Post-Operative Atrial Fibrillation: Current Treatments and Etiologies for a Persistent Surgical Complication
Article Information
Leilani A Lopes1, Devendra K Agrawal2*
1Western University of Health Sciences, College of Osteopathic Medicine of the Pacific – Northwest, Lebanon, OR, USA
2Western University of Health Sciences, College of Osteopathic Medicine of the Pacific, Pomona, CA, USA
*Corresponding Author: Devendra K Agrawal, Department of Translational Research, Western University of Health Sciences, 309 E. Second Street, Pomona, California 91766-1854, USA
Received: 15 March 2022; Accepted: 22 March 2022; Published: 28 March 2022
Citation:
Leilani A. Lopes, Devendra K. Agrawal. Post-Operative Atrial Fibrillation: Current Treatments and Etiologies for a Persistent Surgical Complication. Journal of Surgery and Research 5 (2022): 159-172.
View / Download Pdf Share at FacebookAbstract
Post-operative atrial fibrillation (POAF) is a persistent and serious surgical complication that occur in 20-55% of cardiac surgery cases. POAF may lead to adverse health outcomes such as stroke, thromboembolism, cardiac arrest, and mortality, and may develop long-term. Patients have a 2-fold increase in mortality risk and spend about 3.7 more days in the hospital and $16,000 more in medical costs during their visit. The mechanisms and risk factors of POAF are still poorly understood, yet a strong foundation of how a disease process occurs is needed to provide the most effective treatment. Current mechanisms that are postulated to contribute to POAF include an increase in sympathetic tone, oxidative stress, local and systemic inflammation, a trigger that induces atrial substrate changes, a driver to sustain POAF, and electrolyte disturbances such as hypomagnesemia. While needing more research, current risk factors include age, male sex, history of myocardial infarction or heart failure, hypertension, diabetes, obesity, and COPD. Treatments mostly include prophylaxis of repurposed drugs such as beta-blockers, statins, oral anticoagulants, antiarrhythmics, and Vitamin D and electrolyte supplementation. Autonomic denervation has also been a promising preventative measure for patients undergoing cardiac surgery. This critical review article provides an up-to-date and comprehensive summary of the pathophysiology of POAF, current clinical risk factors and management for POAF and discusses new pathways for further investigation.
Keywords
Atrial fibrillation, Cardiac surgery, Inflammation, Oxidative damage, Post-operative atrial fibrillation, POAF
Atrial fibrillation articles; Cardiac surgery articles; Inflammation articles; Oxidative damage articles; Post-operative atrial fibrillation articles; POAF articles
Atrial fibrillation articles Atrial fibrillation Research articles Atrial fibrillation review articles Atrial fibrillation PubMed articles Atrial fibrillation PubMed Central articles Atrial fibrillation 2023 articles Atrial fibrillation 2024 articles Atrial fibrillation Scopus articles Atrial fibrillation impact factor journals Atrial fibrillation Scopus journals Atrial fibrillation PubMed journals Atrial fibrillation medical journals Atrial fibrillation free journals Atrial fibrillation best journals Atrial fibrillation top journals Atrial fibrillation free medical journals Atrial fibrillation famous journals Atrial fibrillation Google Scholar indexed journals Cardiac surgery articles Cardiac surgery Research articles Cardiac surgery review articles Cardiac surgery PubMed articles Cardiac surgery PubMed Central articles Cardiac surgery 2023 articles Cardiac surgery 2024 articles Cardiac surgery Scopus articles Cardiac surgery impact factor journals Cardiac surgery Scopus journals Cardiac surgery PubMed journals Cardiac surgery medical journals Cardiac surgery free journals Cardiac surgery best journals Cardiac surgery top journals Cardiac surgery free medical journals Cardiac surgery famous journals Cardiac surgery Google Scholar indexed journals Inflammation articles Inflammation Research articles Inflammation review articles Inflammation PubMed articles Inflammation PubMed Central articles Inflammation 2023 articles Inflammation 2024 articles Inflammation Scopus articles Inflammation impact factor journals Inflammation Scopus journals Inflammation PubMed journals Inflammation medical journals Inflammation free journals Inflammation best journals Inflammation top journals Inflammation free medical journals Inflammation famous journals Inflammation Google Scholar indexed journals Oxidative damage articles Oxidative damage Research articles Oxidative damage review articles Oxidative damage PubMed articles Oxidative damage PubMed Central articles Oxidative damage 2023 articles Oxidative damage 2024 articles Oxidative damage Scopus articles Oxidative damage impact factor journals Oxidative damage Scopus journals Oxidative damage PubMed journals Oxidative damage medical journals Oxidative damage free journals Oxidative damage best journals Oxidative damage top journals Oxidative damage free medical journals Oxidative damage famous journals Oxidative damage Google Scholar indexed journals Post-operative atrial fibrillation articles Post-operative atrial fibrillation Research articles Post-operative atrial fibrillation review articles Post-operative atrial fibrillation PubMed articles Post-operative atrial fibrillation PubMed Central articles Post-operative atrial fibrillation 2023 articles Post-operative atrial fibrillation 2024 articles Post-operative atrial fibrillation Scopus articles Post-operative atrial fibrillation impact factor journals Post-operative atrial fibrillation Scopus journals Post-operative atrial fibrillation PubMed journals Post-operative atrial fibrillation medical journals Post-operative atrial fibrillation free journals Post-operative atrial fibrillation best journals Post-operative atrial fibrillation top journals Post-operative atrial fibrillation free medical journals Post-operative atrial fibrillation famous journals Post-operative atrial fibrillation Google Scholar indexed journals End diastolic volume articles End diastolic volume Research articles End diastolic volume review articles End diastolic volume PubMed articles End diastolic volume PubMed Central articles End diastolic volume 2023 articles End diastolic volume 2024 articles End diastolic volume Scopus articles End diastolic volume impact factor journals End diastolic volume Scopus journals End diastolic volume PubMed journals End diastolic volume medical journals End diastolic volume free journals End diastolic volume best journals End diastolic volume top journals End diastolic volume free medical journals End diastolic volume famous journals End diastolic volume Google Scholar indexed journals Oral anticoagulants articles Oral anticoagulants Research articles Oral anticoagulants review articles Oral anticoagulants PubMed articles Oral anticoagulants PubMed Central articles Oral anticoagulants 2023 articles Oral anticoagulants 2024 articles Oral anticoagulants Scopus articles Oral anticoagulants impact factor journals Oral anticoagulants Scopus journals Oral anticoagulants PubMed journals Oral anticoagulants medical journals Oral anticoagulants free journals Oral anticoagulants best journals Oral anticoagulants top journals Oral anticoagulants free medical journals Oral anticoagulants famous journals Oral anticoagulants Google Scholar indexed journals pericardial fluid articles pericardial fluid Research articles pericardial fluid review articles pericardial fluid PubMed articles pericardial fluid PubMed Central articles pericardial fluid 2023 articles pericardial fluid 2024 articles pericardial fluid Scopus articles pericardial fluid impact factor journals pericardial fluid Scopus journals pericardial fluid PubMed journals pericardial fluid medical journals pericardial fluid free journals pericardial fluid best journals pericardial fluid top journals pericardial fluid free medical journals pericardial fluid famous journals pericardial fluid Google Scholar indexed journals Vitamin D receptors articles Vitamin D receptors Research articles Vitamin D receptors review articles Vitamin D receptors PubMed articles Vitamin D receptors PubMed Central articles Vitamin D receptors 2023 articles Vitamin D receptors 2024 articles Vitamin D receptors Scopus articles Vitamin D receptors impact factor journals Vitamin D receptors Scopus journals Vitamin D receptors PubMed journals Vitamin D receptors medical journals Vitamin D receptors free journals Vitamin D receptors best journals Vitamin D receptors top journals Vitamin D receptors free medical journals Vitamin D receptors famous journals Vitamin D receptors Google Scholar indexed journals
Article Details
Abbreviations
AF – Atrial fibrillation
CABG – Coronary artery bypass grafting
CO – Cardiac output
EDV – End diastolic volume
ESV – End systolic volume
OACs – Oral anticoagulants
PCF – pericardial fluid
POAF – Post-operative atrial fibrillation
RAAS - renin-angiotensin-aldosterone system
SAVR – Surgical aortic valve replacement
SV – Stroke volume
TAR – Total arch repair
TAVR – Transcatheter aortic valve replacement
VDRs - Vitamin D receptors
1. Introduction
In spite of many advances in perioperative, surgical care, and medical innovation there have been complications that continue to persist, such as atrial fibrillation occurring post-operation. Post-operative atrial fibrillation (POAF) is a common, expensive, and potentially fatal complication arising in 20-55% of cardiac surgery cases [1,2]. In about 90% of patients who develop POAF it occurs within the first six days post operation, corresponding with the peak of the postoperative systemic inflammation response [3]. POAF is a predictor of early- and long-term cardiovascular complications such as stroke, thromboembolism, infection, cardiac arrest, and the need for reoperation due to internal bleeding [4,5]. Patients who develop POAF have a two-fold increased risk in all-cause 30-day and 6-month mortality and spend 3.7 more days in the hospital on average than patients who do not develop POAF [6-8]. In the United States the clinical and financial burden for POAF complications persists, with an annual healthcare expenditure over $1 billion and extended hospital stays, presenting a significant issue for patient and their families [3,6,9]. Despite an effort to debunk the reason why patients develop atrial fibrillation (AF) following post-surgical intervention, the etiology and risk factors of POAF are poorly understood. Medical prevention and treatment usually follow an understanding of etiology and considering that the mechanism of pathology is still ambiguous, there are limited successful preventative measures and medical treatments for POAF. This critical review provides a comprehensive and up-to-date summary of the etiologies, risk factors, methods, long-term effects, and financial burdens of POAF and discusses the gaps in our knowledge of the underlying cellular and molecular mechanisms of POAF.
2. Methods
In this review, comprehensive literature searches were done on PubMed and Google Scholar from January 2004 through January 2022. Key words included: post-operative atrial fibrillation, cardiothoracic surgery, inflammation, oxidative damage, and atrial fibrillation, POAF treatment. Only the articles in English language were reviewed.
2.1 Proposed Etiology of POAF
2.1.1 Trigger and Atrial Substrate Changes: Post-operative atrial fibrillation typically presents differently from other forms of AF. POAF typically develops within the first 6 days post-operation and then returns to normal sinus rhythm. While the definitive mechanism of POAF has remained elusive, current etiologies that are presumed to contribute to POAF usually require a trigger and a vulnerable atrial substrate change, typically originating from the pulmonary veins and other atrial locations [10,11]. In addition, several biochemical events leading to metabolic derangements in cardiomyocytes affect structural, contractile, and electrophysiological cellular properties in the pathogenesis of AF [12,13].
2.1.2 A Driver to Sustain POAF: Along with a trigger and a vulnerable atrial substrate, another probable mechanism that contributes to POAF is the presence of a driver that will sustain atrial fibrillation in its vulnerable substrate. Ordered re-entry from re-entrant circuits of short cycle length, a rapidly firing ectopic pacemaker (atrial or junctional), or random re-entry by multiple re-entrant wavelengths may sustain POAF in the presence of a vulnerable substrate [11].
2.1.3: Sympathetic tone, Inflammation and Oxidative Stress: Activation of the complement system releases pro-inflammatory cytokines such as C-reactive protein (CRP), interleukin-2 (IL-2) interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) that follow a close time of occurrence to the onset of POAF [14]. These inflammatory markers result in leukocyte activation and release oxidases and nitric oxide. Eventually, this leads to generation of reactive oxygen species that develop into systemic inflammation [15]. Along with systemic inflammation, pericardial fluid (PCF) volume increases after cardiac surgery and causes local inflammation around the heart. Additionally, the myocardium itself contributes to local inflammation by releasing pro-inflammatory cytokines and has a direct effect on the heart itself. Local inflammation that occurs within the pericardial space results in apoptosis of cardiomyocytes as well as a change in electrical activity, which can cause propagation of arrhythmias and altered action potentials [1]. However, icosapent ethyl (IPE), a marine-derived omega-3 fatty acid, has been shown to be a powerful anti-inflammatory medication, yet patients are at a higher risk for developing atrial fibrillation [16]. If POAF is caused by local or systemic inflammation, why do drugs that decrease the levels and activity of inflammatory markers lead to a significantly increased risk of atrial fibrillation? Along with local and systemic inflammation, an overdrive of sympathetic tone has been postulated to contribute to POAF. Sympathetic drive in the heart results from norepinephrine or epinephrine binding to b1-adrenergic receptors, which is stimulated from the cervical and upper thoracic sympathetic chain ganglia to the deep and superficial cardiac plexus. An increase in adrenergic drive and a decrease in vagal tone could shorten the PR interval and atrial wavelength, increase the inotropic state and preload, and may potentially trigger POAF [17-19]. A mechanism of unrelieved pain, which typically occurs after surgery, involves the mobilization of the sympathetic nervous system. Pain, especially unrelieved pain, could be a trigger for atrial fibrillation. However, in a placebo-controlled clinical trial, Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE), the levels of pain and opioid consumption was unremarkable between patients who developed atrial fibrillation post-operation versus patients who did not [20]. Because of the multifactorial nature of atrial fibrillation and delirium, the findings from this study suggest no association between opioid use and atrial fibrillation and delirium. An increase in global cardiac strain is also driven by the activation of sympathetic tone and the presence of inflammatory markers which are increased by transfusions during cardiac surgery. In a recent study 43% of patients developed POAF and showed increased post-operative fluid balance and M-terminal pro-brain natriuretic peptide (NT-ProBNP). Also, anemia pre-operation led to increased transfusion volumes, and suggests that POAF may be triggered by global cardiac strain [20].
2.1.4 Electrolyte Disturbances: Hypomagnesemia is a common feature post-surgery and has been shown to be a biomarker for predicting POAF. TRPM7 channels, which play a vital role in converting fibroblasts to myofibroblasts, are involved in fibrogenesis that occurs in POAF. These channels are typically activated by low concentrations of free magnesium [22]. In the Framingham Heart Study, it was shown low magnesium level after cardiac surgery was an indication for the onset of POAF [23,24]. A meta-analysis showed that treating patients prophylactically with magnesium in the post-operative period only led to a statistically significant decrease in POAF [25], suggesting the use of intravenous magnesium as an alternative to prevent POAF. However, careful randomized clinical studies are required to determine the safety and duration of effect.
2.2 Post-Operative Atrial Fibrillation Risk Factors
POAF after cardiac surgery is a persistent surgical complication and increased in patients who have comorbidities. The most common risk factors that contribute to POAF are hypertension, age, history of myocardial infarction, obesity, heart failure, male sex, type of surgery, atrial enlargement or block or valvular heart disease [18].
2.2.1 Type of Surgery: POAF presents a major risk for any cardiac surgery; however, some surgeries are more likely to induce POAF. Specifically, patients who undergo combined surgeries, such as a combined valve and coronary artery bypass graft (CABG) surgeries, are twice as likely to develop POAF, up to 60% [25]. CABG surgeries alone lead to a higher risk for developing POAF, and patients experience an increased risk of other complications from POAF [26,27]. An observational study following patients for 10 years post-CABG showed an increase in stroke, long-term AF, and overall and cardiac mortality [28]. In the Total Arch Repair (TAR), a challenging cardiac surgery, there is a 32.3% incidence of POAF with the highest risk population found with elderly women who underwent a longer operation. Therefore, the type of surgery and length of the surgery are shown to be significantly correlated with the onset of POAF [29].
2.2.2 Pre-existing Cardiac Comorbidities: An extensive patient history is vital in any cardiac case, especially a surgical case. POAF has many risk factors that include pre-existing cardiac medical conditions or prior episodes such as hypertension, diabetes, chronic obstructive pulmonary disease (COPD), heart failure, and history of myocardial infarction. A meta-analysis showed that these conditions are early predictors of POAF [30,31]. Shown in table 1 are the associated pre-existing cardiac conditions from Yamashita et. al. [30] with their percentage of increased risk to POAF. History of heart failure contributed to the greatest increase in risk for POAF following cardiac surgery. Left atrial enlargement and poor contraction, valvular heart disease and impaired left ventricular function all can contribute to heart failure and aortic stenosis [32,33]. Diabetes had the least increased risk of POAF at 6%, with an odds ratio of 1.06 and (95% CI: 1.00 to 1.13). Dyslipidemia did not show any association with POAF in this meta-analysis [32].
|
Risk Increase for POAF |
Odds Ratio |
|
|
Hypertension |
29% |
1.29 (95% CI: 1.12 to 1.49) |
|
Diabetes |
6% |
1.06 (95% CI: 1.00 to 1.13) |
|
COPD |
36% |
1.36 (95% CI: 1.13 to 1.64) |
|
Heart Failure |
56% |
1.56 (95% CI: 1.31 to 1.86) |
|
Myocardial Infarction |
18% |
1.18 (95% CI: 1.05 to 1.34) |
Table 1: Pre-existing cardiac conditions that predispose patients to POAF. Interpreted from Yamashita et al. (2019).
2.2.3 Non-Cardiac Risk Factors: Increasing age has a strong correlation with the onset of POAF. Literature has discussed that as age advances, the development of an atrial substrate increases which may induce POAF. Age has also been associated with a slowing of conduction rate and increase in fibrosis. Some studies have suggested that male sex is an independent risk factor for developing POAF, however there is conflict in the literature. A comparative study showed that men had an increased postoperative peak lactate and a higher incidence of death after a four-year follow up [34]. Caucasian patients who underwent CABG also have been shown to have a higher incidence of POAF than non-caucasian patients. However, studies that compare race and POAF use a significantly lower patient population of non-white to white patients [35].
2.2.4 Modifiable Risk Factors: Obesity and high BMI is a persistent risk factor in many cardiac pathologies, including POAF. Obesity and high BMI put patients at risk for hypertension and diabetes, and therefore may be a secondary risk factor for developing POAF. A systematic review and meta-analysis showed that of the patients that went cardiac surgery, patients with a high BMI or obesity showed to have a significantly increased risk of developing POAF than patients without these risk factors (p = 0.006) [36].
2.2.5 CHADS2 and CHAD2DS2-VASc score: The CHADS2 (Congestive heart failure, Hypertension, Age (> 65 = 1 point, > 75 = 2 points), Diabetes, previous Stroke/transient ischemic attack (2 points)) and CHAD2DS2-VASc (congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 and sex category (female)) scores are independent predictors of POAF with a strong selectivity and sensitivity for POAF [37]. These scores determine a patient’s stroke risk by combining cardiovascular and non-cardiovascular factors and are useful for predicting POAF. Other scores such as HATCH (hypertension <1 point>, age >75 years <1 point>, stroke or transient ischemic attack <2 points>, chronic obstructive pulmonary disease <1 point>, and heart failure <2 points>) score have been used. However, score using the combination of variables with higher predictive value included in the POAF (COM-AF) has been reported to be more useful for predicting POAF development [11].
2.3 Methods for Reducing POAF Incidence
2.3.1 Beta-Blockers: Beta-1 receptor blockers or antagonists inhibit b1-adrenergic receptors responsible for sympathetic stimulation in the myocardium. One of the etiologies of POAF is by an increase in sympathetic tone, which causes an increase in preload and inotropy, thereby increasing the end diastolic volume (EDV) or decreasing the end systolic volume (ESV), respectfully. Either or both phenomena increase the stroke volume (SV) and cardiac output (CO). Beta-blockers inhibit these receptors, thereby decreasing the SV and CO and decreasing the heart rate, downregulating sympathetic tone [38]. β-Blockers prophylaxis before cardiac surgery has been shown to be one of the most effective treatments for POAF so far [15]. In a study using β-Blockers in combination with Amiodarone, there was a 13.75% decrease in the onset of POAF with an onset frequency of 17.50% compared to β-Blockers alone at 31.25% as shown in figure 1. β-Blockers in combination with Rosuvastatin, which has proved to be less effective, had a higher percentage of patients who developed POAF at 33.75%. While the sample size was small with a total of 240 patients, this may provide insight into personalized therapies for POAF. Another clinical trial should be conducted with Atorvastatin and β-Blockers to see how this treatment compares to β-Blockers combined with Amiodarone [39]. Although effective against POAF and granted a Class I indication, β-Blockers can lead to a variety of adverse outcomes, most commonly bradycardia and hypotension. While β-Blockers are an effective drug against POAF, especially when coupled with Amiodarone, the search continues for the perfect treatment due to the lack of knowledge about the etiology of POAF and some of the adverse effects of β-Blockers. Lanidiolol hydrochloride is a low-dose β-selective blocker that has been shown to give a negative ionotropic effect without the adverse outcomes of β-blockers at a low dose. While debated in literature, The PELTA (Preventive effect of low-dose Lanidiolol on postoperative atrial fibrillation) study showed landiolol has a preventative effect against POAF at low doses in patients who undergo CABG, valvular and aortic surgery [40].
2.3.2 Amiodarone: Amiodarone belongs to the class of antiarrhythmics and is a peripheral and coronary vasodilator [41]. It is granted Class IIA indication by both American and European guidelines and is one of the most effective treatments for POAF [42]. However, amiodarone presents with serious cardiac and extracardiac adverse risks, such as bradycardia, increased liver enzymes, hyperthyroidism, thyrotoxicosis, and interstitial pneumonitis. Accumulation of the drug may also lead to longer lasting side effects after discontinuing the drug [7]. Local epicardial application of amiodarone hydrogel has been shown to reduce the adverse outcomes of amiodarone and have a decrease in POAF incidence from 22-37% to 3.3-8% [7,42]. While these studies were small in sample size (~ 60), they show promise for an effective therapeutic for POAF.
2.3.3 Statin Therapy: Statins, independent from their lipid-lowering properties, also have anti-inflammatory effects. Clinical trials have shown that statins significantly reduce C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), Interleukin- 6 (IL-6) and Interleukin 1 (IL-1) versus placebo. Recently in literature, Atorvastatin and Rosuvastatin have been under study for reducing the incidence of POAF. Benefits are that they have less side-effects than other pharmaceuticals that are being used to mitigate POAF. Some studies have shown that Rosuvastatin given perioperatively had no effect on POAF incidence compared to placebo. However, multiple meta-analyses have shown that statins are effective at preventing POAF in patients undergoing CABG, with Atorvastatin showing effectiveness instead of Rosuvastatin [43,44].
2.3.4 Oral Anticoagulants: POAF onset after CABG surgery has been associated with a higher risk thromboembolism, and recent studies have postulated using oral anti-coagulants (OAC) as a post-operative therapeutic to reduce the risk of thromboembolism once POAF develops [12,45]. Multiple trials, including the SWEDEHEART Registry clinical trial, showed that OAC did not decrease the incidence of thromboembolism in POAF patients and increased the rate of major bleeding [46-48]. Early anticoagulation therapy is a current Class IIA recommendation from international guidelines as preventative treatment for thromboembolism; however, more studies need to be done to determine if the benefits of OAC outweigh the risk of major bleeding or stroke.
2.3.5 Vitamin D Supplementation: Vitamin D is a critical sterol in calcium metabolism and mobilization. Traditionally believed to be limited to bone tissue, Vitamin D receptors (VDRs) have found throughout the body, including cardiomyocytes [49,50]. It has been shown that Vitamin D is invaluable for cardiovascular health, and since most of the United States is deficient in Vitamin D, it has become a recent method for helping prevent cardiomyopathies and infection [51]. Vitamin D has powerful anti-inflammatory abilities such as prostaglandin and cyclooxygenase pathway inhibition, renin-angiotensin-aldosterone system (RAAS) downregulation and anti-inflammatory cytokine upregulation [52]. RAAS activation increases and electrically and structurally remodels the atria, contributing to POAF. In multiple studies, supplementation with Vitamin D lowered the prevalence of POAF via RAAS inhibition [13,52].
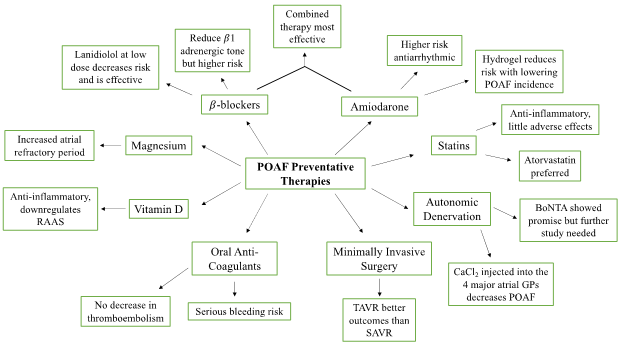
Figure 1: An overview of the proposed preventative therapies for POAF. Pharmacological intervention includes β-Blockers, amiodarone, statins, and oral anti-coagulants. Surgical intervention includes autonomic denervation and minimally invasive surgery. Vitamin D and magnesium supplementation were also proposed as a preventative therapy for reducing the incidence of POAF.
2.3.6 Magnesium Supplementation: Supplementation with Mg significantly increases the refractory period of atrial repolarization. The proposed mechanism for hypomagnesium contributing to POAF is due to dilution from transfusion during surgery [47]. There has been debate in the literature about magnesium supplementation for treatment of POAF, and a meta-analysis showed that post-operative magnesium supplementation was the only time frame that significantly reduced the risk of POAF [24].
2.3.7 Autonomic Denervation: In the epicardial fat pads lie the ganglionated plexi (GPs) that act as integrated centers for autonomic innervation and act on the sinoatrial and atrioventricular node. Since it had been shown that simultaneous or successive stimulation of both the parasympathetic and sympathetic nervous systems in cardiac tissue induce POAF, it was hypothesized whether denervation of GPs adjacent to the pulmonary veins, such as in AF ablation, would decrease the incidence of POAF. A study of 200 patients undergoing CABG had CaCl2 injected into the 4 major atrial GPs and reduced the risk of POAF from 36% to 15%, yet length of hospitalization did not change between the two groups. Heart rate variability data showed that sympathetic and parasympathetic balance was undisturbed by the injection. Onabotulinum toxin A (botulinum toxin type A [BoNTA]) was also tested to denervate GPs in epicardial fat pads. A randomized, placebo-controlled, double-blind clinical trial containing 145 patients showed a decrease incidence of POAF compared to placebo from 47.8% down to 36.5%. Though shown in this trial not to be statistically significant, BoNTA does seem to have promise in reducing POAF, especially due to the injection having little adverse effect (Waldron et. al. 2019). However, other trials have shown that POAF is decreased by almost half (Romanov et. al. 2019). Another study should be conducted with a higher patient population in contrast with CaCl2 on POAF onset post-cardiac surgery.
2.3.8 Opting for Minimally Invasive Cardiac Surgery: Minimally invasive surgery has been postulated to decrease incidence of POAF. CABG surgery has the highest incidence for developing POAF and other long-term effects and methods to reduce the incidence of POAF in CABG patients has been the focus for many clinical trials. A follow-up study comparing minimally invasive direct coronary artery bypass (MIDCAB) showed no correlation to POAF [53]. Transcatheter Aortic Valve Replacement (TAVR) is a method that is increasing in popularity over Surgical Aortic Valve Replacement (SAVR), due to it being less invasive [1,54]. In the PARTNER3 trial, TAVR had a lower incidence of early POAF that patients who underwent SAVR. The study also showed that later onset POAF had a higher incidence of adverse outcomes despite either treatment method [55]. Other studies supported this finding, suggesting that the less invasive TAVR led to a decreased risk of POAF than traditional SAVR procedures [56].
2.4 The Financial and Clinical Burdens of Patients with POAF
While the definitive mechanism and solution for POAF remains elusive, the complication presents a heavy burden for patients, hospitals, and families. One study reported that patients that develop POAF after CABG are twice as likely to die (p < 0.01) [3,4]. Not only does POAF contribute to a higher mortality, but it also causes a large financial burden for patients and their families. A randomized control trial showed that the 1-year cost difference for patients that developed POAF was +$15,593 USD due to a longer hospital stay and complications arising from POAF [4,57]. Therefore, attempts to reduce the incidence of POAF by understanding the mechanism of the complication or finding a consistent preventative measure for reducing POAF could help decrease hospital resource utilization and patient expenses after cardiac surgery [58-65].
3. Conclusion
POAF is a common and expensive complication that occurs in 20-55% of cardiac surgery patients and has increased risk for stroke, thromboembolism, and mortality. Risk factors and etiologies for POAF are poorly understood. However, risk factors that have shown to be significant include type of surgery, age, diabetes, hypertension, history of heart failure, COPD, obesity, male sex, and myocardial infarction. Proposed mechanisms for the development of POAF include atrial substrate changes when exposed to a trigger and/or a driver to sustain POAF, sympathetic stimulation, local and systemic inflammation, oxidative stress, and electrolyte disturbances. Current treatments are typically via drug repositioning such as statins, beta-blockers, antiarrhythmics, oral anticoagulants and restoration of electrolytes. Some research has also been done on autonomic denervation, yet more studies are needed to determine the efficacy and long-term effects of this treatment. POAF also provides a large burden by increasing the cost of a patient’s hospital stay by about $15,593 on average and increasing the use of hospital resources. Due to the prevalence of POAF after cardiac surgery, it is vital that the mechanism of POAF is elucidated so that patients can receive preventative treatment.
Author contribution
Conception and design: DKA, LAL; Literature search, collection of the scientific information, analysis, and interpretation of the data: LAL, DKA; Drafting of the article: LAL; Critical revision and editing of the article for important intellectual content: DKA; Final approval of the submitted article: LAL, DKA.
Funding
This research work was supported by research grants R01 HL144125 and R01 HL147662 to D.K. Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this critical review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
Not applicable; all information is gathered from published articles.
Code Availability
Not applicable
Declarations
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Ethical approval
Not applicable
Consent to participate
Concept for publication
Not applicable
Conflicts of interest
The authors declare no conflicts of interest.
References
- Abdelgawad AME, Hussein MA, Naeim H, et al. A Comparative Study of TAVR versus SAVR in Moderate and High-Risk Surgical Patients: Hospital Outcome and Midterm Results. Heart Surg Forum 22 (2019): E331-E339.
- Albini A, Malavasi VL, Vitolo M, et al. Long-term outcomes of postoperative atrial fibrillation following non cardiac surgery: A systematic review and metanalysis. European Journal of Internal Medicine 85 (2021): 27-33.
- Almassi GH. Postoperative atrial fibrillation; the search goes on. Journal of Surgical Research 198 (2015): 57-58.
- Almassi GH, Wagner TH, Carr B, et al. Postoperative atrial fibrillation impacts on costs and one-year clinical outcomes: the Veterans Affairs Randomized On/Off Bypass Trial. Ann Thorac Surg 99 (2015): 109-114.
- Arsenault KA, Yusuf AM, Crystal E, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev (2013): CD003611.
- Bazarbashi N, Miller M. Icosapent ethyl: drug profile and evidence of reduced residual cardiovascular risk in patients with statin-managed LDL-C cholesterol. Expert Rev Cardiovasc Ther 18 (2020): 175-180.
- Bockeria OL, Kanametov TN, Shvartz VA, et al. Epicardial Application of Hydrogel with Amiodarone for Prevention of Postoperative Atrial Fibrillation in Patients After Coronary Artery Bypass Grafting. J Cardiovasc Transl Res 13 (2020): 191-198.
- Brown S, Larsen N, Thankam FG, et al. Regulatory role of cardiomyocyte metabolism via AMPK activation in modulating atrial structural, contractile, and electrical properties following atrial fibrillation. Can J Physiol Pharmacol 99 (2021): 36-41.
- Brown SM, Larsen NK, Thankam FG, et al. Fetal cardiomyocyte phenotype, ketone body metabolism, and mitochondrial dysfunction in the pathology of atrial fibrillation. Mol Cell Biochem 476 (2021): 1165-1178.
- Budeus M, Hennersdorf M, Perings S, et al. Amiodarone prophylaxis for atrial fibrillation of high-risk patients after coronary bypass grafting: a prospective, double-blinded, placebo-controlled, randomized study. Eur Heart J 27 (2006): 1584-1591.
- Burgos LM, Ramírez AG, Seoane L, et al. New combined risk score to predict atrial fibrillation after cardiac surgery: COM-AF. Ann Card Anaesth 24 (2021): 458-463.
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of Thromboembolism Associated With Atrial Fibrillation Following Noncardiac Surgery. J Am Coll Cardiol 72 (2018): 2027-2036.
- Cerit L, Özcem B, Cerit Z, et al. Preventive Effect of Preoperative Vitamin D Supplementation on Postoperative Atrial Fibrillation. Braz J Cardiovasc Surg 33 (2018): 347-352.
- Chen YL, Zeng M, Liu Y, et al. CHA2DS2-VASc Score for Identifying Patients at High Risk of Postoperative Atrial Fibrillation After Cardiac Surgery: A Meta-analysis. Ann Thorac Surg 109 (2020): 1210-1216.
- Dobrev D, Aguilar M, Heijman J, et al. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol 16 (2019): 417-436.
- Skaria R, Parvaneh S, Zhou S, et al. Path to precision: prevention of post-operative atrial fibrillation. J Thorac Dis 12 (2020): 2735-2746.
- Field ME, Donateo P, Bottoni N, et al. P-Wave Amplitude and PR Changes in Patients With Inappropriate Sinus Tachycardia: Findings Supportive of a Central Mechanism. Journal of the American Heart Association. 7 (2012): e008528.
- Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. Europace 14 (2012): 159-174.
- Ince I, Chiu A, Sagir A, et al. Association of Pain With Atrial Fibrillation and Delirium After Cardiac Surgery: A DECADE Sub-Study. Journal of Cardiothoracic and Vascular Anesthesia 35 (2021): 3021-3026.
- Schnaubelt S, Pilz A, Koller L, et al. The impact of volume substitution on post-operative atrial fibrillation. European Journal of Clinical Investigation 51 (2021): e13456.
- Du J, Xie J, Zhang Z, et al. TRPM7-Mediated Ca2+ Signals Confer Fibrogenesis in Human Atrial Fibrillation. Circulation Research 106 (2010): 992-1003.
- Khan AM, Lubitz SA, Sullivan LM, et al. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation 127 (2013): 33-38.
- Greenberg JW, Lancaster TS, Schuessler RB, et al. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg 52 (2017): 665-672.
- Chaudhary R, Garg J, Turagam M, et al. Role of Prophylactic Magnesium Supplementation in Prevention of Postoperative Atrial Fibrillation in Patients Undergoing Coronary Artery Bypass Grafting: a Systematic Review and Meta-Analysis of 20 Randomized Controlled Trials. J Atr Fibrillation 12 (2019): 2154.
- Lohchab SS, Kumar A. Post-operative atrial fibrillation after off-pump coronary artery bypass grafting. Indian J Thorac Cardiovasc Surg 36 (2020): 4-5.
- Maimari M, Baikoussis NG, Gaitanakis S, et al. Does minimal invasive cardiac surgery reduce the incidence of post-operative atrial fibrillation? Annals of Cardiac Anaesthesia 23 (2020): 7.
- Seo EJ, Hong J, Lee HJ, et al. Perioperative risk factors for new-onset postoperative atrial fibrillation after coronary artery bypass grafting: a systematic review. BMC Cardiovascular Disorders 21 (2021): 418.
- Thorén E, Wernroth ML, Christersson C, et al. Compared with matched controls, patients with postoperative atrial fibrillation (POAF) have increased long-term AF after CABG, and POAF is further associated with increased ischemic stroke, heart failure and mortality even after adjustment for AF. Clin Res Cardiol 109 (2020): 1232-1242.
- Zhao R, Wang Z, Cao F, et al. New-Onset Postoperative Atrial Fibrillation After Total Arch Repair Is Associated With Increased In-Hospital Mortality. J Am Heart Assoc 10 (2021): e021980.
- Yamashita K, Hu N, Ranjan R, et al. Clinical Risk Factors for Postoperative Atrial Fibrillation among Patients after Cardiac Surgery. Thorac Cardiovasc Surg 67 (2019): 107-116.
- Qureshi M, Ahmed A, Massie V, et al. Determinants of atrial fibrillation after cardiac surgery. Rev Cardiovasc Med. 2021;22(2):329-341.
- Rezaei Y, Peighambari MM, Naghshbandi S, et al. Postoperative Atrial Fibrillation Following Cardiac Surgery: From Pathogenesis to Potential Therapies. Am J Cardiovasc Drugs 20 (2020): 19-49.
- Iliescu AC, Salaru DL, Achitei I, et al. Postoperative atrial fibrillation prediction following isolated surgical aortic valve replacement. Anatol J Cardiol 19 (2018): 394-400.
- Fragão-Marques M, Mancio J, Oliveira J, et al. Gender Differences in Predictors and Long-Term Mortality of New-Onset Postoperative Atrial Fibrillation Following Isolated Aortic Valve Replacement Surgery. Ann Thorac Cardiovasc Surg 26 (2020): 342-351.
- Efird JT, Gudimella P, O’Neal WT, et al. Comparison of Risk of Atrial Fibrillation in Black Versus White Patients After Coronary Artery Bypass Grafting. Am J Cardiol 117 (2016): 1095-1100.
- Phan K, Khuong JN, Xu J, et al. Obesity and postoperative atrial fibrillation in patients undergoing cardiac surgery: Systematic review and meta-analysis. International Journal of Cardiology 217 (2016): 49-57.
- Kashani RG, Sareh S, Genovese B, et al. Predicting postoperative atrial fibrillation using CHA2DS2-VASc scores. Journal of Surgical Research. 2015;198(2):267-272.
- Farzam K, Jan A. Beta Blockers. In: StatPearls. StatPearls Publishing (2022).
- Osmanovic E, Ostojic M, Avdic S, et al. Pharmacological Prophylaxis of Atrial Fibrillation After Surgical Myocardial Revascularization. Med Arch 73 (2019): 19-22.
- Sasaki K, Kumagai K, Maeda K, et al. Preventive effect of low-dose landiolol on postoperative atrial fibrillation study (PELTA study). Gen Thorac Cardiovasc Surg 68 (2020): 1240-1251.
- Pádua-Filho WC, Brasil DP, Neves HJ, et al. Effects of metoprolol and amiodarone combination on heart rate, myocardial contractilitybrown and coronary flow: Study in isolated perfused rat hearts. Exp Clin Cardiol 9 (2004): 133-137.
- Wang H, Zhang Y, Xin F, et al. Calcium-Induced Autonomic Denervation in Patients With Post-Operative Atrial Fibrillation. J Am Coll Cardiol 77 (2021): 57-67.
- Elgendy IY, Mahmoud A, Huo T, et al. Meta-analysis of 12 trials evaluating the effects of statins on decreasing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 115 (2015): 1523-1528.
- Nomani H, Mohammadpour AH, Reiner ?, et al. Statin Therapy in Post-Operative Atrial Fibrillation: Focus on the Anti-Inflammatory Effects. J Cardiovasc Dev Dis 8 (2021): 24.
- Sterne JA, Bodalia PN, Bryden PA, et al. Oral anticoagulants for primary prevention, treatment, and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess 21 (2017): 1-386.
- Taha A, Nielsen SJ, Bergfeldt L, et al. New-Onset Atrial Fibrillation After Coronary Artery Bypass Grafting and Long-Term Outcome: A Population-Based Nationwide Study From the SWEDEHEART Registry. J Am Heart Assoc 10 (2021): e017966.
- Rezaei Y, Gholami-Fesharaki M, Dehghani MR, et al. Statin Antiarrhythmic Effect on Atrial Fibrillation in Statin-Naive Patients Undergoing Cardiac Surgery: A Meta-Analysis of Randomized Controlled Trials. J Cardiovasc Pharmacol Ther 21 (2016): 167-176.
- Woldendorp K, Khadra S, Bannon PG, et al. Novel Oral Anticoagulants Compared to Warfarin for Postoperative Atrial Fibrillation After Isolated Coronary Artery Bypass Grafting. Heart Lung Circ 29 (2020): 1832-1838.
- Rai V, Agrawal DK. Role of vitamin D in cardiovascular diseases. Endocrinol Metab Clin North Am 46 (2017): 1039-1059.
- Rai V, Agrawal DK: Immunomodulation of IL-33 and IL-37 with Vitamin D in the Neointima of Coronary Artery: A Comparative Study between Balloon Angioplasty and Stent in Hyperlipidemic Microswine. Int J Mol Sci 22 (2021): 8824.
- Kara H, Yasim A. Effects of high-dose vitamin D supplementation on the occurrence of post-operative atrial fibrillation after coronary artery bypass grafting: randomized controlled trial. Gen Thorac Cardiovasc Surg 68 (2020): 477-484.
- Kunadian V, Ford GA, Bawamia B, et al. Vitamin D deficiency and coronary artery disease: A review of the evidence. American Heart Journal 167 (2014): 283-291.
- Fan G, Liu J, Dong S, et al. Postoperative Atrial Fibrillation after Minimally Invasive Direct Coronary Artery Bypass: A Single-Center, 5-Year Follow-Up Study. Heart Surg Forum 24 (2021): E456-E460.
- Piperata A, Gemelli M, Jorgji V, et al. Good Things Come to Those Who Wait. J Clin Med 9 (2020): 3392.
- Shahim B, Malaisrie SC, George I, et al. Postoperative Atrial Fibrillation or Flutter Following Transcatheter or Surgical Aortic Valve Replacement: PARTNER 3 Trial. JACC Cardiovasc Interv 14 (2021): 1565-1574.
- Jeong HK, Yoon N, Kim JH, et al. Post-operative Atrial Fibrillation Impacts on Outcomes in Transcatheter and Surgical Aortic Valve Replacement. Front Cardiovasc Med 8 (2021): 789548.
- LaPar DJ, Speir AM, Crosby IK, et al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg 98 (2014): 527-533.
- Fakuade FE, Steckmeister V, Seibertz F, et al. Altered atrial cytosolic calcium handling contributes to the development of postoperative atrial fibrillation. Cardiovascular Research 117 (2021): 1790-1801.
- Frendl G, Sodickson AC, Chung MK, et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. The Journal of Thoracic and Cardiovascular Surgery 148 (2014): e153-e193.
- Rosenberg JH, Werner JH, Plitt GD, Net al. Immunopathogenesis and biomarkers of recurrent atrial fibrillation following ablation therapy in patients with preexisting atrial fibrillation. Expert Rev Cardiovasc Therapy 17 (2019): 193-207.
- Lee H, Kim HJ, Yoo JS, et al. Early pharmacologic conversion of atrial fibrillation after off-pump coronary artery bypass grafting. J Thorac Dis 13 (2021): 4072-4082.
- Romanov A, Pokushalov E, Ponomarev D, et al. Long-term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: Three-year follow-up of a randomized study. Heart Rhythm 16 (2019): 172-177.
- Waldron NH, Cooter M, Haney JC, et al. Temporary autonomic modulation with botulinum toxin type A to reduce atrial fibrillation after cardiac surgery. Heart Rhythm 16 (2019): 178-184.
- Ha ACT, Verma S, Mazer CD, et al. Effect of Continuous Electrocardiogram Monitoring on Detection of Undiagnosed Atrial Fibrillation After Hospitalization for Cardiac Surgery: A Randomized Clinical Trial. JAMA Netw Open 4 (2021): e2121867.
- Wang W, Mei YQ, Yuan XH, et al. Clinical efficacy of epicardial application of drug-releasing hydrogels to prevent postoperative atrial fibrillation. The Journal of Thoracic and Cardiovascular Surgery 151 (2016): 80-85.
