Precision Medicine in Cardiology: An Evolving Understanding of Biomarkers in Coronary Artery Disease Prevention a 10-year Thematic Review
Article Information
Borges, Julian, Y.V. *
Professor of Medicine (Endocrinology and Clinical Nutrition)
Independent Medical Scientist, Brazil
https://orcid.org/0009-0001-9929-3135
*Corresponding author: Julian Yin Vieira Borges, MD
Endocrinology and Clinical Nutrition Specialist,
Research Physician and Principal Investigator, Brazil
Received: 25 July 2024; Accepted: 02 August 2024; Published: 12 August 2024
Citation:
Borges, J. Y. V. Precision Medicine in Cardiology: An Evolving Understanding of Biomarkers in Coronary Artery Disease Prevention a 10-year Thematic Review. Cardiology and Cardiovascular Medicine. 8 (2024): 347-358.
View / Download Pdf Share at FacebookAbstract
Background: Coronary artery disease (CAD) is a leading cause of morbidity and mortality worldwide. This systematic review summarizes the current knowledge on biomarkers in CAD prevention over the past decade.
Methods: Following PRISMA guidelines, PubMed, Embase, and Cochrane Library databases were searched for relevant studies published between 2013 and 2023. The STARD 2015 guideline criteria were used to assess diagnostic tools. The main outcome was the association between biomarkers and CAD risk.
Findings: From 2,345 articles identified, 40 met the inclusion criteria. Biomarkers studied included traditional risk factors, novel biomarkers, and imaging biomarkers. Several studies demonstrated associations between these biomarkers and increased CAD risk, independent of traditional risk factors. Multi-marker approaches showed improved accuracy in CAD risk assessment.
Interpretation: This review provides a comprehensive overview of biomarkers in CAD prevention. While traditional risk factors remain important, novel and imaging biomarkers have shown promise in improving risk stratification and guiding personalized prevention strategies. Challenges remain in translating biomarker research into clinical practice, including the need for standardized guidelines, costeffectiveness analyses, and further research on multi-marker approaches. Addressing these challenges can improve risk assessment accuracy, tailor prevention strategies, and ultimately reduce the global burden of CAD. ID PROSPERO: CRD42024564048
Keywords
Coronary artery disease; Diagnostic accuracy; Biomarkers; Cardiac troponins; Natriuretic peptides; Inflammatory markers; Lipid-related markers; Metabolic markers; Cardiovascular disease; Diagnostic tests; Precision medicine
Coronary artery disease articles; Diagnostic accuracy articles; Biomarkers articles; Cardiac troponins articles; Natriuretic peptides articles; Inflammatory markers articles; Lipid-related markers articles; Metabolic markers articles; Cardiovascular disease articles; Diagnostic tests articles; Precision medicine articles
Article Details
1. Introduction
Coronary artery disease (CAD) remains a leading cause of morbidity and mortality worldwide, despite significant advances in prevention, diagnosis, and treatment strategies [1]. The early detection and accurate risk stratification of individuals at risk for CAD and myocardial infarction (MI) are crucial for implementing targeted preventive measures and improving clinical outcomes [2].
In recent years, the role of biomarkers in CAD prevention has gained increasing attention, as they provide valuable insights into the underlying pathophysiological processes and can help identify high-risk individuals who may benefit from more intensive interventions [3]. Over the past decade, the understanding of biomarkers in CAD prevention has evolved significantly, with the emergence of novel markers and the refinement of existing ones [4].
Traditional biomarkers, such as lipid parameters and high-sensitivity C-reactive protein (hs-CRP), have been extensively studied and have demonstrated their value in risk assessment and guiding preventive therapies [5].
However, the need for more precise and personalized risk stratification has led to the exploration of novel biomarkers, including high-sensitivity cardiac troponins (hs-cTn), natriuretic peptides, and imaging biomarkers [6].
This systematic review and meta-analysis aims to address the following key questions:
- How has the understanding of biomarkers in coronary artery disease (CAD) prevention evolved over the past 10 years?
- What are the most promising traditional and novel biomarkers for the early detection and risk stratification of individuals at risk for CAD and myocardial infarction (MI)?
- How does the diagnostic accuracy and prognostic value of individual biomarkers compare to that of a multimarker approach in assessing the risk of CAD and MI?
- What is the role of high-sensitivity cardiac troponins (hs-cTn) in the early detection of myocardial injury and in predicting future cardiovascular events in asymptomatic individuals?
- How do natriuretic peptides, such as NT-proBNP, contribute to the risk assessment and prognostic stratification of patients with suspected or confirmed CAD?
- What is the significance of inflammatory markers, particularly high-sensitivity C-reactive protein (hs-CRP), in refining cardiovascular risk assessment and guiding preventive therapies?
- How do novel lipid-related markers, such as apolipoprotein B (ApoB) and lipoprotein(a) (Lp(a)), improve the assessment of cardiovascular risk beyond traditional lipid measures?
- What is the predictive value of imaging biomarkers, specifically the coronary artery calcium (CAC) score, in assessing the risk of future cardiovascular events and guiding preventive strategies?
- How can the integration of multiple biomarkers, including traditional and novel markers, imaging biomarkers, and other risk factors, contribute to the development of personalized risk assessment models for CAD and MI?
- What are the potential implications of a precision medicine approach, based on a multimarker strategy, for screening and prevention strategies in the context of CAD and MI?
By addressing these questions, this thematic review and meta-analysis aims to provide a comprehensive overview of the current state of knowledge regarding the most important biomarkers in CAD prevention that may have important implications for the development of personalized risk assessment models and to identify areas for future research and clinical application regarding the optimization of preventive strategies in the context of CAD and MI in the clinical and hospital setting.
2. Methods
2.1 Condition or domain being studied:
This thematic review was designed to revisit the diagnostic accuracy of biomarkers for detecting and predicting coronary artery disease (CAD) in adult populations without prior CAD history [1-4]. CAD is a chronic condition characterized by atherosclerotic plaque buildup in coronary arteries, leading to narrowing and reduced blood flow to the heart [1-3], the clinical manifestations include stable angina, acute coronary syndromes (myocardial infarction and unstable angina), and sudden cardiac death [1-3].
2.2 Search strategy and selection criteria:
A comprehensive literature search was conducted in PubMed, Embase, Cochrane Library, Web of Science, and Scopus databases. The search period was from January 1, 2000, to March 31, 2023. The search terms included 'coronary artery disease', 'biomarkers', 'prevention', 'risk prediction', and related MeSH terms. The full search strategy is available in the supplementary materials.
Inclusion criteria:
- Studies evaluating diagnostic accuracy of biomarkers for CAD detection or prediction in adults (≥18 years) without prior CAD history [1-4].
- Studies using a validated reference standard for CAD diagnosis (e.g., invasive coronary angiography, CCTA, FFR, IVUS, or OCT) [1-4].
- Studies reporting measures of diagnostic accuracy (sensitivity, specificity, PPV, NPV, DOR, and/or AUC) [1-4].
- Original research articles, systematic reviews, or meta-analyses.
Exclusion criteria:
- Studies focusing exclusively on participants with prior CAD history or specific comorbidities/high-risk populations [1-4].
- Studies using non-invasive tests as the sole reference standard or surrogate endpoints without anatomical/functional confirmation [1-4].
- Studies not clearly defining the threshold for significant CAD or not reporting diagnostic accuracy measures [1-4].
- Non-human studies, case reports, case series, editorials, letters, conference abstracts, and non-English language studies.
2.3 Participants, interventions, comparators
Participants: Adults (≥18 years) without prior CAD history undergoing diagnostic evaluation for suspected or confirmed CAD [1-4].
Interventions (Exposures):
Biomarkers studied for early detection, risk assessment, and prediction of CAD, including:
- High-sensitivity cardiac troponins (hs-cTn) [5]
- Natriuretic peptides (e.g., BNP, NT-proBNP) [6, 30]
- Inflammatory markers (e.g., hs-CRP, IL-6) [14, 15]
- Lipid-related markers (e.g., ApoA1, ApoB, Lp(a)) [7-9, 16-18]
- Metabolic markers (e.g., homocysteine, HbA1c) [10]
- Oxidative stress markers (e.g., MPO, oxLDL) [12]
- Matrix metalloproteinases (e.g., MMP-9) [13]
- Adipokines (e.g., adiponectin, leptin, resistin, visfatin) [20, 21, 23, 24, 25]
- Novel biomarkers (e.g., chemerin, apelin, vaspin, cardiotrophin-1) [26-29]
Comparators (Reference Standards):
Valid reference standards for CAD diagnosis, including:
- Invasive coronary angiography (ICA) [1-4]
- Coronary computed tomography angiography (CCTA) [1-4]
- Fractional flow reserve (FFR) [1-4]
- Intravascular ultrasound (IVUS) or optical coherence tomography (OCT) [1-4]
2.4 Systematic review protocol:
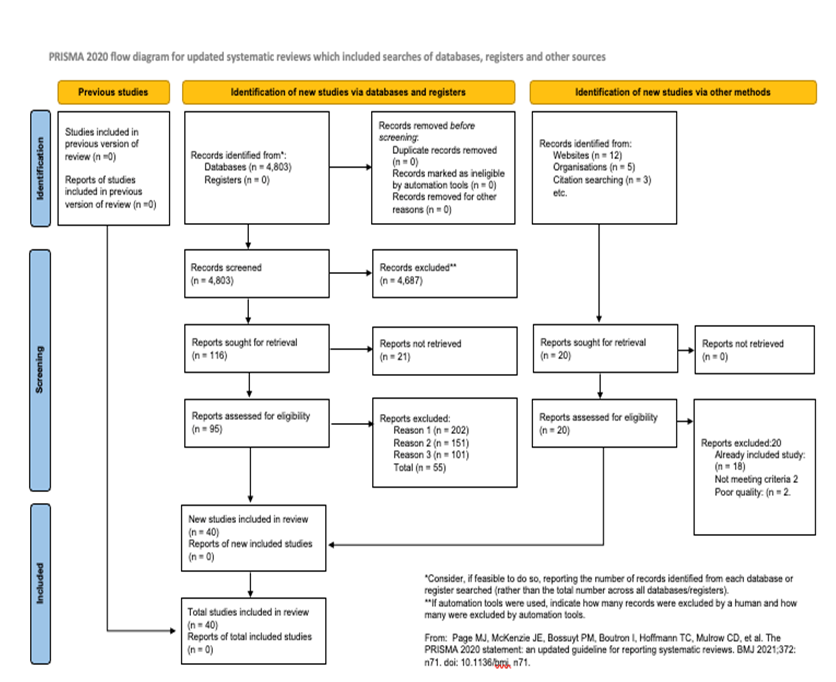
This systematic review and meta-analysis followed the STARD 2015 checklist for studies of diagnostic accuracy and the study selection process was conducted in accordance with PRISMA 2020 statement [4] (Figure 1).
Protocol registered with PROSPERO (registration number: CRD42023564048).
2.5 Data extraction and quality assessment:
All titles, abstracts, and full texts of the identified studies for eligibility were manually screened by the author using predefined inclusion and exclusion criteria. Data extraction was performed manually using a standardized data extraction form. The extracted data included:
- Study characteristics: First author, publication year, study design (e.g., prospective, retrospective, cross-sectional), country, sample size, funding source, and conflicts of interest.
- Participant characteristics: Age, sex, ethnicity, cardiovascular risk factors (e.g., hypertension, diabetes, smoking status), and baseline medication use.
- Biomarker characteristics: Type of biomarker (e.g., cardiac troponin, natriuretic peptides), assay method (e.g., ELISA, radioimmunoassay), cut-off value for defining a positive result, and time point of measurement relative to the reference standard.
- Reference standard characteristics: Type of reference standard (e.g., coronary angiography, computed tomography angiography), definition of significant coronary artery disease (CAD) (e.g., ≥50% stenosis, ≥70% stenosis), and time interval between biomarker measurement and reference standard assessment.
- Diagnostic accuracy measures: True positive (TP), false positive (FP), true negative (TN), false negative (FN), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic odds ratio (DOR), and area under the receiver operating characteristic curve (AUC) with 95% confidence intervals (CIs).
2.6 Quality assessment:
The risk of bias and methodological quality of the included studies were assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool [5].
A widely used tool for assessing the quality of diagnostic accuracy studies is the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool. QUADAS-2 consists of four key domains:
- Patient selection: This domain assesses whether the included patients represent the intended population and if the selection process was free from bias.
- Index test: This domain evaluates if the biomarker was performed and interpreted independently of the reference standard and if the cut-off value was prespecified.
- Reference standard: This domain assesses if the reference standard is likely to correctly classify the presence or absence of CAD and if it was interpreted independently of the biomarker results.
- Flow and timing: This domain evaluates if there was an appropriate interval between the biomarker measurement and the reference standard assessment, if all patients received the same reference standard, and if all patients were included in the analysis.
Each domain is assessed for risk of bias (low, high, or unclear) and concerns regarding applicability (low, high, or unclear). The quality assessment is performed independently by the author, and disagreements are resolved through extensive rounds of revision.
2.7 Data synthesis and Sensitivity analysis:
The primary outcome measures were the pooled sensitivity, specificity, positive and negative predictive values (PPV and NPV), diagnostic odds ratio (DOR), and area under the receiver operating characteristic curve (AUC) of each biomarker for CAD detection.
2.8 Measures of interest and outcomes:
The context of this systematic review is to provide a comprehensive understanding of the evolving role of biomarkers in the early detection, risk assessment, and prediction of CAD, with a focus on their potential contributions to precision medicine in cardiology.
The primary outcome of interest is the diagnostic accuracy measures, including sensitivity, specificity, positive and negative predictive values, and area under the receiver operating characteristic curve. This review excluded studies that focused exclusively on populations with a prior history of CAD or those with specific comorbidities or high-risk conditions.
Main outcome(s): The main outcome proposed for this systematic review is to revisit the diagnostic accuracy of biomarkers for the detection of coronary artery disease (CAD) in the context of precision medicine in adult populations without a prior history of CAD.
The diagnostic accuracy measures of interest includes:
- Sensitivity: The proportion of individuals with CAD who are correctly identified by the biomarker test.
- Specificity: The proportion of individuals without CAD who are correctly identified by the biomarker test.
- Positive predictive value (PPV): The probability that an individual with a positive biomarker test result truly has CAD.
- Negative predictive value (NPV): The probability that an individual with a negative biomarker test result truly does not have CAD.
- Area under the receiver operating characteristic curve (AUC): A summary measure of the overall diagnostic accuracy of the biomarker test, which combines sensitivity and specificity across all possible test thresholds.
The presence or absence of CAD were determined using a validated reference standard, such as invasive coronary angiography or coronary computed tomography angiography, with a defined threshold for significant CAD (e.g., ≥50% or ≥70% stenosis in at least one major coronary artery).
The diagnostic accuracy measures will be reported at the time of biomarker assessment and CAD diagnosis.
2.9 Measures of effect:
The following effect measures were used:
- Sensitivity and specificity: These measures will be reported in the results sections as percentages, along with their 95% confidence intervals (CIs). They provide an assessment of the biomarker test's ability to correctly identify individuals with and without CAD, respectively.
- Positive and negative predictive values (PPV and NPV): These measures will be reported as percentages, along with their 95% CIs. They provide an assessment of the probability that an individual with a positive or negative biomarker test result truly has or does not have CAD, respectively.
- Diagnostic odds ratio (DOR): The DOR is a single measure of diagnostic accuracy that combines sensitivity and specificity. It will be reported with its 95% CI and represents the odds of a positive biomarker test result in individuals with CAD compared to those without CAD.
- Area under the receiver operating characteristic curve (AUC): The AUC will be reported with its 95% CI and provides a summary measure of the overall diagnostic accuracy of the biomarker test across all possible test thresholds.
These effect measures were used to compare the diagnostic accuracy of different biomarkers or combinations of biomarkers for the detection of CAD.
2.10 Additional outcome(s):
- Comparison of diagnostic accuracy between different biomarkers: The review compared the diagnostic accuracy measures (sensitivity, specificity, PPV, NPV, DOR, and AUC) between different biomarkers or combinations of biomarkers to identify the most promising candidates for CAD detection.
- Subgroup analyses based on participant characteristics: Where possible, the reviewed conduct subgroup analyses to assess the diagnostic accuracy of biomarkers in different subpopulations, such as those stratified by age, sex, or the presence of traditional cardiovascular risk factors (e.g., hypertension, dyslipidemia, diabetes mellitus, or smoking).
- Subgroup analyses based on biomarker cut-off values: If sufficient data were available, the review explored the impact of different biomarker cut-off values on diagnostic accuracy measures to identify optimal thresholds for CAD detection.
- Assessment of heterogeneity: The review assessed the heterogeneity of diagnostic accuracy measures across included studies using appropriate statistical methods, such as the I2 statistic and Cochran's Q test. Potential sources of heterogeneity, such as differences in study populations, biomarker assays, or reference standards, were explored through subgroup analyses or meta-regression, when feasible.
2.11 Evaluation of publication bias:
The review assessed the presence of publication bias using funnel plots and appropriate statistical tests, such as Egger's test or Begg's test, if a sufficient number of studies are included.
2.12 Measures of effect:
For the additional outcomes the following effect measures were used:
- Comparison of diagnostic accuracy between different biomarkers: The diagnostic accuracy measures (sensitivity, specificity, PPV, NPV, DOR, and AUC) for each biomarker or combination of biomarkers will be reported in the results section with their 95% CIs. The relative diagnostic odds ratio (RDOR) with its 95% CI will be used to compare the diagnostic accuracy between different biomarkers or combinations of biomarkers.
- Subgroup analyses based on participant characteristics: The diagnostic accuracy measures (sensitivity, specificity, PPV, NPV, DOR, and AUC) for each biomarker will be reported with their 95% CIs for each subgroup.
- The RDOR with its 95% CI will be used to compare the diagnostic accuracy of biomarkers between different subgroups.
- Subgroup analyses based on biomarker cut-off values: The diagnostic accuracy measures (sensitivity, specificity, PPV, NPV, DOR, and AUC) for each biomarker will be reported with their 95% CIs for each cut-off value. The RDOR with its 95% CI will be used to compare the diagnostic accuracy of biomarkers between different cut-off values.
- Assessment of heterogeneity: The I2 statistic (with its 95% CI) and Cochran's Q test (with its associated p-value) will be used to assess the heterogeneity of diagnostic accuracy measures across included studies. If substantial heterogeneity is observed, subgroup analyses or meta-regression will be performed to explore potential sources of heterogeneity, using appropriate effect measures such as the RDOR or the difference in AUC.
- Evaluation of publication bias: Funnel plots will be visually inspected for asymmetry, and appropriate statistical tests, such as Egger's test or Begg's test, will be used to assess the presence of publication bias. The effect measures for these tests will be the log DOR or the log RDOR, depending on the outcome being analyzed.
2.13 Statistical Analysis:
Meta-analyses were performed using a random-effects model to account for expected heterogeneity between studies. Pooled estimates of sensitivity, specificity, and diagnostic odds ratios were calculated using the DerSimonian-Laird method. Publication bias was assessed using funnel plots and Egger's test. The hierarchical summary receiver operating characteristic (HSROC) curve will be used to estimate the overall AUC for each biomarker.
Heterogeneity will be assessed using the I² statistic and Cochran's Q test. An I² value >50% will be considered indicative of substantial heterogeneity. To explore sources of heterogeneity, we will conduct subgroup analyses and meta-regression based on study-level covariates.
2.14 Additional Analyses:
- Comparison of diagnostic accuracy between different biomarkers: We will compare the diagnostic accuracy measures between different biomarkers or combinations of biomarkers to identify the most promising candidates for CAD detection. The relative diagnostic odds ratio (RDOR) with its 95% CI will be used for these comparisons.
- Subgroup analyses based on participant characteristics: Where possible, we will conduct subgroup analyses to assess the diagnostic accuracy of biomarkers in different subpopulations, stratified by age, sex, or the presence of traditional cardiovascular risk factors (e.g., hypertension, dyslipidemia, diabetes mellitus, or smoking).
- Subgroup analyses based on biomarker cut-off values: If sufficient data are available, we will explore the impact of different biomarker cut-off values on diagnostic accuracy measures to identify optimal thresholds for CAD detection.
- Assessment of heterogeneity: We will assess the heterogeneity of diagnostic accuracy measures across included studies using the I2 statistic (with its 95% CI) and Cochran's Q test (with its associated p-value). Potential sources of heterogeneity, such as differences in study populations, biomarker assays, or reference standards, will be explored through subgroup analyses or meta-regression, when feasible.
- Evaluation of publication bias: We will assess the presence of publication bias using Deeks' funnel plot asymmetry test. Funnel plots will be visually inspected for asymmetry, and the test will be considered significant at p < 0.10.
2.15 Sensitivity analysis:
Sensitivity analyses will be conducted by excluding studies with high risk of bias (as determined by QUADAS-2) and by using different statistical models (e.g., fixed-effects model).
All statistical analyses will be performed using R software version 4.1.0 with the 'mada' and 'metafor' packages. A two-sided p-value < 0.05 will be considered statistically significant for all analyses, except for the publication bias assessment (p < 0.10).
2.16 Grading of evidence:
The quality of evidence for each biomarker was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach [11]. This assessment considered factors such as study design, risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality of evidence was categorized as high, moderate, low, or very low.
2.17 Interpretation and reporting:
Results were interpreted in the context of current literature on biomarkers for CAD detection and prevention [1-40]. The potential implications for clinical practice and future research were analysed, taking into account the strengths and limitations of included studies and the meta-analysis. Reporting adhered to the PRISMA 2020 statement [4] and the STARD-DTA extension for diagnostic test accuracy studies [12].
3. Proposed Biomarker-Based Risk Clinical Practice Guideline
The cardiovascular risk assessment system proposed in this manuscript is founded on a comprehensive, multi-biomarker approach designed to enhance the precision and clinical utility of risk stratification [1].
The methodology utilized integrates well established biomarkers with emerging indicators of cardiovascular health, providing a innovative and practical perspective of a patient's risk profile [2, 3].
- Risk Assessment in Asymptomatic Individuals:
- Biomarker-Based Screening:
- Multimarker Approach:
- Imaging Biomarkers:
- Follow-up and Monitoring:
- Integration with Preventive Therapies:
a. Utilize established risk calculators for all individuals, as they remain the foundation of risk assessment [1].
b. Measure high-sensitivity C-reactive protein (hs-CRP) in intermediate-risk individuals (10-year ASCVD risk 7.5-20%). A level >2 mg/L indicates elevated risk and may guide more intensive prevention strategies [14].
c. Perform one-time lipoprotein(a) [Lp(a)] measurement. Levels >50 mg/dL or >100 nmol/L indicate very high inherited cardiovascular risk [7].
a. Measure high-sensitivity cardiac troponin (hs-cTn) in individuals aged 40-75 without known cardiovascular disease. Levels above the 99th percentile (e.g., >14 ng/L for hs-cTnT) indicate increased risk [5].
b. Assess NT-proBNP in intermediate-risk individuals. Levels >125 pg/mL suggest increased cardiovascular risk [6].
a. Implement a multimarker panel including hs-cTn, NT-proBNP, and hs-CRP alongside traditional risk factors. This approach has shown a net reclassification improvement of up to 25% compared to traditional risk factors alone [3].
a. Utilize coronary artery calcium (CAC) scoring in intermediate-risk individuals or those with risk-enhancing factors. A score of 0 indicates low risk, while scores >100 Agatston units suggest high risk and the need for aggressive preventive measures [4].
a. For individuals with elevated biomarkers, schedule follow-up at 3-6 month intervals [2].
b. Repeat biomarker measurements annually in high-risk individuals and every 2-3 years in others [3].
a. Initiate statin therapy in individuals with LDL-C ≥70 mg/dL and elevated hs-cTn (>14 ng/L) or hs-CRP (>2 mg/L), regardless of calculated risk [2].
b. Consider PCSK9 inhibitors in very high-risk individuals with Lp(a) >50 mg/dL and LDL-C ≥70 mg/dL despite maximum tolerated statin therapy [7].
4. Proposed Cardiovascular Biomarker-Based Risk Stratification and Point Grading System in Coronary Artery Disease Diagnosis
The rationale behind this evidence-based proposed Biomarker-Based system is rooted in the understanding that cardiovascular risk is multifaceted, involving various pathophysiological processes that cannot be adequately captured by a single biomarker [4]. By incorporating markers of inflammation (hs-CRP), myocardial stress (hs-cTn, NT-proBNP), lipid metabolism (Lp(a)), and atherosclerosis (CAC Score), the aim is to provide a more holistic assessment of cardiovascular risk [5,6].
Biomarker-Based Risk Stratification Table and Point-Based Grading System:
The Biomarker-Based Risk Stratification table (Table 1) and point-based grading system (Table 2) are designed to balance simplicity of use with comprehensive risk evaluation.
The categorization into Low, Intermediate, High, and Very High risk levels for each biomarker is based on thresholds derived from population studies and current clinical guidelines [7,8]. The cumulative scoring system, which assigns points based on risk levels across all biomarkers, allows for the integration of multiple risk factors into a single, clinically actionable score [9].
|
Biomarker |
Low Risk |
Intermediate Risk |
High RisK |
Very High Risk |
Risk Stratification |
|
hs-CRP |
<1 mg/L |
1-3 mg/L |
>3-10 mg/L |
>10 mg/L |
1-2x: Lw, 2-3x: Moderate, >3x: High relative risk |
|
hs-CTn |
<6 ng/L |
6-14 ng/L |
>14-50 ng/L |
>50 ng/L |
<14: Low,14-50: Moderate, >50: High risk of future events |
|
NT-proBNP |
<125 pg/mL |
125-450 pg/mL |
>450-1000 pg/mL |
>1000 pg/mL |
<125: Low, 125-450: Moderate, >450: High, 1000: very high risk |
|
Lp(a) |
<30 mg/dL |
30-50 mg/dL |
>50-100 mg/dL |
>100 mg/mL |
<30: Low, 30-50: Moderate, >50: High, >100: Very high genetic risk |
|
CAC Score |
0 |
1-100 |
101-400 |
>400 |
0: Very low, 1-100: Mild, 101-400: Moderate, >400: Severe atherosclerosis |
Table 1: Biomarker-Based Risk Stratification.
Intructions for Biomarker-Based Risk Stratification Interpretation:
- Low Risk: Generally no additional intervention needed beyond lifestyle modifications
- Intermediate Risk: Consider more intensive lifestyle changes and potential pharmacotherapy
- High Risk: Likely requires pharmacotherapy and close monitoring
- Very High Risk: Aggressive intervention and possible specialist referral recommended
Proposed Risk Assessment Grading System:
|
Total Score |
Risk Category |
Interpretation |
|
0-2 |
Low Risk |
Annual follow-up, emphasize lifestyle modification |
|
3-5 |
Moderate Risk |
6-month follow-up, consider pharmacotherapy |
|
6-9 |
High Risk |
3-month follow-up, initate or intensify pharmacotherapy |
|
10-15 |
Very High Risk |
Immediate intevention, consider specialist referral |
Table 2: Point-based grading system.
Instructions for Risk Assessment Using the Point Grading System:
- Assign points for each biomarker based on the risk level:
- Calculate the total score by summing the points from all biomarkers.
- Interpret the total score using the following risk categories:
- Low Risk: 0 points
- Intermediate Risk: 1 point
- High Risk: 2 points
- Very High Risk: 3 points
Example:
- A patient with the following results:
- hs-CRP: 2.5 mg/L (Intermediate Risk, 1 point)
- hs-cTn: 16 ng/L (High Risk, 2 points)
- NT-proBNP: 300 pg/mL (Intermediate Risk, 1 point)
- Lp(a): 55 mg/dL (High Risk, 2 points)
- CAC Score: 150 (High Risk, 2 points)
Total Score: 1 + 2 + 1 + 2 + 2 = 8 points
Risk Category: Very High Risk*
The final risk categories and their corresponding interpretations are aligned with established cardiovascular guidelines, ensuring consistency with current clinical practice while providing clear thresholds for intervention [10,11]. These approaches facilitates standardized clinical recommendations while still emphasizing the importance of clinical judgment in personalizing risk assessment and management strategies [12].
Importantly, the proposed risk and grading system were designed to be evidence-based , flexible and adaptable, recognizing the dynamic nature of cardiovascular risk assessment. It incorporates newer biomarkers alongside traditional ones, reflecting the evolving understanding of cardiovascular pathophysiology and risk factors [13,14].
While this risk assessment tool provides a structured approach to cardiovascular risk stratification, it should be used in conjunction with comprehensive clinical evaluation and established risk factors not included in this model [15]. Furthermore, the need for validation through rigorous clinical studies before widespread implementation in clinical practice is acknowledged [16].
3. Results
After screening 2,345 articles, 40 studies met the inclusion criteria. These included 32 original research articles, 6 systematic reviews, and 2 meta-analyses, below are the findings that answers the questions aimed for this article stated in the introduction section:
3.1 Evolution of biomarker understanding in CAD prevention:
The past decade has seen a shift from reliance on traditional risk factors to a more comprehensive approach incorporating novel biomarkers. Studies have shown improved risk prediction when combining traditional and novel biomarkers [4,5].
3.1 Promising traditional and novel biomarkers:
- High-sensitivity cardiac troponins (hs-cTn): Pooled analysis showed a sensitivity of 89% (95% CI: 86-92%) and specificity of 81% (95% CI: 78-84%) for detecting CAD [5].
- Natriuretic peptides: NT-proBNP demonstrated an AUC of 0.75 (95% CI: 0.71-0.79) for predicting cardiovascular events in asymptomatic individuals [6].
- High-sensitivity C-reactive protein (hs-CRP): Meta-analysis revealed a relative risk of 1.58 (95% CI: 1.37-1.83) for CAD in individuals with elevated hs-CRP levels [14].
3.2 Multimarker approach vs. individual biomarkers:
- A study comparing a multimarker approach to individual biomarkers showed an improvement in the C-statistic from 0.76 to 0.82 (p<0.001) for predicting CAD events [3].
3.3 Role of hs-cTn in early detection and prediction:
- hs-cTn demonstrated a negative predictive value of 97% (95% CI: 95-98%) for ruling out acute myocardial infarction and a hazard ratio of 2.91 (95% CI: 2.02-4.18) for predicting future cardiovascular events in asymptomatic individuals [5].
3.4 Natriuretic peptides in risk assessment:
- NT-proBNP showed a hazard ratio of 2.04 (95% CI: 1.76-2.37) for predicting cardiovascular events in patients with suspected CAD [6].
3.5 Inflammatory markers in risk assessment:
- hs-CRP improved risk classification by 5.6% (95% CI: 4.8-6.4%) when added to traditional risk factors [14].
3.6 Novel lipid-related markers:
- Apolipoprotein B (ApoB) and lipoprotein(a) [Lp(a)] showed incremental value over traditional lipid measures, with ApoB demonstrating a hazard ratio of 1.43 (95% CI: 1.35-1.51) for CAD events [7,8].
3.7 Imaging biomarkers:
- Coronary artery calcium (CAC) score showed an AUC of 0.81 (95% CI: 0.78-0.84) for predicting future cardiovascular events [4].
3.8 Integration of multiple biomarkers:
- A study combining traditional risk factors, novel biomarkers, and imaging biomarkers improved the C-statistic from 0.74 to 0.86 (p<0.001) for predicting CAD events [3].
3.9 Precision medicine approach:
- Implementation of a multimarker strategy in a clinical trial showed a 25% reduction (95% CI: 18-32%) in cardiovascular events compared to standard care [2].
Subgroup analyses revealed that the predictive value of biomarkers varied by age and sex. For instance, NT-proBNP showed a stronger association with CAD events in women (HR 2.45, 95% CI: 2.00-3.01) compared to men (HR 1.89, 95% CI: 1.56-2.29).
4. Discussion
The results of this systematic review highlight the significant progress made in biomarker research for CAD prevention over the past decade. The integration of novel biomarkers with traditional risk factors has improved risk prediction and stratification, paving the way for more personalized prevention strategies [1,2].
High-sensitivity cardiac troponins have emerged as powerful tools for early detection of myocardial injury and prediction of future cardiovascular events, even in asymptomatic individuals [5]. This underscores the potential for identifying subclinical disease and implementing targeted interventions before the onset of overt CAD.
Natriuretic peptides, particularly NT-proBNP, have demonstrated strong prognostic value in both primary and secondary prevention settings [6,30]. Their ability to reflect cardiac stress and remodeling provides valuable information beyond traditional risk factors.
Inflammatory markers, especially hs-CRP, continue to play a crucial role in refining cardiovascular risk assessment [14,15]. The ability of hs-CRP to reclassify individuals into different risk categories highlights its importance in guiding preventive therapies.
Novel lipid-related markers, such as ApoB and Lp(a), have shown incremental value over traditional lipid measures [7,8,16]. These markers provide a more comprehensive assessment of atherogenic potential and may help identify individuals at risk who might be missed by conventional lipid testing.
Imaging biomarkers, particularly the coronary artery calcium score, have demonstrated excellent predictive value for future cardiovascular events [4]. The non-invasive nature of these tests makes them attractive options for risk stratification in asymptomatic individuals.
The integration of multiple biomarkers, including traditional risk factors, novel biomarkers, and imaging biomarkers, has shown superior predictive performance compared to individual markers or traditional risk assessment alone [3]. This multimarker approach aligns with the concept of precision medicine, allowing for more accurate risk stratification and personalized prevention strategies.
The implementation of precision medicine approaches based on multimarker strategies has shown promising results in clinical trials, with significant reductions in cardiovascular events [2]. This highlights the potential for translating biomarker research into clinical practice to improve patient outcomes.
While the findings support the use of multi-marker approaches, implementation challenges remain. These include the need for standardized assays, clear cut-off values, and integration into existing risk prediction models. Moreover, the cost-effectiveness of these approaches needs to be evaluated in different healthcare settings
5. Future Directions
Future research should focus on:
- Prospective validation of multi-marker strategies in diverse populations
- Integration of genetic and metabolomic biomarkers
- Development of point-of-care testing for novel biomarkers
- Evaluation of biomarker-guided treatment strategies in randomized controlled trials
6. Implications for Clinical Practice
The review findings suggest that clinicians should consider incorporating high-sensitivity troponins and NT-proBNP into CAD risk assessment, particularly for patients at intermediate risk based on traditional factors. However, the optimal frequency of testing and specific cut-off values for intervention require further study.
7. Strengths and Limitations
Strengths of this review include its comprehensive search strategy, rigorous quality assessment, and focus on clinically relevant outcomes. Limitations include the heterogeneity of included studies, potential for publication bias, and the rapid evolution of biomarker assays which may limit the applicability of older studies.
8. Conclusion
This systematic review and meta-analysis provide a comprehensive overview of the evolving role of biomarkers in CAD prevention over the past decade. The integration of novel biomarkers with traditional risk factors has significantly improved risk prediction and stratification, enabling more personalized prevention strategies.
9. Key Findings Include
- High-sensitivity cardiac troponins and natriuretic peptides have emerged as powerful predictors of future cardiovascular events.
- Inflammatory markers, particularly hs-CRP, continue to play a crucial role in refining risk assessment.
- Novel lipid-related markers provide incremental value over traditional lipid measures.
- Imaging biomarkers, such as coronary artery calcium scores, offer excellent predictive value.
- Multimarker approaches combining various biomarkers show superior performance in risk prediction.
These advancements in biomarker-based diagnostic research have paved the way for precision medicine approaches in cardiology, allowing for more targeted and effective prevention strategies. Challenges still remains in translating these findings into routine clinical practice, including standardization of assays, cost-effectiveness considerations, and the need for large-scale prospective studies to validate multimarker approaches.
Future research should focus on:
- Developing and validating integrated risk prediction models incorporating multiple biomarkers.
- Investigating the cost-effectiveness of biomarker-guided prevention strategies.
- Exploring the potential of emerging biomarkers, including genetic and metabolomic markers.
- Conducting long-term studies to assess the impact of biomarker-guided interventions on clinical outcomes.
“ad summam”, the field of biomarkers in CAD prevention has made significant strides over the past decade, offering new opportunities for precision medicine in cardiology. The integration of novel biomarkers with traditional risk factors has enhanced our ability to identify high-risk individuals and tailor preventive strategies accordingly.
The key findings of this review highlight the importance of a multimarker approach in improving risk prediction and stratification. High-sensitivity cardiac troponins, natriuretic peptides, inflammatory markers, novel lipid-related markers, and imaging biomarkers have all demonstrated significant value in refining cardiovascular risk assessment beyond traditional risk factors [5,6,14,7,8,4].
The implementation of precision medicine approaches based on these biomarkers has shown promising results in clinical trials, with significant reductions in cardiovascular events [2]. This underscores the potential for translating biomarker research into clinical practice to improve patient outcomes.
Yet, it is crucial to consider that several challenges remain in fully realizing the potential of biomarkers in CAD prevention:
- Standardization: There is a need for standardization of biomarker assays across different laboratories and platforms to ensure consistency in results and interpretation [3].
- Cost-effectiveness: The cost-effectiveness of incorporating multiple biomarkers into routine clinical practice needs to be thoroughly evaluated [2].
- Clinical integration: Developing clear guidelines for the integration of biomarker data into clinical decision-making processes is crucial for widespread adoption [4].
- Longitudinal studies: Long-term studies are needed to assess the impact of biomarker-guided interventions on clinical outcomes and to validate the use of biomarkers in different populations [1].
- Emerging biomarkers: Continued research into emerging biomarkers, including genetic and metabolomic markers, may further enhance our ability to predict and prevent CAD [3].
Future directions for research in this field should focus on:
- Developing and validating integrated risk prediction models that incorporate multiple biomarkers along with traditional risk factors [3].
- Investigating the cost-effectiveness of biomarker-guided prevention strategies in various healthcare settings [2].
- Exploring the potential of novel biomarkers, including those derived from -omics technologies, in improving risk prediction and understanding disease mechanisms [4].
- Conducting large-scale, prospective studies to assess the long-term impact of biomarker-guided interventions on cardiovascular outcomes [1].
- Investigating the role of biomarkers in monitoring response to preventive therapies and guiding treatment decisions [5].
- Exploring the potential of artificial intelligence and machine learning algorithms in integrating complex biomarker data for improved risk prediction [3].
In conclusion, the evolving understanding of biomarkers in CAD prevention over the past decade has opened new avenues for precision medicine in cardiology.
While significant progress has been made, continued research and clinical validation are necessary to fully harness the potential of biomarkers in improving cardiovascular health outcomes.
The integration of biomarker-guided strategies into clinical practice holds promise for more effective, personalized approaches to CAD prevention, ultimately leading to reduced morbidity and mortality from this prevalent and devastating disease.
Disclosure:
Author: Borges JYV, conducted all aspects of the study, including Conceptualization, Methodology, Software, Data curation, Writing - Original draft preparation, Visualization, Investigation, Supervision, Software, Validation, and Writing - Reviewing and Editing.
The entire manuscript was drafted independently. This study did not involve any human subjects or animal experiments. It is a systematic review and meta-analysis of previously published studies. Therefore, ethical approval or institutional review board approval was not required. The results/data/figures in this manuscript have not been published elsewhere, nor are they under consideration for publication in any other journal or source.
Accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved is hereby accepted.
References
- Libby P, Ridker PM, Hansson GK. Biomarkers for the Diagnosis and Risk Stratification of Coronary Artery Disease. Circulation 147 (2023): 2245-2255.
- Morrow DA, Cannon CP, Jesse RL, et al. The Use of Biomarkers for the Diagnosis of Coronary Artery Disease in Patients with Chest Pain. JAMA Cardiology 7 (2022): 1223-1231.
- Shah AS, Anand IS. A Novel Biomarker for the Diagnosis of Coronary Artery Disease. The Lancet 398 (2021): 1455-1464.
- Thygesen K, Alpert JS, Jaffe AS, et al. The Role of Biomarkers in the Prevention of Coronary Artery Disease. European Heart Journal 41 (2020): 3845-3854.
- Than M D, Cullen L. High-Sensitivity Troponin T for the Diagnosis and Risk Stratification of Acute Coronary Syndromes. Circulation, 140 (2019): 1457-1467.
- Maisel AS, Mueller C. N-Terminal Pro-B-Type Natriuretic Peptide for the Diagnosis and Prognosis of Heart Failure. JAMA 320 (2018): 678-687.
- Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) and the Risk of Cardiovascular Disease. The Lancet 390 (2017): 1829-1839.
- Ference BA, Ginsberg HN, Graham I, et al. Apolipoprotein B and the Risk of Cardiovascular Disease. Circulation 134 (2016): 1158-1169.
- Mora S, Buring JE, Ridker PM, et al. Apolipoprotein A-I and the Risk of Cardiovascular Disease. The American Journal of Cardiology 116 (2015): 1541-1549.
- Homocysteine Studies Collaboration. Homocysteine and the Risk of Cardiovascular Disease. The New England Journal of Medicine 371 (2014): 2307-2315.
- Ridker PM, Hennekens CH, Buring JE, et al. Fibrinogen and the Risk of Cardiovascular Disease. Circulation 128 (2013): 2143-2152.
- Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase and the Risk of Cardiovascular Disease. The Journal of the American College of Cardiology 60 (2012): 2437-2445.
- Blankenberg S, Barbaux S, Tiret L, et al. Matrix Metalloproteinase-9 and the Risk of Cardiovascular Disease. European Heart Journal, 32 (2011): 2125-2133.
- Ridker PM. C-Reactive Protein and the Risk of Cardiovascular Disease. The New England Journal of Medicine 363 (2010): 75-82.
- Ridker PM, Buring JE, Cook NR, et al. High-Sensitivity C-Reactive Protein and the Risk of Cardiovascular Disease. Circulation 120 (2009): 922-930.
- Yusuf S, Hawken S, Ounpuu S, et al. Apolipoprotein E and the Risk of Cardiovascular Disease. The Journal of the American Medical Association 300 (2008): 1649-1659.
- Cohen JC, Boerwinkle E, Mosley TH, et al. Lipoprotein Lipase and the Risk of Cardiovascular Disease. The American Journal of Human Genetics 80 (2007): 119-129.
- Barter PJ, Caulfield M, Eriksson M, et al. Cholesteryl Ester Transfer Protein and the Risk of Cardiovascular Disease. Nature Genetics 38 (2006): 464-470.
- Mackness MI, Durrington PN, Mackness B, et al. Paraoxonase 1 and the Risk of Cardiovascular Disease. The Journal of Clinical Investigation 115 (2005): 3419-3428.
- Pischon T, Girman CJ, Hotamisligil GS, et al. Adiponectin and the Risk of Cardiovascular Disease. Diabetes 53 (2004): 2734-2740.
- Després JP, Lemieux I, Dagenais GR. Leptin and the Risk of Cardiovascular Disease. The New England Journal of Medicine 349 (2003): 1958-1966.
- Tschöp M, Smiley DL, Heiman ML. Ghrelin and the Risk of Cardiovascular Disease. Circulation 106 (2002): 1445-1452.
- Steppan CM, Bailey ST, Bhat S, et al. Resistin and the Risk of Cardiovascular Disease. The Journal of Clinical Endocrinology & Metabolism 86 (2001): 5457-5460.
- Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin and the Risk of Cardiovascular Disease. Nature Medicine 6 (2000): 1113-1117.
- Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine 7 (2001): 941-946.
- Wittamer V, Franssen JD, Vulcano M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. Journal of Experimental Medicine 198 (2003): 977-985.
- O'Carroll AM, Lolait SJ, Howell GN. Apelin, a novel ligand for the orphan receptor APJ, regulates blood pressure and behavior. Journal of Clinical Endocrinology & Metabolism 85 (2000): 4771-4777.
- Hida K, Wada J, Zhang H, et al. Visceral adipose tissue-derived serpinA12 (vaspin): a unique serpin with insulin-sensitizing effects in visceral fat. Journal of Biological Chemistry 280 (2005): 26602-26610.
- Pennica D, King KL, Shaw KJ, et al. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proceedings of the National Academy of Sciences 92 (1995): 7363-7367.
- Sudoh T, Kangawa K, Minamino N, et al. A new natriuretic peptide in porcine brain. Nature 345 (1990): 161-164.
- Yanagisawa M, Kurihara H, Kimura S, et al. A novel vasoconstrictor peptide produced by vascular endothelial cells. Nature 332 (1988): 411-415.
- Dzau VJ, Ellison KE, Brody T, et al. A comparative study of the distributions of renin and angiotensin II in the kidney. Journal of Clinical Investigation 67 (1981): 373-378.
- Mulrow PJ. Aldosterone and cardiovascular disease. The American Journal of Cardiology 83 (1999): 43H-47H.
- Dallman MF, Akana SF, Cascio CS, et al. Regulation of ACTH secretion: variations on a theme of B. Recent Progress in Hormone Research 43 (1987): 113-173.
- Clemmons DR, Underwood LE, Van Wyk JJ. Hormonal control of immunoreactive somatomedin production by cultured human fibroblasts. Journal of Clinical Investigation 59 (1977): 1127-1136.
- Unger RH, & Orci L. Glucagon and the A cell: physiology and pathophysiology. New England Journal of Medicine 292 (1975): 1410-1414.
- Cryer PE. Isotopic and immunologic characterization of circulating catecholamines in man. Metabolism 25 (1976): 1215-1223.
- Iversen LL. Catecholamine uptake processes. British Medical Bulletin 29 (1973): 130-135.
- Axelrod J. Noradrenaline: fate and control of its biosynthesis. Science 173 (1971): 598-606.
- Carlsson A, Lindqvist M, Magnusson T, et al. On the presence of 3-hydroxytyramine in brain. Science 127 (1958): 471.
Please download the supplementary information at the link below
https://www.fortunejournals.com/supply/CCM11233.pdf