognosis is of great significance[7]. Commonly used inflammatory indicators, such as white blood cell count, C-reactive protein, erythrocyte sedimentation rate and PCT, have been widely used in the early diagnosis of sepsis, of which PCT is internationally recognized as an effective monitoring indicator of sepsis due to its high sensitivity and specificity, and is also of significance in the evaluation of prognosis[8].
Procalcitonin (PCT) is a glycoprotein precursor polypeptide of calcitonin. Under normal conditions, all PCT are cleared by the body to undetectable levels in the serum of healthy individuals. However, in severe bacterial infection, macrophages and monocytes in the liver, lymphocytes and endocrine cells in the lung and intestinal tissues can also synthesize and secrete procalcitonin under the action of endotoxin, TNF-α and IL-6, serum PCT can even rise rapidly to above 100 ng / mL[9, 10], and there is a dynamic trend in the body. During the inflammatory process caused by sepsis, the initial absolute peak value of PCT occurs at 6-24 hours and has a half-life of around 24---35h. PCT has high sensitivity and specificity in the diagnosis of sepsis and has been reported to be used as an important diagnostic indicator for sepsis[11]. Most studies have shown that a single measurement of PCT has a poor predictive value for mortality in patients with sepsis. ICU mortality is associated with sustained high PCT levels, suggesting that dynamic changes in PCT may be a more valuable biomarker for the diagnosis of sepsis patients[12, 13]. Dynamic assessment of PCT can provide more information about patient survival. The concept of PCTc has been introduced in several studies as a tool to monitor the evolution of PCT levels during sepsis[14, 15]. However, there are few studies on the predictive value of PCTc for prognosis in patients with sepsis. This study is aimed to compare the correlation between PCTc and prognosis at 48 hours and 72 hours after sepsis treatment.
Materials and methods
Inclusion and exclusion criteria
Patients with sepsis (including sepsis and septic shock) admitted to the ICU of DongE Hospital Affiliated to Shandong First Medical University from September 1, 2015 to September 1, 2018 was selected in this study. Inclusion criteria: (1) Age more than18 years; (2) All patients were in accordance with the diagnostic criteria of the 2016 Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock[16]; (3) hospital ICU stay > 72h. (4) Patients with complete recorded clinical data. Exclusion criteria: (1) Patients with irreversible conditions when admitted to ICU, such as malignant tumors and chronic renal insufficiency, etc. (2) Patients with immune system disorders or blood system diseases. (3) Pregnant women (4) Patients who have discontinued the treatment.
Ethics
The study protocol was in accordance with the ethics standards and gained approval from the ethics committee (approval number: 2018-035). This was a retrospective study in which the informed consent was exempted by the Ethics Committee.
Data collection
This is a retrospective cohort study, we estimated the sample size before data collection. Clinical data of eligible patients were collected from electronic medical records, including age, gender, diagnosis, combined underlying diseases, acute physiology and chronic health evaluation (APACHE II) score, laboratory results including all PCT measurements, source of infection, the pathogenic microorganisms infected and patient outcome. For all patients, pathological specimens (blood, urine, sputum and secretions, etc.) were collected for testing before administration of antibiotics. These data was entry cross-checked.
PCT measurement
For PCT measurement, we used COBAS e 601 (Hoffmann-La Roche, Inc, Basel, Switzerland) (the reagent was also a product of Roche). Serum PCT measured initially at the time of diagnosis of sepsis was considered as the baseline value of PCT (denoted as PCT-1). Subsequently, serum PCT levels were measured again on 2nd, 3rd and 4th day, which were denoted as PCT-2, PCT-3, and PCT-4, respectively. Procalcitonin clearance was calculated after 48 and 72 hours and was expressed as PCTc. For example, 48-hour procalcitonin clearance (PCTc-48) = (PCT-1) – (PCT-3) /( PCT-1) * 100%[6]. Similarly, the 72-hour PCTc (PCTc-72) was calculated. The maximum PCT value during the treatment was denoted as PCTmax.
Methods
The subjects were divided into survival and death groups according to their survival duration at 28 days. The predictive value of APACHE II score, PCT-1, PCTc-48 and PCTc-72 were analyzed for assessing the prognosis in patients with sepsis and septic shock. In addition, the patients were grouped according to their site of infection, so as to compare the predictive value of different infection sites. Moreover, the prognosis in ICU patients with sepsis caused by infection at different sites was analyzed based on their different PCTc levels.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software. Measured data in accordance with normal distribution (such as age, APACHE II score, PCT-1 and PCTmax) were expressed as mean ± standard deviation (mean ±SD), and were compared using the t test. Meanwhile, measured data in accordance with non-normal distribution (such as PCTc-48 and PCTc-72) were expressed as median and quartile, and were compared using Rank sum test. The receiver operating characteristic curve (ROC) was plotted and the area under the ROC (AUC) was used to analyze the optimal cut-off values as well as the sensitivities and specificities of PCTc-48, PCTc-72 and APACHE II score for predicting prognosis. The APACHE II score and PCTc are opposite in terms of evaluation for the prognosis in patients with sepsis, that is, the lower the APACHE II score, the lower the risk of death, while the higher the PCTc, the lower the risk of death. We used 100-APACHE II score to represent the predictive value of APACHE II score to develop the ROC curve. Factors with statistical significance by univariate analysis were included in the logistic regression analysis and investigated the influencing factors affecting the prognosis of ICU patients with sepsis by adopting the forward (Forward: LR) method (inclusion criteria 0.05, exclusion criteria 0.10). P<0.05 was considered statistically significant.
Results
Clinical and demographic data of patients
Clinical and demographic data of 138 patients were analyzed, which included 79 cases of sepsis and 59 cases of septic shock. The overall 28-day mortality rate was 23.2% (32/138). The age, gender, APACHE II score, combined underlying diseases, infection site, and pathogenic microorganisms causing the disease in the survival and death groups are shown in Table 1. There were no significant differences in age, gender, and combined underlying diseases between the two groups (all P>0.05), indicating that the baseline data were comparable.
As shown in Table 1, the APACHE II score of the death group was higher than that of the survival group, and the difference was statistically significant (P=0.007), which suggested that the APACHE II score has predictive prognostic value for evaluation in patients with sepsis. Meanwhile, there were no significant difference in PCT-1 and PCTmax between the two groups (all P>0.05), which indicated that the PCT-1 and PCTmax have poor predictive value for prognostic evaluation in patients with sepsis.
We have compared the two groups in terms of the pathogens causing sepsis, such as Gram positive cocci, Gram negative bacilli, fungi, non-cultivable bacteria and multiple bacterial infections (≥2 pathogenic microorganisms were cultured). The results showed that there was no significant difference in prognosis between the two groups (P=0.744), suggesting that the pathogens causing sepsis has no relation to the prognosis in patients (Table 1).
|
Indicator |
Survival (n=106) |
Death (n=32) |
Total (n=138) |
P value |
|
Age (years) |
72.6±14.25 |
75.09±13.36 |
73.18±14.04 |
P=0.381 |
|
Gender |
P=0.493 |
|||
|
Male |
59(55.7) |
20(62.5) |
79 (57.2) |
|
|
Female |
47(44.3) |
12(37.5) |
59 (42.8) |
|
|
Sepsis |
64?60.4? |
15 (46.9) |
79 (57.2) |
|
|
Septic shock |
42?39.6? |
17 (53.1) |
59 (42.8) |
|
|
APACHE II |
18.52±8.46 |
23.09±7.60 |
P=0.007 |
|
|
PCT-1 (ng/ml) |
47.28±37.25 |
35.03±42.09 |
P=0.116 |
|
|
PCTmax (ng/ml) |
48.27±37.11 |
43.74±40.69 |
P=0.555 |
|
|
Classification of underlying diseases |
P=0.110 |
|||
|
Cardio-cerebrovascular diseases |
25 (23.6) |
8 (25.0) |
33 (23.9) |
|
|
Diabetes |
24 (22.6) |
3 (9.4) |
27 (19.6) |
|
|
Tumour |
9 (8.5) |
7 (21.9) |
16 (11.6) |
|
|
No underlying diseases |
48 (45.3) |
14 (43.8) |
62 (44.9) |
|
|
Pathogen |
P=0.744 |
|||
|
Gram positive cocci |
7 (6.6) |
3 (9.4) |
10 (7.3) |
|
|
Gram negative bacilli |
47 (44.3) |
13 (40.6) |
60 (43.5) |
|
|
Fungi |
0 (0.0) |
3 (9.4) |
3 (2.2) |
|
|
Non-cultivable bacteria |
32 (30.2) |
7 (21.9) |
39 (28.3) |
|
|
Multiple bacterial infections |
20 (18.9) |
6 (18.8) |
26 (18.83) |
Table 1. General characteristics of patients in two groups n(%)
APACHE II: Acute Physiology and Chronic Health Evaluation score; PCT-1 (ng/ml): PCT value at the time of diagnosis of sepsis; PCTmax (ng/ml): maximum PCT value during the treatment.
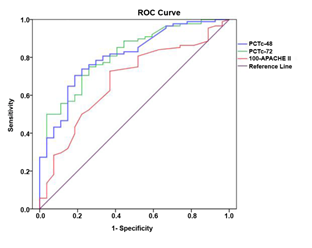
There were statistically significant differences in PCTc-48 and PCTc-72 between the two groups (P<0.001), which indicated that both PCTc-48 and PCTc-72 are predictive values for prognostic evaluation of patients with sepsis (Table 2). For evaluation of the prognosis in patients with sepsis, in terms of the predictive values of PCTc-48 and PCTc-72, ROC curve was plotted and analyzed (Figure 1 and Table 3). The analysis showed that all of the PCTc-48, PCTc-72, and 100-APACHE II score had good predictive value for evaluation of prognosis in patients with sepsis, but the predictive value of PCTc-48 and PCTc-72 was better than the APACHE II score. The optimal cut-off values, sensitivity and specificity for PCTc-48 to predict prognosis were respectively 42.24%, 0.703 and 0.821, and the AUC of ROC was 0.811, 95% CI (0.724, 0.898), whereas the optimal cut-off value, sensitivity and specificity for PCTc-72 to predict prognosis were respectively 60.84%, 0.747 and 0.750, and the AUC of ROC was 0.818, 95% CI (0.734, 0.902).
|
Indicator |
n |
Survival group |
n |
Death group |
P |
|
PCTc-48 |
104 |
57.51 (36.06, 70.73) |
32 |
6.6 (-35.33, 43.93) |
<0.001 |
|
PCTc-72 |
91 |
77.47 (59.84, 86.31) |
28 |
22.30 (-62.38, 63.44) |
<0.001 |
Table 2. Comparisons of PCTc-48 and PCTc-72 between the two groups
- PCTc-48: procalcitonin clearance after 48h
- PCTc-72: procalcitonin clearance after 72h
|
Indicator |
AUC |
95% CI |
Cut-off (%) |
Sensitivity |
Specificity |
Positive predictive value (%) |
Negative predictive value (%) |
Youden index |
|
|
Lower limit |
Upper limit |
||||||||
|
PCTc-48 |
0.811 |
0.724 |
0.898 |
42.24 |
0.703 |
0.821 |
80.72 |
77.78 |
0.525 |
|
PCTc-72 |
0.818 |
0.734 |
0.902 |
60.84 |
0.747 |
0.750 |
94.03 |
76.92 |
0.497 |
|
100-APACHE?score |
0.669 |
0.557 |
0.781 |
77.50 |
0.736 |
0.607 |
66.67 |
43.90 |
0.343 |
Table 3. The predictive value of PCTc-48, PCTc-72 and 100-APACHE II score for prognostic evaluation in patients with sepsis
The cut-off value refers to the value corresponding to the largest Yoden index
Yoden index = sensitivity + specificity -1
PCTc-48: procalcitonin clearance after 48h; PCTc-72: procalcitonin clearance after 72h; APACHE II: Acute Physiology and Chronic Health Evaluation score.
PCTc-48: procalcitonin clearance after 48h; PCTc-72: procalcitonin clearance after 72h; APACHE II: Acute Physiology and Chronic Health Evaluation score.
In terms of the site of infection leading to sepsis, there were 48 cases (34.8%) in the lungs, 62 cases (44.9%) in the abdomen (including gastrointestinal infections and biliary system infections), 24 cases in the urinary system (17.4%) and 4 cases (2.9%) in other parts (including skin and soft tissue infections, unclear source of infection). Based on the site of infection, the patients were divided into different sub-groups such as pulmonary infection, abdominal infection and urinary system infection groups. Patients with infection at other sites were excluded due to small number of cases (they were excluded in other statistical analyses in the present study as well). Statistical analysis of these sub-groups showed that there was significant difference (P<0.001) in prognosis among patients with sepsis caused by infection at different sites, of which patients with urinary system infection showed the best prognosis, and those with pulmonary infection had the worst prognosis. Multiple comparisons revealed that there was significant difference in prognosis between patients with infection at lung and urinary system (P<0.05), and between lung and abdomen (P<0.001), while there was no significant difference between patients with infection at abdomen and urinary system (P>0.05) (Table 4).
|
Indicator |
Survival (n=103) |
Death (n=31) |
Sum (n=138) |
P |
|
Lunga |
27 (56.25) |
21 (43.75) |
48 (100.00) |
<0.001 |
|
Abdomenb |
54 (87.1) |
8 (12.9) |
62 (100.00) |
|
|
Urinary system c |
22 (91.67) |
2 (8.33) |
24 (100.00) |
|
|
Total |
103 (76.87) |
31 (23.13) |
134 (100.00) |
Table 4. Comparison of prognosis between different infection sites n (%)
Note: A significant difference existed between a and c (P<0.05), a and b (P<0.001) and b and c (P>0.05).
As shown in Table 4, there is significant difference in prognosis among patients with sepsis caused by infection at different sites. We further compared PCTc among patients with sepsis caused by infection at different sites, and the results (Table 5) revealed that there was significant difference in PCTc (including PCTc-48 and PCTc-72) among patients with sepsis caused by infection at different sites (all P < 0.05).
|
Infection site |
PCTc-48 |
PCTc-72 |
|
Lung |
0.33 (0.01, 0.58) |
0.59 (0.17, 0.75) |
|
Abdomen |
0.54 (0.36, 0.68) |
0.78 (0.62, 0.88) |
|
Urinary system |
0.59 (0.00, 0.67) |
0.79 (0.41, 0.86) |
|
P Value |
0.010 |
0.006 |
Table 5. Comparison of PCTc among patients with sepsis caused by infection at different sites
PCTc: procalcitonin clearance; PCTc-48: procalcitonin clearance after 48h; PCTc-72: procalcitonin clearance after 72h
Multivariate logistic regression analysis was conducted to determine whether PCTc is an independent risk factor for the prognosis in ICU patients with sepsis. As to PCTc-48, death was considered as a dependent variable, and factors that were confirmed statistically significant in univariate analysis (PCTc-48, site of infection) were enrolled into multivariate logistic regression analysis. The results showed that factors with statistical significance included: PCTc-48 (OR=0.253, 95% CI (0.102, 0.629)), pulmonary infection (OR=12.136, 95% CI (1.648, 89.354)) and abdominal infection (OR=2.928), 95% CI (0.372, 23.017) by considering the urinary system infection as the control. Therefore, after controlling other influencing factors, PCTc-48 remained as the main factor affecting the prognosis in ICU patients with sepsis and is also regarded as a protective factor, where the higher the PCTc, the greater the chances of survival. At the same time, compared with other sites, pulmonary infection is a major factor affecting the prognosis of ICU patients with sepsis(Table 6). The same results were also obtained in the analysis of PCTc-72 as an independent risk factor affecting the prognosis in ICU patients with sepsis, which were more convincing than PCTc-48 (Table 7).
|
Indicator |
P value |
OR |
95% CI. for OR |
|
|
Lower |
Upper |
|||
|
PCTc-48 |
0.003 |
0.253 |
0.102 |
0.629 |
|
Infection site (urinary system) |
0.003 |
|||
|
Infection site (lung) |
0.014 |
12.136 |
1.648 |
89.354 |
|
Infection site (abdomen) |
0.307 |
2.928 |
0.372 |
23.017 |
|
Constant |
0.010 |
0.085 |
||
Table 6. Multivariate logistic regression analysis of factors affecting prognosis in ICU patients with sepsis (PCTc-48 as an independent risk factor)
PCTc-48: procalcitonin clearance after 48h; OR: odds ratio; 95% CI: 95%confidence interval.
|
Indicator |
P value |
OR |
95% C.I. for OR |
|
|
Lower |
Upper |
|||
|
PCTc-72 |
0.001 |
0.147 |
0.047 |
0.457 |
|
Infection site (urinary system) |
0.001 |
|||
|
Infection site (lung) |
0.009 |
38.504 |
2.501 |
592.838 |
|
Infection site (abdomen) |
0.192 |
6.656 |
0.387 |
114.603 |
|
Constant |
0.013 |
0.046 |
||
PCTc-72: procalcitonin clearance after 72h; OR: odds ratio; 95% CI: 95%confidence interval.
Table 7. Multivariate logistic regression analysis of factors affecting prognosis in ICU patients with sepsis (PCTc-72 as an independent risk factor).
Discussion
Sepsis and its complications are the leading causes of ICU death. Although the Surviving Sepsis Campaign[16] has brought substantial improvements to the patient's survival, it has failed to include markers that could identify the prognosis in patients. In this study, we have continuously analyzed the PCT concentrations and PCTc was calculated as a biomarker in patients with sepsis, so as to determine the prognosis in patients at an early stage. We found that both PCTc-48 and PCTc-72 had good predictive value for prognostic evaluation in patients with sepsis. Simultaneoulsy, we found that both PCTc and the site of infection leading to sepsis were risk factors for the prognosis in patients with sepsis, of which patients with pulmonary infection in had worst prognosis compared with urinary tract infection and abdominal infection.
Currently, many indicators are commonly used to evaluate the conditions of critically ill patients. Among them, the APACHE II score is a commonly used clinical evaluation index for the severity of ICU patients. The results of this study also found that the APACHE II score of patients in the death group was significantly higher than that in the survival group (P=0.007), which was consistent with previous reports[17]. In the meantime, a prospective observational study revealed that the initial SOFA score and the ΔSOFA score (the difference between the SOFA score on the third day after treatment and the SOFA score at admission) were confirmed to be accurate predictors for prognosis in patients with severe sepsis and septic shock[10]. PCT was considered as a special biomarker for bacterial infections with enhanced sensitivity and specificity for the diagnosis and prognosis of sepsis[18, 19]. A number of studies have shown that a single-monitoring of PCT level is not well correlated with the prognosis in patients with sepsis[20, 21]. This could be verified through the results in the present study, which clearly indicated that the baseline PCT and the maximum PCT had poor prognostic evaluation in patients with sepsis. Additionally, the specific kinetics of PCT in inflammatory response induced by sepsis shows a peak at 6-24 hours, a half-life of about 24-35 hours, and followed by a decline, which is almost unaffected by renal functions[22]. Hence, dynamic monitoring of PCT trends can be an effective indicator for evaluation of prognosis in patients with sepsis[18, 23-25]. A retrospective study involving 156 sepsis patients in two ICU centers in the United States showed that, PCT changes were independently associated with hospital mortality during 72 hours of ICU stay[26].
Most of the previously published studies had focused on the relationship between PCTc-48, PCTc-72 and prognosis, without including PCTc-24, which was associated with the kinetic characteristics of PCT as mentioned above. Therefore, we also investigated the relationship between PCTc-48, PCTc-72 and prognosis. J.C Ruiz-Rodrígueza et al. have proposed the importance of continuous monitoring of PCT concentrations in patients with septic shock and multiple organ failure. They were the pioneer to develop the concept of procalcitonin clearance (PCTc) as a biomarker for timely evaluation of prognosis in patients with septic shock, and also put forward that a 48-hour PCTc of greater than 50% was associated with a good prognosis[14]. A large-scale multi-center MOSES (multicenter procalcitonin MOnitoring SEpsis) study in the United States found that a 80% reduction in PCT concentration within 4 days after admission was an important independent prognostic factor for sepsis, where the 28-day all-cause mortality rate of ineligible patients was two times that of eligible patients[19]. Philipp Schuetz et al. have reported that, the predictive value by kinetics of PCT during the first 72 hours of ICU therapy in patients with sepsis exceeded that of clinical risk scoring. Moreover, they also proposed that the dynamic monitoring of PCT could help doctors to timely take decision on whether to continue the intensive therapy or shift from ICU to ward[26]. Therefore, determining the optimal cut-off value of PCTc is meaningful and mandatory. Investigation by Schuetz et al.[26] revealed that a cut-off value of an 80% decline in PCT within 72 hours was associated with a sudden increase in mortality (9.5 vs 47.8%), with a negative predictive value of 0.90 and a sensitivity of 0.91. Suberviola et al.[27] have also showed the optimal threshold of 70% for PCTc-72 in predicting survival. In this study, we have confirmed that both PCTc-48 and PCTc-72 showed good predictive value for evaluation of the
prognosis in patients with sepsis, and could be used as prognostic predictors for patients with sepsis with cut-off values of 42.24% and 60.84%, respectively. However, these cut-off values were lower than those reported in literature, which might be due to smaller number of septic shock patients included in this study.
In addition, we found that pathogenic microorganisms causing sepsis were not associated with the prognosis in patients with sepsis, while the site of infection leading to sepsis was directly associated with the prognosis. Patients with urinary system infections showed the best prognosis, and those with pulmonary infections showed the worst prognosis (P<0.001). In order to avoid the influence of pathogen type between different infected sites, we made further statistical analysis and the results showed that no difference in pathogen types between different infection sites(P=0.694). Moreover, a statistically significant difference in PCTc among patients with infection at different sites (all P<0.05) were also observed. In the present study, multivariate logistic regression analysis also found that PCTc and the site of infection leading to sepsis were two important factors influencing the prognosis in ICU patients with sepsis. At present, the correlation of site of infection and pathogens with prognosis in sepsis patients has not been explored.
Through this study, we confirmed that the initial and maximum concentration of PCT is of no prognostic value, and that PCTc-48 and PCTc-72 are greatly associated with the prognosis in patients with sepsis. But, there are limitations in the present study. First and foremost, our sample size was small, and the survival and death groups had imbalanced number of cases, which might have resulted in the possibility of bias. Second, this was a single-center study, and the results were subjected to confirmation by large-scale multicenter prospective studies. Third, some patients had self-administration of antibiotics before admitting the ICU, which definitely had a certain impact on their PCT levels.
Conclusion
In summary, PCTc has a good value for prognostic evaluation of the patients with sepsis. PCTc-48 and PCTc-72 with optimal cut-off values of 42.24% and 60.84%, respectively, can be considered as prognostic predictors for patients with sepsis during the treatment, with enhanced clinical applications. In addition, sepsis patients with pulmonary infection showed the worst prognosis compared with urinary tract infection and abdominal infection. Hence, we concluded that both PCTc and site of infection are risk factors for prognosis in ICU patients with sepsis.
Other Information
Funding sources
None.
Acknowledgement
None.
Conflicts of Interest
All authors declare that they have no any conflict of interests.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Reference
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315 (2016): 801-810.
- Esper AM, Martin GS. Extending international sepsis epidemiology: the impact of organ dysfunction. Critical care 13 (2009):
- Rangel-Frausto MS, Pittet D, Costigan M, et al. The natural history of the systemic inflammatory response syndrome (SIRS): a prospective study. Jama 273 (1995): 117-1
- Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive care medicine 26 (2000): S064-S0
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical care medicine 29 (2001): 1303-13
- Campaign SS, Dellinger RP, Levy MM, et al. International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41 (2013): 580-637.
- Garnacho-Montero J, Huici-Moreno MJ, Gutiérrez-Pizarraya A, et al. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Critical care 18 (2014):
- Jain S, Sinha S, Sharma SK, et al. Procalcitonin as a prognostic marker for sepsis: a prospective observational study. BMC research notes 7 (2014):
- Assicot M, Bohuon C, Gendrel D, et al. High serum procalcitonin concentrations in patients with sepsis and infection. The Lancet 341 (1993): 515-51
- de Azevedo JR, Torres OJ, Beraldi RA, et al. Prognostic evaluation of severe sepsis and septic shock: procalcitonin clearance vs Δ Sequential Organ Failure Assessment. Journal of critical care 30 (2015): 219-e9.
- Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. The Lancet infectious diseases 13 (2013): 426-4
- Seligman R, Meisner M, Lisboa TC, et al. Decreases in procalcitonin and C-reactive protein are strong predictors of survival in ventilator-associated pneumonia. Critical Care 10 (2006):
- Jensen JU, Heslet L, Jensen TH, et al. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Critical care medicine 34 (2006): 2596-2602.
- Ruiz-Rodríguez JC, Caballero J, Ruiz-Sanmartin A, et al. Usefulness of procalcitonin clearance as a prognostic biomarker in septic shock. A prospective pilot study. Medicina Intensiva (English Edition) 36 (2012): 475-4
- Suberviola B, Castellanos-Ortega A, González-Castro A, et al. Prognostic value of procalcitonin, C-reactive protein and leukocytes in septic shock. Medicina Intensiva (English Edition) 36 (2012): 177-184.
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive care medicine 43 (2017): 304-3
- Jiang L, Feng B, Gao D, et al. Plasma concentrations of copeptin, C-reactive protein and procalcitonin are positively correlated with APACHE II scores in patients with sepsis. Journal of International Medical Research 43 (2015): 188-1
- Lipinska-Gediga M, Mierzchata-Pasierb M, Durek G. Procalcitonin kinetics–prognostic and diagnostic significance in septic patients. Arch Med Sci 12 (2016): 112–11
- Schuetz P, Birkhahn R, Sherwin R, et al. Serial procalcitonin predicts mortality in severe sepsis patients: results from the multicenter procalcitonin MOnitoring SEpsis (MOSES) study. Critical care medicine 45 (2017):
- Jensen JU, Heslet L, Jensen TH, et al. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Critical care medicine 34 (2006): 2596-2
- Meng FS, Su L, Tang YQ, et al. Serum procalcitonin at the time of admission to the ICU as a predictor of short-term mortality. Clinical biochemistry 42 (2009): 1025-10
- Meisner M, Schmidt J, Hüttner H, et al. The natural elimination rate of procalcitonin in patients with normal and impaired renal function. Intensive care medicine 26 (2000): S212-S21
- Huang MY, Chen CY, Chien JH, et al. Serum procalcitonin and procalcitonin clearance as a prognostic biomarker in patients with severe sepsis and septic shock. BioMed research international (2016).
- Yu H, Qi Z, Hang C, et al. Evaluating the value of dynamic procalcitonin and presepsin measurements for patients with severe sepsis. The American journal of emergency medicine 35 (2017): 835-8
- Liu D, Su L, Han G, et al. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PloS one 10 (2015).
- Schuetz P, Maurer P, Punjabi V, et al. Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Critical Care 17 (2013):
- Nor M, Basri M, Md Ralib A. Procalcitonin clearance for early prediction of survival in critically ill patients with severe sepsis. Critical care research and practice (2014).