Predicting Required COVID-19 Vaccine Coverage and its Impact in Sierra Leone Using Mathematical Models
Article Information
Joseph AL Kamara, Abdul A Kamara*, Sallieu K Samura
Department of Mathematics and Statistic, Fourah Bay College, University of Sierra Leone, Freetown, Sierra Leone
*Corresponding author: Abdul A Kamara, Department of Mathematics and Statistic, Fourah Bay College, University of Sierra Leone, Freetown, Sierra Leone.
Received: 26 December 2021; Accepted: 12 January 2022; Published: 27 January 2022
Citation: Joseph AL Kamara, Abdul A Kamara, Sallieu K Samura. Predicting Required COVID-19 Vaccine Coverage and its Impact in Sierra Leone Using Mathematical Models. Archives of Clinical and Biomedical Research 6 (2022): 85-93.
View / Download Pdf Share at FacebookAbstract
In this article, we predict the required vaccine coverage for the COVID-19 outbreak in Sierra Leone. We also, investigate the impact of facemask and vaccine coverage on the spread of the COVID-19 virus using modified Symptomatic-Asymptomatic Infection Transmissions Susceptible-Latent-Infectious-Asymptomatic-Recovered (SLIAR) model. We derived an explicit formula for the basic reproduction number and used it to understand the dynamics of the disease when it is greater than unity. We also used the Maximum Likelihood modelling technique to estimate the basic reproduction number using incidence data. Numerically, we show that 58 per cent of the Sierra Leone national population required vaccination for the COVID-19 virus. Also, the SLIAR with vaccine model results reveals that the impact of using facemask is very challenging to understand and the vaccine coverage decrease the infected transmission rate but cannot completely stop the infection.
Keywords
Asymptomatic-symptomatic infection; COVID-19; Mathematical models; Vaccine coverage
Asymptomatic-symptomatic infection articles; COVID-19 articles; Mathematical models articles; Vaccine coverage articles
Article Details
1. Introduction
The 2019 coronavirus (COVID-19) outbreak has been a global pandemic with over 140,000,000 cases and more than 3,000,000 deaths [1]. Because of its high rate of infection and mode of transmission many control strategies have been implemented like social distancing, use of face masks, handwashing, and vaccine. Facemask is used because the virus of the COVID-19 can transmit through sneezing and coughing [2, 3]. Regular handwashing because human frequently touches their face which is the fastest transition point of the virus [4]. Social distancing because the virus can spread at a distance less than 6.1ft [5]. The vaccine available currently is used to reduce the severity of illness for an infected person but cannot prevent for getting infected. The fear imposed by the COVID-19 is that people without symptoms whether they can show symptoms or not, as long as they have been exposed to the virus they can cause transmission [6-9]. That is, there is an asymptomatic form of transmission. Although the recovery rate of the COVID-19 is encouraging, the deaths rate in most developed nations is alarming and because of that, the use of the vaccine has been the focus by many developed nations to reduce the panic the COVID-19 virus causes globally. Many vaccines have been used which developing countries like Sierra Leone is also a beneficiary. In February 2021, the People Republic of China donated 200,000 doses of vaccine [10] and 96,000 doses of the AstraZeneca-Oxford COVID-19 vaccine shipped via the COVAX facility was donated on March 8, 2021, [11] to fight the COVID-19 virus in Sierra Leone. The targeted populace are frontline health workers and people aged 18 years and above healthy individuals [11, 12]. However, whilst many countries are preventing the third wave of the COVID-19, there is concern among public health officials that Sierra Leone is on the verge of a third wave of COVID-19 infections [12]. New cases are surging and have hit highs record since June 1, 2021 [12]. Like many African countries, citizens in Sierra Leone have been reluctant to take the COVID-19 vaccine. As of June 16, 2021, the estimated vaccine total doses given is 91,789 and people fully vaccinated is 13,831 which is about 0.2 per cent of the Sierra Leone population [13]. Among the recent restriction by the Sierra Leone government as of June 17, 2021, are; age for vaccination has been revised further to thirty (30) years and educational institutions shall comply with mandatory face masks, hand-washing and social distance protocols [12]. As a researcher, our main concern is the fraction of the population that must be successfully vaccinated to minimize the severity of sickness and death for those with the infectious agent so that the government policy which states that government ministries, departments and agencies will only be accessed by employees and members of the public with proof of at least one dose of COVID-19 vaccination [12] can be revisited.
A mathematical model has been an effective tool to understand disease dynamics [14]. The state compartments in the system are demonstrated using differential equations from which an expression of the basic reproduction number can be derived [14]. The basic reproduction number (R_0 ) is a value that determines on average how many individuals an infected person infect during the infectious period. If R_0>1, it believes that the disease will persist and becomes endemic and if R_0<1, the disease will die out and the system becomes disease-free [15]. After the R_0 has been determined, the basic reproductive number under vaccination which is the number of secondary cases caused by one primary case introduced into a population in which a proportion have been vaccinated [16] can also be determined. Also, the critical vaccination proportion that will achieve to reduce fear of deaths or severe sickness of the virus, for which the basic reproductive number under vaccination is equal to unity [16] can be determined.
In this article, we modified a symptomatic-asymptomatic infection transmission Susceptible-Latent-Infectious-Asymptomatic-Recovered (SLIAR) model for Influenza outbreak with a Vaccine (V), used of facemask and handwashing. The symptomatic-asymptomatic infection transmission SLIAR model is common in the literature of SARS-CoV-1 [17, 18] due to its mode of transmission as it is done in COVID-19. That is why scientists referred to the COVID-19 as SAR-CoV-2 [19, 20] as they get to know more about the COVID-19 virus. We aim to use the mathematical SLIARV model to inspect the impact of facemask, and vaccine coverages using epidemic parameters in Sierra Leone. We also, aim to use Maximum Likelihood modelling [21] to estimate the R_0 from Sierra Leone daily reported incidence data and use it to estimate the proportion of people to vaccinate for the SARS-CoV-2 in Sierra Leone. By considering the vaccine coverage period in Sierra Leone, the estimated R_0 is 2.4 showing that 58 per cent of the national population is required to vaccinate for the COVID-19 virus. Also, our mathematical model results show that the impact of using facemask is very challenging to understand and the vaccine coverage decrease the infected transmission rate but cannot completely eradicate the infection.
1.1. The model
In this article, we consider a varying population SLIARV model since the SARS-CoV-2 has been going on for more than a year and there is no restriction of movement in and out of Sierra Leone during the time of writing. The model is demonstrated using the differential equation given as.
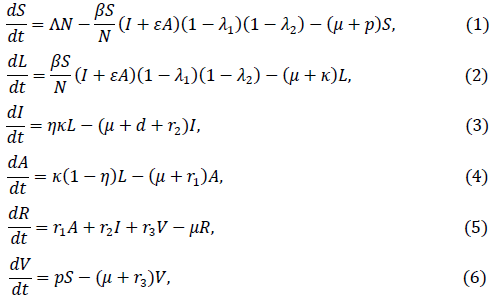
Where S = S(t) is the susceptible, L = L(t) is the latent, I = S(t)is the symptomatic infection, A = A(t) is the asymptomatically infection, R = R(t) recovered and V = V(t) vaccination populations at time t. The total population is N = S + L + I + A + R + V, ∧, is the rate of birth/migration into the S population, ꞵ is the symptomatic infective transmission coefficient and the infectiveness due to an asymptomatic person is reduced by the proportion ε. The rate of facemask and handwashing are denoted as λ1 and λ2 respectively, whereas μ and p are the natural death rate and proportion of vaccinated individuals respectively. The incubation period rate is measured by k and the probability of being symptomatic infective is measured by η. Death related to the disease is measured by the rate d, the recovered rate from populations of A,I and V are respectively denoted as r1,r2 and r3. For simplicity equations (1) to (6) is converted to proportional form given as
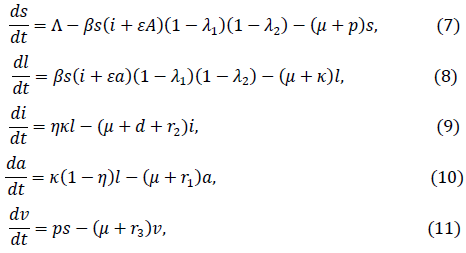
Where s = S/N ,l = L/N, i = I/N, a = A/N, v = V/N and equation (6) is excluded for further analysis since it does not influence the other equations. It is easy to see that if λ1 = λ2 = p = 0, equations (7) to (11) will be in the same form as the influenza model in [18]. However, it is not difficult to see that the feasible region of the model (2).
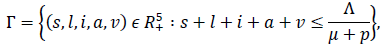
is positive invariant and attracting sets that attracts all solutions of model (2) with nonnegative initial condition. Also, it is not difficult to see that model (2) has disease-free equilibrium (DFE),
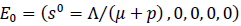 and using the next-generation matrix method [15] we get
and using the next-generation matrix method [15] we get
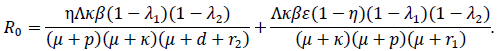
It is well known that the disease will die out whenever R0 < 1; or continue to persist in the population whenever R0 > 1. For a perfect vaccine that confers life-long protection, the basic reproductive number under vaccination (R0p)can be determined as (1 - p)R0, and the vaccination proportion is determined by (1 - 1/R0)[16].
2. Data
In this section, we use R-software to run simulations for our SLIAR-V model and the values of the parameters are taken from epidemiological data for Sierra Leone. Data of this study is taken from the global change data lab, Our World in Data [22]. It is a scientific online publication that focuses on large global problems such as disease, inequality, climate change, poverty, and war. In terms of disease, the database recorded new and cumulative cases, and deaths, reproduction rate, countries population and ages as well as other significant data relevant for disease analysis. Our datasets do not consider whether the infectious cases are from abroad or inland but as a representative of the country infected populations. Also, we assumed that a healthy or infected person is an individual that did not receive any dose of the COVID-19 vaccine. However, in Sierra Leone as of June 20, 2021, the average number of handwashing facilities is 19.275 [22] and 1.4 per 10000 healthcare workers as of 2016 [23]. Because healthcare workers frequently wash their hands, we estimated as the ratio of average handwashing facilities and average healthcare workers. Sierra Leone has administered at least 91,789 doses of COVID vaccine as of June 16, 2021, which is 0.6% of the country’s population if assuming every person needs two doses to be fully vaccinated [13]. Therefore, our assumed initial estimate of and also, since there is no report of reinfection after vaccination, we assumed all those who are vaccinated move to the recovery state with permanent immunity. The lifespan of Sierra Leone as of 2019 is 54.7 years [24], hence μ = 1/(54.7 × 365) days. There are 82 deaths out of 4816 infected cases as of June 20, 2021 [25], therefore we estimate deaths related to the disease as d = 82/4816. The incubation period is 14 days and the recovery period from visibly infectious has an average of 5.8 days [26]. We also assume the rate of moving from an asymptomatic state to a recovery rate to be 14 days since it is the same observed period used for quarantine individuals. Table 1 gives the value of the parameter used in the numerical simulation,
|
Parameters |
Description |
Values |
Source |
| λ1 |
Facemask |
0.001 |
Assumed |
| λ2 |
Handwashing |
0.001 |
[22] |
| p |
Vaccinated proportion |
0.006 |
[13] |
| ε |
Latency transmission efficacy |
0.5 |
[14] |
| μ |
Natural death rate |
0.0005 |
[24] |
| d |
Death related to disease |
0.02 |
Calculated |
| k |
Incubation period |
0.07 |
[26] |
| r1 |
Asymptomatic recovery |
0.07 |
Assumed |
| r2 |
Visibly infectious recovery |
0.712 |
Assumed |
| r3 |
Vaccinated recovery |
0.006 |
Assumed |
| Λ |
Birth rate |
0.0042 |
[27] |
| η |
Symptomatic infective rate |
0.05 |
Assumed |
| β |
Transmission rate |
0.533 |
[14] |
Table 1: The Sierra Leone demography and epidemiological parameter values.
3. Numerical Simulations
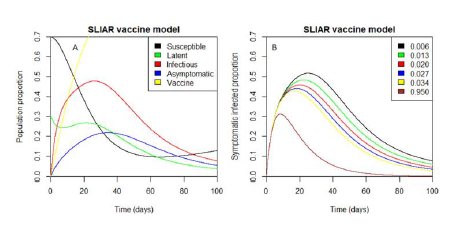
Using the parameter values from Table 1, we get R_0=2.4, which is the R_0 values for Sierra Leone, when simulating the incidence data from January 1, 2021, to June 20, 2021. Using Maximum Likelihood modelling as described in [21], the estimated R_0=2.4 with confidence interval [2.248304 , 2.670513]. The estimate of the basic reproductive number under vaccination is then (1-p) R_0=(1-0.006).(2.4)=2.4. However, the critical vaccination proportion, for which the basic reproductive number under vaccination is equal to unity is determined as 1-1⁄R_0 =1-0.42=0.58. That is, based on the current transmission rate, if approximately 58% of the Sierra Leone population is vaccinated, reducing fear of death or severe sickness of the COVID-19 infection will not be a challenge. Furthermore, we now focus our analysis on the impact of the control measured (p,λ_1,λ_2 ) considered in the SLIAR vaccine model. Firstly, we simulate the SLIAR-V model without any adjustment of the values of the parameters in Table 1 to observe the behaviour of the individual population trajectory, especially the visibly infectious compartment. It is observed in Figure.1A that the symptomatic infected trajectory increases with increasing time despite the use of facemask, handwashing and vaccine parameters. Also, it observes that when I and A trajectories increase the susceptible decreases, indicating their negative effect to the susceptible populations if they are not controlled.
Figure 1B show the impact of increasing the vaccination rate constant by 0.7 %. It is well known that when vaccine coverage is large and immunity is lifelong, the prevalence of infection decreases. This is observed in Figure 1B as the vaccination rate increases. The trajectories peaks of the symptomatic infected proportion decrease but it does not reach zero even at 95% coverage. Moreover, even though the vaccine coverage increases equally with facemask and handwashing coverages (Figure 2A), the symptomatic infected proportion trajectories peaks decrease but do not reach zero. This shows that large vaccine coverage reduces the infection spread, but does not lead to complete eradication of the disease as demonstrated in [28].
In Figure 2B, it is observed that despite the 0.7% increment of the facemask coverage the same trajectories are produced and it is difficult to give an insightful characterization of the epidemic curve. This shows that there is a problem of identifying the importance of using facemask with the symptomatic-asymptomatic infections SLIAR vaccine model.
4. Discussion
This paper aims to extend a basic symptomatic-asymptomatic infection SLIAR model without control of Influenzas propagation [17, 18] to model the use of facemask, handwashing and vaccination as a control strategy for the COVID-19 pandemic. We investigate the effects of the facemask and vaccination rate on the spread of the coronavirus infection in Sierra Leone. We have derived an explicit formula for the basic reproduction number (R_0), which has been the key parameter in our model. It is well known that when R_0 > 1, there exists a unique endemic equilibrium, which indicates that the disease is a threat to the population. Numerical simulations are conducted to illustrate that the infection maintain in the population with time when R_0 is great than unity (see Figure 1A). In terms of calculating R_0, we show that the R_0 estimated from the SLIAR vaccine model is equivalent to the estimated R_0 for Sierra Leone COVID-19 outbreak using Maximum Likelihood modeling [21]. It shows that from January 1, 2021, to June 20, 2021, the estimated R_0 is 2.4, indicating that an infectious person can infect an average of two persons in his/her infectious period. Approximately, our estimated R_0 is in agreement with reproduction rate of Our World in Data [22]. Because the transmission rate is alarming in Sierra Leone, we estimated the critical vaccination proportion of people to reduce the panic caused by the virus as 58%, which is far above the targeted population the vaccine program focus on in Sierra Leone. According to the World Population Prospects 2019, Volume II [24], 59.4% of the country population is between 15 years to older ages. This shows that 18 years and above is less than 58%, as our targeted estimate. Therefore, targeting 18 years and above, for the vaccine coverage in Sierra Leone will impose a serious challenge to the COVID-19 disease outbreak. Hence, the policy of vaccinating just a portion of the susceptible population needs to be revisited by including young ages of an individual to tackle the COVID-19 in Sierra Leone. The predicting vaccine coverage in this article can only be reached if the targeted population can be reduced to at least age 15 years using the World Population Prospects 2019, Volume II data [24] or the 2018 Sierra Leone Integrated Household Survey age estimates [29]. Using the SLIAR vaccine model the vaccination coverage only involves those in the susceptible population. In terms of controlling the spread of COVID-19 by facemask, we show that the importance of using facemask cannot be identified, as the same trajectory produce each time the coverage proportion increases. By controlling the spread of the COVID-19 virus by vaccination, we also show that even though vaccination reduces the infection spread, it does not completely eradicate the virus in the community when the coverage is high which is in agreement with [28].
Declarations
Availability of data materials. The authors can confirm that all relevant data sources are included in the article.
Competing interests. The authors declare that they have no competing interests,
Funding
Not applicable.
References
- Johns Hopkins University Center for Systems Science and Engineering (CSSE): Coronavirus COVID-19 Global Cases.
- Centers for Disease Control and Prevention: How COVID-19 Spreads.
- World Health Organisation: How COVID-19 Spreads.
- Centers for Disease Control and Prevention: Transmission of Coronavirus Disease 2019 (COVID-19). Centers Dis Control Prev Bull (2020).
- World Health Organisation: Coronavirus disease (COVID-19): How is it transmitted? (2020).
- Anastassopoulou C, Russo L, Tsakris A, et al Data-based analysis, modelling and forecasting of the COVID-19 outbreak. PLoS One 3 (2020): 1-21.
- Furukawa NW, Furukawa NW, Brooks JT, et al. Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 while Presymptomatic or Asymptomatic. Emerg Infect Dis 26 (2020).
- Li C, Ji F, Wang L, et al. Asymptomatic and Human-to-Human Transmission of SARS-CoV-2 in a 2-Family Cluster, Xuzhou, China. Emerg Infect Dis 26 (2020).
- Kimball A, Hatfield KM, Arons, et al. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility-King County, Washington, March 2020. MMWR. Morb Mortal Wkly Rep (2020).
- China donates 200,000 doses of COVID-19 vaccine to Sierra Leone (2021).
- World Health Organisation: COVID-19 vaccines shipped by COVAX arrive in Sierra Leone (2021).
- The Sierra Leone Ministry of Information and Communication: COVID-19 Update (2021).
- Sierra Leone: the latest coronavirus counts, charts and maps (2021).
- Kamara AA, Mouanguissa LN, Barasa GO. Mathematical modelling of the COVID-19 pandemic with demographic effects. J Egypt Math Soc 29, (2021).
- Van Den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission (2002).
- Nikbakht R, Baneshi MR, Bahrampour A. Estimation of the basic reproduction number and vaccination coverage of influenza in the United States (2017-18). J. Res. Health Sci 18 (2018): 1-6.
- Arino J, Brauer F, Van Den Driessche P, et al, Simple models for containment of a pandemic. JR Soc Interface 3 (2006): 453-457.
- Lamichhane S, Chen Y. Global asymptotic stability of a compartmental model for a pandemic. J. Egypt. Math. Soc 23 (2015): 251-255.
- Du Z, Xu X, Wu Y, et al. Serial Interval of COVID-19 among Publicly Reported Confirmed Cases. Emerg Infect Dis 26 (2020).
- World Health Organization: Transmission of SARS-CoV-2: implications for infection prevention precautions (2020).
- Obadia T, Haneef R, Boëlle PY. The R0 package: A toolbox to estimate reproduction numbers for epidemic outbreaks. BMC Med Inform Decis Mak 12 (2012).
- covid-19-data_public_data at master • owid_covid-19-data (2021)
- Robinson C. Primary health care and family medicine in Sierra Leone. African J. Prim. Heal. Care Fam. Med 11 (2019): 1-3.
- United Nations Department of Economic and Social Affairs Population Division: World Population Prospects 2019: Demographic Profiles (2019).
- Sierra Leone COVID-4,042 Cases and 79 Deaths (2021).
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med (2020).
- Statistics Sierra Leone: Sierra Leone Demographic and Health Survey 2019 key indicators (2019).
- Nkamba LN, Manga TT, Agouanet F, et al. Mathematical model to assess vaccination and effective contact rate impact in the spread of tuberculosis. J. Biol. Dyn 13 (2019): 26-42.
- Statistics Sierra Leone: Sierra Leone Integrated House Hold Survey. Stat. Sierra Leone Bull (2019).