Predictive Factors of Early Pregnancy Loss During In Vitro Fertilization/ Intracytoplasmic Sperm Injection (Ivf/Icsi): Retrospective Study on 1806 Embryo Transfers
Article Information
Karine Morcel1, Philippe Merviel1, Pandora James1, Sarah Bouée1, Mathilde Le Guillou1, Diane Pertuisel1, Jean-Jacques Chabaud1, Sylvie Roche1, Aurore Perrin2, Hortense Drapier2, Damien Beauvillard2
1Obstetrics-Gynecology and Reproductive Medicine department, ART center, Brest University Hospital, 2 avenue Foch, 29200 Brest – France
2Reproductive Laboratory department, ART center, Brest University Hospital, 2 avenue Foch, 29200 Brest – France
*Corresponding Author: Philippe Merviel, MD, PhD, Department of Gynecology, Obstetrics and Reproductive Medicine, Brest University Hospital, 2 avenue Foch, F-29200 Brest, France.
Received: 22 August 2023; Accepted: 26 August 2023; Published: 04 September 2023
Citation: Karine Morcel, Philippe Merviel, Pandora James, Sarah Bouée, Mathilde Le Guillou, Diane Pertuisel, Jean-Jacques Chabaud, Sylvie Roche, Aurore Perrin, Hortense Drapier, Damien Beauvillard. Predictive Factors of Early Pregnancy Loss During In Vitro Fertilization/ Intracytoplasmic Sperm Injection (Ivf/Icsi): Retrospective Study on 1806 Embryo Transfers. Obstetrics and Gynecology Research. 6 (2023): 241-250.
View / Download Pdf Share at FacebookAbstract
Early pregnancy loss (EPL) is a spontaneous miscarriage of a clinical pregnancy during the first trimester. Several factors of EPL have been studied but results were discordant. We performed a retrospective study in our ART center, comparing baseline data and IVF/ICSI outcomes between cycles with EPL, ongoing pregnancy and without pregnancy. Ectopic pregnancies and biochemical pregnancies (without visualization of a gestational sac on ultrasound) were excluded. The aim of this study is to compare these different cycles, and analyze the risk factors for EPL.
We included 2555 IVF/ICSI cycles leading to 2193 oocyte pick-ups and 1806 embryo transfers. Several characteristics (women’s age, infertility diagnosis and duration, estradiol level on the day of hCG-trigger, endometrial thickness, day of embryo transfer) appeared to be risk factors of EPL in univariate analysis. Only women’s age has a significant (p < 0.001) influence in multivariate analysis on the rate of EPL, with an OR: 1.71 if the woman’s age ≥ 35 years old (reference < 35 y.o = 1), 2.96 if ≥ 38 y.o and 5.31 if ≥ 40 y.o.
In this study, we observed an increase in EPL rate by 4.15% per year in women over 35 years of age.
Keywords
Early pregnancy loss – IVF/ICSI – Ongoing pregnancy – predictive factor
Early pregnancy loss articles Early pregnancy loss Research articles Early pregnancy loss review articles Early pregnancy loss PubMed articles Early pregnancy loss PubMed Central articles Early pregnancy loss 2023 articles Early pregnancy loss 2024 articles Early pregnancy loss Scopus articles Early pregnancy loss impact factor journals Early pregnancy loss Scopus journals Early pregnancy loss PubMed journals Early pregnancy loss medical journals Early pregnancy loss free journals Early pregnancy loss best journals Early pregnancy loss top journals Early pregnancy loss free medical journals Early pregnancy loss famous journals Early pregnancy loss Google Scholar indexed journals IVF articles IVF Research articles IVF review articles IVF PubMed articles IVF PubMed Central articles IVF 2023 articles IVF 2024 articles IVF Scopus articles IVF impact factor journals IVF Scopus journals IVF PubMed journals IVF medical journals IVF free journals IVF best journals IVF top journals IVF free medical journals IVF famous journals IVF Google Scholar indexed journals ICSI articles ICSI Research articles ICSI review articles ICSI PubMed articles ICSI PubMed Central articles ICSI 2023 articles ICSI 2024 articles ICSI Scopus articles ICSI impact factor journals ICSI Scopus journals ICSI PubMed journals ICSI medical journals ICSI free journals ICSI best journals ICSI top journals ICSI free medical journals ICSI famous journals ICSI Google Scholar indexed journals Ongoing pregnancy articles Ongoing pregnancy Research articles Ongoing pregnancy review articles Ongoing pregnancy PubMed articles Ongoing pregnancy PubMed Central articles Ongoing pregnancy 2023 articles Ongoing pregnancy 2024 articles Ongoing pregnancy Scopus articles Ongoing pregnancy impact factor journals Ongoing pregnancy Scopus journals Ongoing pregnancy PubMed journals Ongoing pregnancy medical journals Ongoing pregnancy free journals Ongoing pregnancy best journals Ongoing pregnancy top journals Ongoing pregnancy free medical journals Ongoing pregnancy famous journals Ongoing pregnancy Google Scholar indexed journals predictive factor articles predictive factor Research articles predictive factor review articles predictive factor PubMed articles predictive factor PubMed Central articles predictive factor 2023 articles predictive factor 2024 articles predictive factor Scopus articles predictive factor impact factor journals predictive factor Scopus journals predictive factor PubMed journals predictive factor medical journals predictive factor free journals predictive factor best journals predictive factor top journals predictive factor free medical journals predictive factor famous journals predictive factor Google Scholar indexed journals Assisted Reproductive Technology articles Assisted Reproductive Technology Research articles Assisted Reproductive Technology review articles Assisted Reproductive Technology PubMed articles Assisted Reproductive Technology PubMed Central articles Assisted Reproductive Technology 2023 articles Assisted Reproductive Technology 2024 articles Assisted Reproductive Technology Scopus articles Assisted Reproductive Technology impact factor journals Assisted Reproductive Technology Scopus journals Assisted Reproductive Technology PubMed journals Assisted Reproductive Technology medical journals Assisted Reproductive Technology free journals Assisted Reproductive Technology best journals Assisted Reproductive Technology top journals Assisted Reproductive Technology free medical journals Assisted Reproductive Technology famous journals Assisted Reproductive Technology Google Scholar indexed journals BMI articles BMI Research articles BMI review articles BMI PubMed articles BMI PubMed Central articles BMI 2023 articles BMI 2024 articles BMI Scopus articles BMI impact factor journals BMI Scopus journals BMI PubMed journals BMI medical journals BMI free journals BMI best journals BMI top journals BMI free medical journals BMI famous journals BMI Google Scholar indexed journals Polycystic Ovary Syndrome articles Polycystic Ovary Syndrome Research articles Polycystic Ovary Syndrome review articles Polycystic Ovary Syndrome PubMed articles Polycystic Ovary Syndrome PubMed Central articles Polycystic Ovary Syndrome 2023 articles Polycystic Ovary Syndrome 2024 articles Polycystic Ovary Syndrome Scopus articles Polycystic Ovary Syndrome impact factor journals Polycystic Ovary Syndrome Scopus journals Polycystic Ovary Syndrome PubMed journals Polycystic Ovary Syndrome medical journals Polycystic Ovary Syndrome free journals Polycystic Ovary Syndrome best journals Polycystic Ovary Syndrome top journals Polycystic Ovary Syndrome free medical journals Polycystic Ovary Syndrome famous journals Polycystic Ovary Syndrome Google Scholar indexed journals embryo transfer articles embryo transfer Research articles embryo transfer review articles embryo transfer PubMed articles embryo transfer PubMed Central articles embryo transfer 2023 articles embryo transfer 2024 articles embryo transfer Scopus articles embryo transfer impact factor journals embryo transfer Scopus journals embryo transfer PubMed journals embryo transfer medical journals embryo transfer free journals embryo transfer best journals embryo transfer top journals embryo transfer free medical journals embryo transfer famous journals embryo transfer Google Scholar indexed journals biochemical pregnancy articles biochemical pregnancy Research articles biochemical pregnancy review articles biochemical pregnancy PubMed articles biochemical pregnancy PubMed Central articles biochemical pregnancy 2023 articles biochemical pregnancy 2024 articles biochemical pregnancy Scopus articles biochemical pregnancy impact factor journals biochemical pregnancy Scopus journals biochemical pregnancy PubMed journals biochemical pregnancy medical journals biochemical pregnancy free journals biochemical pregnancy best journals biochemical pregnancy top journals biochemical pregnancy free medical journals biochemical pregnancy famous journals biochemical pregnancy Google Scholar indexed journals
Article Details
List of abbreviations
ART: Assisted Reproductive Technology; AUC-ROC: Area under the curve - receiver operating curve; BMI: Body Mass Index; c: cycle; CI 95%: Confidence Interval at 95%; CPR: clinical pregnancy rate; dhCG: Day of hCG; /d: per day; E2: Estradiol-17β; EPL: early pregnancy loss; IU: International Unit; IVF/ICSI: In Vitro Fertilization/Intracytoplasmic Sperm Injection; LBR: Live Birth Rate; MII: Mature Oocyte in Metaphase II; N: Number; No P: No Pregnancy; NS: Not Significant; OP: ongoing pregnancy; OPU: Oocyte pick-up; OR: Odd Ratio; PCOS: Polycystic Ovary Syndrome; PGD: Preimplantation Genetic Diagnosis; PL: Pregnancy Loss; t: transfer; WA: Woman Age; y: year; y.o: years old
Introduction
Assisted reproductive technologies (ART) are used to treat infertility and obtain a healthy child. In vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI), one of the ARTs, is a procedure with many steps, i/ oocytes obtained after controlled ovarian stimulation, ii/ fertilization by spermatozoa, iii/ intrauterine embryo transfer, thus allowing to study the risk factors of EPL at each stage.
Although the clinical pregnancy rate (CPR) has improved over the last decade, the live birth rate (LBR) remains low, only 38.1% in IVF/ICSI procedure [1]. Pregnancy loss (PL) was the spontaneous miscarriage of an embryo in the first trimester, including non-visualized pregnancy on ultrasound (biochemical pregnancy), ectopic pregnancy, and early clinical pregnancy loss (EPL). Several risk factors for EPL have been identified, such as maternal age, specially after 35 years old (y.o) [2,3], overweight and obese women [4], in case of polycystic ovary syndrome (PCOS) [5,6], thin endometrial thickness [7], low ovarian reserve [8,9], and ovulatory dysfunction [10], but the results of these studies are often subject to debate. The main objective of this retrospective study was to compare demographic, clinical and biological characteristics in the EPL, ongoing pregnancy and no pregnancy groups after IVF/ICSI and fresh embryo transfer, and to analyze risk factors for EPL. In a second phase, we studied the rates of biochemical pregnancies, ectopic pregnancies, early pregnancy loss according to the women's age ranges (< 35, ≥ 35, ≥ 38, and ≥ 40 y.o).
Material and methods
After an infertility evaluation (for women, serum assays of FSH, LH, estradiol-17β (E2), prolactin and AMH on the second or third day of the menstrual cycle, pelvic ultrasonography, hysterosalpingography followed by laparoscopy and/or hysteroscopy if the results were abnormal; for men a spermogram and a sperm motility test), all couples were enrolled in IVF/ICSI at the ART center in Brest University Hospital. The antral follicle count (AFC) was not a necessary variable for including and treating patients. All hormonal blood tests and semen examinations were performed in the same laboratory.
Inclusion and exclusion criteria
The inclusion criteria were women aged 18-42 y.o and men aged 18-59 y.o, with written consent for infertility treatment. Tubal infertility and endometriosis were established after laparoscopy, uterine infertility (polyp, myoma, malformation, synechiae) after hysteroscopy and treatment of these cases, and the diagnosis of polycystic ovary syndrome (PCOS) was in accordance with the Rotterdam criteria. Masculine infertility is defined as a total mobile count ≤ 1 million spermatozoa. Mixed infertility is the combination of male and female infertility factors. We excluded cycles involving donor gametes or embryos.
Procedure
The long GnRH agonist protocol begins on the 20th day of the previous cycle with the daily injection of triptorelin 0.1 mg per day (/d) (Decapeptyl®, Ipsen Pharma, Paris, France), followed 14 days later by an assessment of ovarian desensitization (estradiol level < 50 pg/ml, LH < 3 IU/l and progesterone < 1 ng/ml). When this stage is reached, we decreased the triptorelin to 0.05 mg/d and associate it with a gonadotropin (Fostimon®, Genevrier, Sophia-Antipolis, France; Gonal-F®, Merck, Lyon, France; Puregon®, MSD, Levallois-Perret, France; or Menopur®, Ferring SAS, St Prex, Switzerland) whose dose depends on the women’s age, the basal hormonal balance and the previous ovarian stimulation results.
The short antagonist protocol consists of starting a gondotropin on the 2nd day of the cycle and adding ganirelix (Orgalutran®, MSD, Levallois-Perret, France) when the the follicle size exceeded 14 mm or when the E2 level was over 400 pg/ml. The first evaluation, combined hormone assays (E2, LH, and progesterone) and a vaginal ultrasound examination of the number and size of follicles and the endometrial maturation (thickness and pattern), is carried out on the 5th day of stimulation for the antagonist protocol and on the 7th day for the agonsite protocol, with an adaptation of the gonadotropin doses according to the results. This evaluation was repeated every two or three days, depending on the follicular growth. The triggering of ovulation by 250 µg of recombinant human chorionic gonadotropin (Ovitrelle®, Merck, Lyon, France) is decided when at least three follicles ≥ 17 mm diameter and endometrium thickness > 7 mm with a triple-line pattern were observed, and oocyte pick-up is performed 35-37 hours after. The support of the luteal phase is started on the evening of the follicular puncture, with 400 mg/d of intravaginal micronized progesterone (Utrogestan®; Besins International, Paris, France), until the β-hCG dosage 15 days after.
After observing fertilization 18h after incubation, the embryos are then maintained in vitro culture 2 to 3 days (D2 or D3 cleaved embryos) or 5 to 6 days (D5 or D6 blastocysts), according to their evolution. The classification of cleaved embryos is that of the French IVF biologists association (BLEFCO), and the blastocysts were evaluated according to Gardner’s classification (supplementary file). One to two embryos (exceptionally three) were transferred with a Frydman catheter (CCD Laboratories, Paris, France), and the other good embryos were vitrified. The oocyte or embryo “freeze-all” events were decided in cases with (i) an elevated (> 1.5 ng/ml) plasma progesterone level on the hCG trigger day, (ii) ovarian hyperstimulation (> 3500 pg/ml), (iii) a lack of spermatozoa on the day of oocyte retrieval.
The no pregnancy group (No P) consisted of women with a negative β-hCG assay (< 5 IU/l) 2 weeks after embryo transfer. Clinical pregnancy (CP) was confirmed by a gestational sac on ultrasound 3 weeks after embryo transfer and a β-hCG level above 1000 IU/l. Ongoing pregnancy (OP) was defined as a pregnancy > 12 weeks of gestation. Early pregnancy loss (EPL) was a miscarriage of a clinical pregnancy before 12 weeks of gestation.
Ectopic pregnancy and women with a biochemical pregnancy (β-hCG assay between 100 and 1000 IU/l without gestational sac on ultrasound) were excluded from the main analysis.
Statistical analysis
Statistical comparisons were conducted using either the Student t test or a Mann-Whitney U test for continuous variables and a Chi 2 test or Fisher exact test for qualitative variables. The multivariate analysis included all data for which there was a significant difference between EPL and OP groups (woman's age, duration of infertility, infertility diagnosis, E2 level on the day of hCG trigger, endometrial thickness, number of embryos transferred, day of embryo transfer). The rate of EPL in women < 35 y.o was used as a reference. We used the XLSTAT® add-in (Addinsoft, Paris, France) program for statistical analysis. The statistical significance was set at p < 0.05.
Ethical approval and consent to participate
All the subjects have signed consent to the infertility treatment (ART French legislation). For this study, an informed consent was obtained from all subjects and/or their legal guardians. The consents of each subject are available in their medical record and with the corresponding author. This study was approved by the Brest Institutional Review Board (2020, June 18) as an ancillary study under the authorization reference B2020CE.43.
Results
We studied 2555 IVF/ICSI cycles (c) at the ART center of Brest university hospital between 2015 and 2020. These cycles resulted in 2193 oocyte pick-ups (OPU) (85.8% of the cycles) and 1806 embryo transfers (t) (82.3% of the OPU). We have observed 362 cancelations during ovarian stimulation (14.1% of the cycles), 24 oocyte pick-ups without oocytes (1.1%), 75 freeze all procedures (3.4%) and 288 embryo culture failures (13.1%). After removing biochemical pregnancies (n: 74; 10.9%) and ectopic pregnancies (n: 26; 3.8%), we obtained 574 clinical pregnancies (31.7%/t), of which 140 early pregnancy losses (24.4%) and 434 ongoing pregnancies (24.0%/t). Figure 1 summarizes the flow chart of this study.
|
EPL (A) |
OP (B) |
No P (C) |
p |
|
|
Number |
140 |
434 |
1132 |
|
|
Woman age y.o |
31.6 ± 5.7 |
29.8 ± 4.2 |
29.9 ± 4.6 |
A-B***; A-C*** |
|
Man age y.o |
36.5 ± 7.3 |
36.9 ± 10.5 |
35.1 ± 7.0 |
A-C*; B-C*** |
|
Woman BMI kg/m2 |
25.7 ± 4.8 |
24.8 ± 5.0 |
24.0 ± 4.5 |
A-C***; B-C** |
|
Man BMI kg/m2 |
25.2 ± 4.8 |
25.6 ± 3.6 |
26.5 ± 4.9 |
A-C**; B-C*** |
|
Woman tobacco use % |
27.8 |
27.6 |
23.1 |
NS |
|
Man tobacco use % |
35.0 |
34.8 |
28.7 |
NS |
|
Infertility diagnosis % Tubal PCOS Endometriosis Uterine Male factor Mixed |
14.2 11.4 8.5 6.4 59.2 21.2 |
14.2 17.0 4.6 3.2 61.0 9.9 |
17.0 12.0 2.9 4.5 57.5 6.1 |
NS A-B**; B-C** A-C*** NS NS A-B***; A-C***; B-C** |
|
Basal FSH (IU/l) Basal AMH (ng/ml) |
6.5 ± 1.7 1.9 ± 1.3 |
6.7 ± 2.3 2.0 ± 1.2 |
7.4 ± 1.9 1.6 ± 1.1 |
A-C***; B-C*** A-C**; B-C*** |
|
Pregnancies without ART %/couple Pregnancy losses %/pregnancy |
24.2 15.2 |
23.7 18.1 |
21.2 15.8 |
NS NS |
|
Pregnancies with previous ART %/couple Pregnancylosses %/pregnancy |
14.2 20.0 |
17.2 18.6 |
7.0 15.0 |
A-C***; B-C*** NS |
|
Infertility duration y |
4.2 ± 2.5 |
3.6 ± 1.8 |
4.0 ± 2.3 |
A-B**; B-C*** |
Legends : EPL : early clinical pregnancy loss ; OP : ongoing pregnancy ; No P : no pregnancy ; y.o : years old ; BMI : body mass index ; PCOS : polycystic ovarian syndrome ; ART : assisted reproductive technology ; y : years
Statistical analysis p: * : < 0.05 ; ** : < 0.01 ; *** : < 0.001 ; NS : not significant
Table 1: Baseline data of the different groups : early pregnancy loss, ongoing pregnancy and no pregnancy
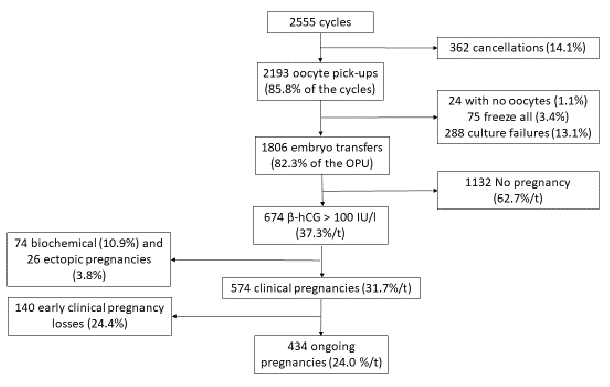
Figure 1: Flow chart of the study
Table 1 reports the baseline characteristics of couples with EPL, OP and without pregnancy. The clinical pregnancy rate was significantly higher in couples in which were found higher male age, higher female BMI, lower male BMI, lower FSH and AMH levels at day 2 or 3 of the menstrual cycle, and those who had a previous pregnancy after ART. There was no difference between the three groups concerning the history of EPL in previous spontaneous pregnancies or pregnancies obtained after ART. Couples with EPL showed significantly higher female age (p < 0.001), longer duration of infertility (p < 0.01), more frequent mixed cause of infertility (p < 0.001), and less PCOS (p < 0.01) compared with couples with an ongoing pregnancy.
|
EPL (A) |
OP (B) |
No P (C) |
p |
|
|
Number |
140 |
434 |
1132 |
|
|
Cycle rank |
1.6 ± 0.7 |
1.7 ± 1.1 |
1.9 ± 1.2 |
A-C***; B-C** |
|
Agonist protocol % |
59.2 |
65.6 |
79.5 |
A-C***; B-C*** |
|
Total FSH dose IU |
2326 ± 1360 |
2129 ± 979 |
2676 ± 1374 |
A-C**; B-C*** |
|
Stimulation duration day |
10.9 ± 1.9 |
11.2 ± 2.1 |
11.6 ± 2.4 |
A-C***; B-C*** |
|
Estradiol at dhCG pg/ml |
2639 ± 896 |
2401 ± 1108 |
2189 ± 853 |
A-B*; A-C***; B-C*** |
|
Endom thickness mm |
10.7 ± 2.0 |
11.1 ± 2.4 |
10.7 ± 2.1 |
A-B*; B-C** |
|
Total oocytes |
13.3 ± 4.9 |
14.1 ± 5.9 |
9.4 ± 4.9 |
A-C***; B-C*** |
|
MII oocytes |
10.0 ± 3.6 |
9.9 ± 4.7 |
6.6 ± 4.0 |
A-C***; B-C*** |
|
Fertilized oocytes |
6.1 ± 2.8 |
6.1 ± 3.1 |
3.0 ± 2.3 |
A-C***; B-C*** |
|
Total embryos |
6.5 ± 2.8 |
6.5 ± 2.8 |
3.0 ± 2.6 |
A-C***; B-C*** |
|
Fertilization + clivage rate % |
65.5 |
65.6 |
45.8 |
A-C***; B-C*** |
|
Embryo grades A % B % C % D % |
40.0 18.0 30.0 12.0 |
47.0 20.0 26.0 7.0 |
37.7 21.5 30.4 10.4 |
NS NS NS NS |
|
Embryo transferred |
2.0 ± 0.4 |
1.7 ± 0.4 |
1.2 ± 0.4 |
A-B***; A-C***; B-C*** |
|
Twin pregnancy % |
10.1 |
|||
|
Day embryo transfer Day 2 % Day 3 % Day 5 or 6 % |
70.0 22.0 8.0 |
52.0 33.0 15.0 |
70.7 18.9 10.4 |
A-B***; B-C** A-B*; B-C*** A-B*; B-C* |
|
Couple with cryopreserved embryo %/transfer m ± SD |
36.4 3.6 ± 0.5 |
48.3 3.7 ± 0.4 |
20.6 3.4 ± 0.6 |
A-B*; A-C***; B-C*** A-B*; A-C***; B-C*** |
Legends : EPL : early clinical pregnancy loss ; OP : ongoing pregnancy ; No P : no pregnancy ; IU : international unit ; dhCG : day of hCG administration ; MII : mature oocyte (metaphase II)
Statistical analysis p: * : < 0.05 ; ** : < 0.01 ; *** : < 0.001 ; NS : not significant
Table 2: IVF/ICSI procedures and outcomes in the different groups: early pregnancy loss, ongoing pregnancy and no pregnancy
Table 2 shows the clinical and biological results of IVF/ICSI cycles. Clinical pregnancy rate was significantly higher in couples with lower cycle rank, agonist protocol, total gonadotropin dose, stimulation duration, and higher E2 level on hCG day, oocyte retrieved, embryo obtained, number of embryos transferred and embryos frozen. Couples with EPL had significantly higher E2 levels on the day of hCG (p < 0.05), less endometrial thickness (p < 0.05), more embryos transferred (p < 0.001) and a cleavage stage at D2 (p < 0.001) and at D3 (p < 0.05) compared to couples with an ongoing pregnancy.
|
WA < 35 years old |
WA ≥ 35 years old |
WA ≥ 38 years old |
WA ≥ 40 years old |
|
|
N cycles (c) |
1171 |
1384 |
963 |
653 |
|
N transfers (t) |
899 (76.7%/c) |
907 (65.5%/c) |
539 (55.9%/c) |
320 (49.0%/c) |
|
Without pregnancy (No P) |
530 |
602 |
383 |
226 |
|
Total pregnancies (β-hCG > 100 IU/l) |
369 (41.0%/t) |
305 (33.6%/t)** |
156 (28.9%/t)*** |
94 (29.3%/t)*** |
|
Biochemical pregnancies (% of total pregnancies) |
31 (8.4) |
43 (14.0)* |
35 (22.4)*** |
29 (30.8)*** |
|
Ectopic pregnancies (% of total pregnancies) |
15 (4.0) |
11 (3.6) NS |
7 (4.4) NS |
3 (3.2) NS |
|
Clinical pregnancies (CP) |
323 (35.9%/t) |
251 (27.7%/t)*** |
114 (21.1%/t)*** |
62 (19.4%/t)*** |
|
Early pregnancy losses (EPL) (% of clinical pregnancies) |
57 (17.6) |
83 (33.0)*** |
51 (44.7)*** |
34 (54.8)*** |
|
Ongoing pregnancies (OP) |
266 (29.5%/t) |
168 (18.5%/t)*** |
63 (11.6%/t)*** |
28 (8.7%/t)*** |
Legends: WA : woman age ; N : number; c: cycle; t: transfer
Statistical analysis between women < 35 and ≥ 35 y.o, < 35 and ≥ 38 y.o, < 35 and ≥ 40 y.o: *: p < 0.05; **: p < 0.01; ***: p < 0.001; NS: not significant
Table 3: Data of total pregnancies, biochemical and extra-uterine pregnancies, ongoing pregnancies and early clinical pregnancy losses in age classes
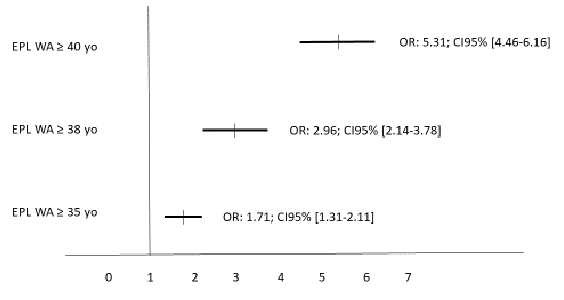
Figure 2: Relative risks of EPL in the different woman age groups in a multivariate analysis. The EPL rate in women < 35 yo served as the reference (= 1). The multivariate analysis included the woman age, infertility duration, type of infertility, estradiol level on hCG day, endometrial thickness, number of embryo transferred and day of embryo transfer.
Table 3 present the data of the type of pregnancy according to the different woman age ranges. In multivariate analysis (figure 2), only the woman's age significantly influenced the rate of EPL with an odd-ratio (OR) of 1.71 (95%CI: 1.31-2.11; p < 0.01) (≥ 35 versus < 35 years of age), 2.96 (95%CI: 2.14-3.78; p < 0.001) (≥ 38 versus < 35 years of age), and 5.31 (95%CI: 4.46-6.16; p < 0.001) (≥ 40 versus < 35 years). Thus, we calculated that each additional year beyond the woman's age of 35 years led an increase in EPL by 4.15% per year.
Discussion
In this study, we report a pregnancy loss rate of 35.3% (10.9% biochemical pregnancies and 24.4% of EPL), similar to the rates reported in the United States between 1999-2002 [11] (29%) and by Goldstein where the rate of spontaneous first trimester miscarriage (like EPL) was 15-30% in the general population [12]. In contrast, Yang [7] showed a very low pregnancy loss rate of 19.7% (5.5% biochemical pregnancies and 8.9% of EPL). Two possible explanations for this reduction of pregnancy loss were a different luteal phase support by vaginal progesterone gel 90 mg/d (Crinone®, Merck, Lyon, France) and the transfer of more embryos (3 embryos in 23.6% of cases). After identification of embryonic heart activity, the rate of late pregnancy loss remains around 3-4% [13].
Clinical studies have confirmed that EPL increased with the maternal age, especially after 35 y.o [2,3]. Yang [7], in 15210 pregnancies following an embryo transfer (LBR 77.6%), showed a multivariate OR in women aged ≥ 35 years of 1.49 (CI95%: 1.22-1.83) and ≥ 40 years of 3.82 (CI95%: 2.65-5.51) compared with women < 35 years. This author showed that the risk of EPL increases by 22% per year in a woman older than 36 years of age. These values were similar to the results of our study (≥ 35 yo: 1.71 and ≥ 40: 5.31). The main causes of EPL with the maternal age appear to be chromosomal aneuploidy [14,15] and decreased ovarian reserve [16]. Preimplantation genetic diagnosis (PGD) before embryo transfer may reduce the EPL in relation with aneuploidy. After PGD, Verlinsky [17] reports a 4-fold reduction in the rate of EPL when the couple has a Mendelian anomaly, and Grifo [18] shows a 35-6% reduction of EPL. However, the safety and the risks of this technology need to be further investigated. Regarding paternal age, Marsidi [19] showed that the increase in EPL rate observed above 45 y.o disappeared when women were younger than 35 y.o, in relation with the best oocyte quality. In the Yi’s study [20], the mean maternal age (32.6 +/- 4.3 vs. 30.5 +/- 4.2, p < 0.001) and the infertility duration (5 [3-8.5] vs. 4 [3-7]; p: 0.03) were significantly higher in the EPL group than in the OP group. This result may be attributed to oocyte deterioration and endocrine variations that occur with advanced maternal age [21]. Yang [7] showed that PCOS was an independent risk factor for late pregnancy loss, but not for EPL (OR: 1.14; CI95%: 0.94-1.37). The relationship between PCOS and EPL could be secondary to high secretions of LH and testosterone in these patients [22]. However, GnRH agonist therapy has not shown a beneficial effect on pregnancy outcomes [23]. In contrast, Rai in 2000 [24] showed that neither a LH level > 10 IU/l nor a testosterone level > 3 nmoles/l were associated with an increase of EPL. Obesity, which is common in this condition, could be an independent and confounding risk factor of miscarriage.
Munch [25] showed that high doses of gonadotropins decreased the probability of pregnancy after fresh embryo transfers but not after frozen embryo transfers, suggesting an impact on the endometrium and not on embryo quality. Supraphysiological levels of estradiol may affect implantation and migration of trophoblasts [26], as shown by the difference in endometrial gene expression in this case [27,28]. Thus, the proportion of euploid oocytes is directly and positively related to the number of mature oocytes and inversely related to the total FSH dose per oocyte and per mature oocyte [29,30,31]. Similarly, in the Kaleli’s study, the FSH dose is related to the aneuploidy in the granulosa cells [32]. Serum estradiol level reflects follicular maturation and oocyte quality, and has a significant influence on endometrial maturation [33]. According to Taheri [34], the serum E2 level on the hCG day appeared to be a negative predictive factor for live births (OR [95%CI]: 0.99 [0.99-1]). According to Blazar’s study [35], the serum E2 cut-off for pregnancy is 2500 pg/ml. In the study by Li [36], the optimal serum E2 level on the trigger day for obtaining a pregnancy after a fresh embryo transfer is between 1000 and 3148 pg/ml (AUC-ROC: 0.721).
Studies have concluded that endometrial thickness (with a threshold of 10 mm) is strongly associated with pregnancy outcomes after IVF/ICSI treatment [37]. For Yang [7], a negative correlation was observed between endometrial thickness and EPL (OR 0.95; CI95% 0.92-0.97), and del Carmen Nogales [38] observed a significant association between endometrial thickness and EPL (OR: 0.65; 95% CI: 0.52-0.77, p: 0.04). For Liu [39], the pregnancy loss rate increased with each millimeter decline in fresh cycles, but Yang [7] showed that there was no significant difference when the endometrial thickness was greater than 8 mm (as in the Vuong’s study [40]). Zhao [41] reported embryo implantation rates over 30% when endometrial thickness was between 8 and 16 mm (AUC: 0.596; CI95%: 0.571-0.621), and the meta-analysis by Kasius [42] showed that the pregnancy rate increased with endometrial thickness (starting at 7 mm, in a univariate analysis). However, Sundström [43] reported a pregnancy with an endometrial thickness of 4 mm and Check [44] with an endometrial thickness of 3.6 mm. Remohi [45] showed that 60% of women with an endometrial thickness between 6.0 and 6.9 mm had successful pregnancies.
In our study, embryo quality is not a risk factor for EPL, as in the del Carmen Nogales’s study [38] which reported an EPL rate of 13.1% with no difference between embryo quality classes A (11.3%), B (12.8%), C (11.8% and D (12.5%). Non blastocyst transfers have a higher risk of abnormal implantation than blastocyst transfers (OR: 1.19 ; CI95% 1.17-1.21) [45]. Our ART center policy is to transfer embryos at the blastocyst stage (day 5 or 6) whenever possible. Wang [46] showed that fresh blastocyst transfers had a lowest risk of biochemical pregnancy and pregnancy loss than cleaved embryo transfers in women < 40 y.o. However this study did not account for cleavage stage vs. blastocyst, which has a significant interaction with the outcomes and may explain the differences. Women who transferred ≥ 2 embryos showed lower risk of EPL than women who transferred a single embryo: multivariate OR for 2 embryo transferred : 0.63 (CI 95% 0.49-0.82) and for 3 : 0.47 (CI95% 0.34-0.66) [7]. A meta-analysis of three trials of 633 cycles in women 27-33 years old demonstrated higher clinical and ongoing pregnancy rates in frozen/thawed embryo transfers compared with fresh embryo transfers [47]. For Yang [7], there was an increased risk of EPL after frozen-thawed ET versus fresh ET (OR 1.12 ; CI 1.01-1.24). Hipp [48] reported an increased risk of first-trimester pregnancy loss after the transfer of frozen embryos compared with fresh embryos in women < 38 y.o, and this difference persisted in women < 30 y.o.
This study did not show an impact of BMI on the EPL risk (26.8% of women were overweight and 16% were obese in all cycles), as in our previous study where we did not find an association between clinical pregnancy and live birth rates and different BMI classes [49]. Similarly, Brunet [50] reported that after adjustment, obesity, including classes II/III, had no impact on EPL rates. Provost [4] showed that pregnancy outcomes were most favorable in low or normal weight cohorts but progressively worsened with increasing BMI in fresh cycles. For Yang [7], the multivariate OR in case of BMI ≥ 30 was 1.47 (CI95% 1.14-1.91) but no significant among BMI 25-30. Others studies have shown that obesity is a risk factor for EPL after IVF/ICSI [51,52]. Impaired stromal decidualization in obese women has been observed [53], in relation to insulin and leptin resistance, and decreased glycodelin, IGF BP1 (insulin-like growth factor binding protein 1) and PAI 1 (plasminogen activator inhibitor 1).
In this study, uterine infertility was defined by a history of polyp, myoma, malformation or synechiae, treated by operative hysteroscopy with subsequent uterine cavity control. Yang [7] showed a multivariate OR of 1.77 (CI95% 1.32-2.38) when there was endo-uterine pathology. However, for congenital uterine malformations other than a septal uterus, the surgery fails to increase the live birth rate or decrease EPL [54].
In this study, clinical pregnancy rates were significantly higher in couples who had a prior pregnancy after ART. Magnusson et al [55] evaluated the probability of a live birth in a Swedish series of 77956 fresh transfers between 2007 and 2013. The ideal number of oocytes retrieved was between 10 and 20 (adjusted OR: 1.064), but this variable was less predictive than having previously had a child through IVF (OR: 1.462), but was more predictive than the woman's age (OR: 0.936).
Other factors may also play a role in embryo development, such as vitamin B, which is involved in the synthesis and methylation of nucleic acids and proteins [56]. Methyl tetrahydrofolate reductase (MTHFR) is one of the key steps in folate metabolism, and the c.677 C>T mutation in the MTHFR gene can alter folate concentration and lead to the development of EPL, as shown in a previous study [57].
One of the strengths of our study is its large sample size - more than 1800 IVF/ICSI embryo transfers in a single assisted reproduction center. Secondly, there is limited literature on risk factors for EPL after IVF/ICSI, and in this study we performed a multivariate analysis of a number of risk factors for EPL according to women's age. However, our study also had several limitations. First, our study was retrospective, had significant demographic differences, and did not take into account cumulative pregnancy rates. Secondly, we did not take into account some confounding factors that had a significant effect on the probability of pregnancy (e.g., poor responders or low-quality embryo transfers); the only predictor of EPL was the woman's age, which prompted us to analyze our data by age class.
Conclusion
In this study, we found in univariate analysis the common risk factors for EPL (woman's age, duration of infertility, diagnosis of infertility, estradiol level on the day of hCG trigger, endometrial thickness, number of embryos transferred, and day of embryo transfer), but in multivariate analysis, only the woman's age was retained as a significant risk factor. Thus, we calculated that each additional year beyond the age of 35 years led to an increase in EPL rate by 4.15% per year. Further prospective studies are needed to confirm these results.
Acknowledgements
The authors thanks Pandora James for her comments, suggestions and critical reading of the manuscript.
Declarations and statements
Consent to publish
The manuscript has been read and approved by all authors. The authors confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Availability of data and materials
The material contained in this manuscript has not been published, has not been submitted or is not being submitted elsewhere. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors report no conflicts of interest in relation to the present study.
Funding
Not applicable
Authors' contributions
- Karine Morcel, Philippe Merviel: substantial contributions to the conception, design of the work, the acquisition, analysis and interpretation of data; have drafted the work or substantively revised it
- Pandora James: comments, suggestions and critical reading of the manuscript
- Sarah Bouée, Mathilde Le Guillou, Diane Pertuisel, Jean-Jacques Chabaud, Sylvie Roche: the acquisition of clinical data
- Aurore Perrin, Hortense Drapier, Damien Beauvillard: the acquisition of biological data
Each author has approved the submitted version and has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
References
- Sunderam S, et al. Assisted reproductive technology surveillance--United States, 2009. MMWR Surveill Summ 61 (2012): 1-23.
- Mikwar M, MacFarlane A.J. & Marchetti F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat Res Rev Mutat Res 785 (2020): 108320.
- Spandorfer S.D, Davis O.K, Barmat L.I, et al. Relationship between maternal age and aneuploidy in in vitro fertilization pregnancy loss. Fertil Steril 81 (2004): 1265-1269.
- Provost M.P, et al. Pregnancy outcomes decline with increasing body mass index: analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008-2010 Society for Assisted Reproductive Technology registry. Fertil Steril 105 (2016): 663-669.
- Fedorcsak P, Dale P.O, Storeng R, et al. The impact of obesity and insulin resistance on the outcome of IVF or ICSI in women with polycystic ovarian syndrome. Hum Reprod 16 (2001): 1086-1091.
- Wang J.X, Davies M.J, Norman R.J. Polycystic ovarian syndrome and the risk of spontaneous abortion following assisted reproductive technology treatment. Hum Reprod 16 (2001): 2606-2609.
- Yang A.M., et al. Risk Factors for Different Types of Pregnancy Losses: Analysis of 15,210 Pregnancies After Embryo Transfer. Front Endocrinol 12 (2021): 683236.
- Haadsma M.L., et al. Miscarriage risk for IVF pregnancies in poor responders to ovarian hyperstimulation. Reprod Biomed Online 20 (2010): 191-200.
- De Sutter P, Dhont M. Poor response after hormonal stimulation for in vitro fertilization is not related to ovarian aging. Fertil Steril 79 (2003): 1294-1298.
- Balen A.H, Tan S.L, MacDougall J et al. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Hum Reprod 8 (1993): 959-964.
- Farr S.L, Schieve L.A, Jamieson D.J. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999-2002. Am J Epidemiol 165 (2007): 1380-1388.
- Goldstein S.R. Embryonic death in early pregnancy: a new look at the first trimester. Obstet Gynecol 84 (1994): 294-297.
- Rauch E.R, Schattman G.L, Christos P.J, et al. Embryonic heart rate as a predictor of first-trimester pregnancy loss in infertility patients after in vitro fertilization. Fertil Steril 91 (2009): 2451-2454.
- Hu L., et al. Influencing factors of pregnancy loss and survival probability of clinical pregnancies conceived through assisted reproductive technology. Reprod Biol Endocrinol 16 (2018): 74.
- Franasiak J.M, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril 101 (2014): 656-663.
- Ziebe S, et al. Embryo quality and developmental potential is compromised by age. Acta Obstet Gynecol Scand 80 (2001): 169-174.
- Verlinsky Y., et al. Over a decade of experience with preimplantation genetic diagnosis: a multicenter report. Fertil Steril 82 (2004): 292-294.
- Grifo J, Talebian S, Keegan D, et al. Ten-year experience with preimplantation genetic diagnosis (PGD) at the New York University School of Medicine Fertility Center. Fertil Steril 88 (2007): 978-981.
- Marsidi A.M, Kipling L.M, Kawwass J.F et al. Influence of paternal age on assisted reproductive technology cycles and perinatal outcomes. Fertil Steril 116 (2021): 380-387.
- Yi Y., et al. A logistic model to predict early pregnancy los following in vitro fertilization based on 2601 infertility patients. Reprod Biol Endocrinol 14 (2016): 15.
- Cabry R., et al. Management of infertility in women over 40. Maturitas 78 (2014): 17-21.
- Regan L, Owen E.J, Jacobs H.S. Hypersecretion of luteinising hormone, infertility and miscarriage. Lancet 336 (1990): 1141-1144.
- Clifford K, Rai R, Watson H, et al. Does suppressing luteinizing hormone secretion reduce the miscarriage rate: results of randomized trial. BMJ 312 (1996): 1508-1511.
- Rai R, Backos M, Rushworth F, et al. Polycystic ovaries and recurrent miscarriage - a reappraisal. Hum Reprod 15 (2000): 612-615.
- Munch E.M, Sparks A.E, Zimmerman M.B, et al: High FSH dosing is associated with reduced live birth rate in fresh but not subsequent frozen embryo transfers. Hum Reprod 32 (2017): 1402-1409.
- Lee B, et al. Function and Hormonal Regulation of GATA3 in Human First Trimester Placentation. Biol Reprod 95 (2016): 113.
- Liu Y, Lee K.F, Ng E.H, et al. Gene expression profiling of human peri-implantation endometria between natural and stimulated cycles. Fertil Steril 90 (2008): 2152-2164.
- Horcajadas J.A, et al. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod 11 (2005): 195-205.
- Haaf T., et al. A high oocyte yield for intracytoplasmic sperm injection treatment is associated with an increased chromosome error rate. Fertil Steril 91 (2009): 733-738.
- Gianaroli L, et al. Predicting aneuploidy in human oocytes key factors which affect the meiotic process. Hum Reprod 25 (2010): 2374-2386.
- Baart E.B, et al. Milder ovarian stimulation for in-vitro fertlization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod 22 (2007): 980-988.
- Kaleli S. et al. High rate of aneuploidy in luteinized granulosa cells obtained from follicular fluid in women who underwent controlled ovarian hyperstimulation. Fertil Steril 84 (2005): 802-804.
- Anifandis G, et al. Estradiol and leptin as conditional prognostic IVF markers. Reproduction 129 (2005): 531-534.
- Taheri F, et al. The determination of estradiol to cumulus oocyte complex (COC) number ratio: Does it predict the outcomes of ART cycles? J Reprod Infertil 21 (2020): 11-16.
- Blazar A.S, Hogan J.W, Frankfurter D, et al. Serum estradiol positively predicts outcomes in patients undergoing in vitro fertilization. Fertil Steril 81 (2004): 1707-1709.
- Li H.W, Lee V.C, Ho P.C, et al: Ovarian sensitivity index is a better measure of ovarian responsiveness to gonadotrophin stimulation than the number of oocytes during in-vitro fertilization treatment. J Assist Reprod Genet 31 (2014): 199-203.
- Gallos I.D, et al. Optimal endometrial thickness to maximize live births and minimize pregnancy losses: Analysis of 25,767 fresh embryo transfers. Reprod Biomed Online 37 (2018): 542-548.
- Del Carmen Nogales M, et al, Association between clinical and IVF laboratory parameters and miscarriage after single euploid embryo transfers. Reprod Biol Endocrinol 19 (2021): 186.
- Liu K.E, Hartman M, Hartman A, et al. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod 33 (2018): 1883-1888.
- Vuong L.N, et al. Live birth rates with a freeze-only strategy versus fresh embryo transfer: secondary analysis of a randomized clinical trial. Reprod Biomed Online 38 (2019): 387-396.
- Zhao J, Zhang Q, Wang Y, et al. Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 IVF cycle. Reprod Biomed Online 29 (2014): 291-298.
- Kasius A, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update 20 (2014): 530-541.
- Sundström, P. Establishement of a successful pregnancy following in-vitro fertilization with an endometrial thickness of no more than 4 mm. Hum Reprod 13 (1998): 1550-1552.
- Check J.H. Cohen R. Live fetus following embryo transfer in a woman with diminished egg reserve whose maximal endometrial thickness was less than 4 mm. Clin Exp Obstet Gynecol 38 (2011): 330-332.
- Remohi J, et al. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod 12 (1997): 2271-2276.
- Wang E.T, et al. Abnormal implantation after fresh and frozen in vitro fertilization cycles. Fertil Steril 107 (2017): 1153-1158.
- Roque M, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril 99 (2013): 156-162.
- Hipp H, et al. First trimester pregnancy loss after fresh and frozen in vitro fertilization cycles. Fertil Steril 105 (2016): 722-728.
- Merviel P, et al. Effects of female body mass index on the outcome of in vitro fertilization/intracytoplasmic sperm injection or intrauterine insemination. Clin Obstet Gynecol Reprod Med (2020).
- Brunet C, et al. Impact of Women Obesity and Obesity Severity on Live Birth Rate after In Vitro Fertilization. J Clin Med 9 (2020): 2414.
- Fedorcsak P, Storeng R, Dale P.O, et al. Obesity is a risk factor for early pregnancy loss after IVF or ICSI. Acta Obstet Gynecol Scand 79 (2000): 43-48.
- Cui N, et al. Impact of Body Mass Index on Outcomes of In Vitro Fertilization/Intracytoplasmic Sperm Injection Among Polycystic Ovarian Syndrome Patients. Cell Physiol Biochem 39 (2016): 1723-1734.
- 53 Snider A.P, Wood J.R. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction 158 (2019): R79-R90.
- ESHRE Guideline Group on RPL, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open, 2018: hoy004 (2018).
- Magnusson A, Källen K, Thurin-Kjellberg A. et al: The number of oocytes retrieved during IVF: a balance between efficacy and safety. Hum Reprod 33 (2018): 58-64.
- Laanpere M, et al. Folate-metabolizing gene variants and pregnancy outcome of IVF. Reprod Biomed Online 22 (2011): 603-614.
- Merviel P, et al. Comparison of two preventive treatments for patients with recurrent miscarriages carrying a C677T methylenetetrahydrofolate reductase mutation: 5-year experience. J Int Med Res 45 (2017): 1720-1730.
