Post Systolic Motion – A Marker for Ischemia in Left Bundle Branch Block
Article Information
Praveen Babu R1*, Arun kumar S1, Sakthivel V1
1Department of Medicine, Vinayaka mission medical college and hospital, Karaikal, Puducherry, affliated to Vinayaka Mission’s Research Foundation (deemed to be university), Salem - 636308, Tamil Nadu, India.
*Corresponding author: Praveen Babu R, Department of Medicine, Vinayaka mission medical college and hospital, Karaikal, Puducherry, affliated to Vinayaka Mission’s Research Foundation (deemed to be university), Salem - 636308, Tamil Nadu, India.
Received: 03 August 2022; Accepted: 18 August 2022; Published: 20 August 2022
Citation: Praveen Babu R, Arun kumar S, Sakthivel V. Post Systolic Motion – A Marker for Ischemia in Left Bundle Branch Block. Cardiology and Cardiovascular Medicine 6 (2022): 456-460.
View / Download Pdf Share at FacebookAbstract
Background: To diagnose ischemia in patients with left bundle branch block (LBBB) patients non-invasively is always a diagnostic challenge. Many of the non-invasive modalities like stress test, nuclear imaging, cardiac CT and MRI that are routinely used to detect ischemia in recent times have their own limitations when used in patients with LBBB. Tissue Doppler imaging (TDI) has shown promising results in detecting ischemia in LBBB patients in various studies.
Methods: The study population was divided into two groups. Group one included 22 patients with LBBB with left anterior descending artery (LAD) stenosis > 50%. Group two includes 29 patients with LBBB with no or < 50% LAD stenosis. Both groups were subjected to TDI.
Results: TDI showed low myocardial systolic velocities (Sm), high late diastolic velocities (Am) and high post-systolic motion (PSM) in patients with LAD stenosis. PSM > 6.3 m/s and Sm/ PSM ratio ≤ 0.8 detected LAD stenosis with 77% sensitivity and 96% specificity.
Conclusions: TDI may be useful to identify ischemia in patients with LBBB.
Keywords
LBBB; LAD Stenosis; Post-Systolic Motion (PSM)
Article Details
1. Introduction
Left bundle branch block (LBBB) is usually considered pathological and indicative of coronary artery disease (CAD) in a resting ECG unless proved otherwise. But actually prevalence of CAD in patients with LBBB varies between 30-52% [1,2]. LBBB can also occur in non-ischemic conditions like aortic stenosis, dilated cardiomyopathy, hypertension and older age [3]. Non-invasive modalities commonly used to assess CAD are found to have several drawbacks and false positive results in patients with LBBB. Tissue Doppler imaging (TDI) identifies ischemia by exhibiting variations in myocardial tissue systolic and diastolic velocities. Studies have shown that myocardial systolic and diastolic velocities and post systolic motion measured by TDI can be used as an early marker to identify ischemia in patients with LBBB [4-8]. Since there is a paucity of data concerning the above parameters, we designed a prospective case control study to test whether the above parameters can be used as an early marker to identify ischemia in patients with LBBB.
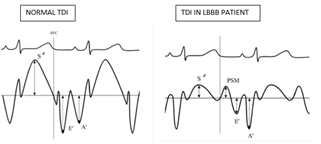
S’ - Peak systolic velocity, PSM – Post-systolic motion, E’ – Peak velocity of early ventricular filling, A’ – Peak velocity of atrial contraction.
2. Materials and Methods
We conducted a prospective case control study in Vinayaka mission medical college and research centre, Karaikal, between august 2020 to July 2021. 51 patients who came to cardiac OP with LBBB and who had underwent coronary angiogram (CAG) were enrolled for the study. Patients were divided into two groups based on their CAG findings. Group one includes 22 patients, who had significant left anterior descending artery (LAD) stenosis > 50%. Group two includes 29 patients who had no LAD stenosis or ≤ 50% stenosis. Patients with valvular heart diseases, congenital heart diseases, pacemakers and abnormal A-V pathways were excluded from the study. Both groups were subjected to 2D Echo, Doppler echo and TDI using Esoate my Lab Gamma machine equipped with a phased array transducer. The following TDI measurements, myocardial systolic velocity (Sm), myocardial early diastolic velocity (Em), myocardial late diastolic velocity (Am) were measured. Statistical analyses was done using software namely SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0 and R environment ver.2.11.1.
3. Results
|
Baseline characteristics |
Group I (With LAD stenosis) |
Group II (Without LAD stenosis) |
P value |
|
Age |
60.77 ± 8.45 |
59.55 ± 7.53 |
0.59 |
|
Gender · Male · Female |
15 7 |
21 8 |
0.743 |
|
Hypertension |
16 |
22 |
0.799 |
|
Diabetes mellitus |
12 |
14 |
0.657 |
|
Dyslipidemia |
13 |
15 |
0.692 |
|
Family history of CAD |
7 |
10 |
0.842 |
|
Body mass index |
26.39 ± 2.76 |
25.47 ± 2.36 |
0.208 |
|
Body surface area |
1.85 ± 0.16 |
1.79 ± 0.21 |
0.233 |
|
Systolic BP (mm HG) |
130.64 ± 12.79 |
129.66 ± 13.63 |
0.79 |
|
Diastolic BP (mm HG) |
79.82 ± 8.57 |
81.24 ± 7.99 |
0.544 |
|
Heart rate (beats/min) |
70.27 ± 7.57 |
76 ± 8.4 |
0.15* |
Table 1: Baseline Characteristics of Study Population.
Table-1 shows that baseline characteristics are comparable between the groups. Though p value for heart rate was statistically significant, when considered clinically it is insignificant value falls within normal limits.
|
Echo M-MODE |
Group I (With LAD stenosis) |
Group II (Without LAD stenosis) |
P value |
|
IVS (mm) |
9.45 ± 1.34 |
10.93 ± 2 |
0.004** |
|
PWT (mm) |
9.55 ± 1.22 |
10.52 ± 1.77 |
0.032* |
|
LVDD (mm) |
56.14 ± 3.77 |
51.24 ± 4.16 |
< 0.001** |
|
LVSD (mm) |
40.45 ± 3.33 |
37.17 ± 4.18 |
0.004** |
|
EF (%) |
55.64 ± 5.85 |
55.1 ± 5.7 |
0.745 |
|
LV MASS (g) |
207.36±36.17 |
210.38±48.83 |
0.809 |
|
LVMI (g/m²) |
111.50±23.66 |
116.66±24.6 |
0.455 |
Table 2: Echo M-MODE Analysis.
(IVS – interventricular septal thickness, PWT – posterior wall thickness, LVDD – left ventricular end diastolic diameter, LVSD – left ventricular end systolic diameter, EF – ejection fraction, LV mass – left ventricular mass, LVMI – left ventricular mass index)
Table- 2 shows the results of 2D – Echocardiographic M-mode analysis between the two groups. Patients with LAD stenosis had a significantly lower septal wall thickness (p=0.04) and posterior wall thickness (p=0.032) and a greater LV - end diastolic (p<0.001) and LV – end systolic dimensions (p=0.004).
|
Echo Doppler |
Group I (With LAD stenosis) |
Group II (Without LAD stenosis) |
P value |
|
E (m/s) |
55.55±5.79 |
55.10±6.75 |
0.807 |
|
A (m/s) |
52.95±6.98 |
57.00±9.21 |
0.092 |
|
E/A |
1.06±0.11 |
0.99±0.23 |
0.247 |
|
DT (ms) |
200.18±17.51 |
197.45±27.85 |
0.688 |
|
IVRT (ms) |
89.59±11.28 |
97.14±13.23 |
0.037* |
Table 3: Echo Doppler Analysis.
(E – early diastolic filling velocity, A- atrial filling velocity, DT – deceleration time and IVRT- iso-volumic relaxation time)
Table- 3 shows the results of Echo Doppler analysis. Patient with LAD stenosis showed a mild reduction in iso-volumic relaxation time when compared to other group. No differences were found in other variables between the two groups.
|
Tissue Doppler Imaging - MID Inter Ventricular Septum |
Group I (With LAD stenosis) |
Group II (Without LAD stenosis) |
P value |
|
Sm (cm/s) |
4.68±0.71 |
6.70±1.02 |
<0.001** |
|
Em (cm/s) |
6.89±0.99 |
6.17±1.02 |
0.015* |
|
Am (cm/s) |
9.85±1.06 |
7.89±1.16 |
<0.001** |
|
PSM (cm/s) |
8.66±2.43 |
4.71±1.09 |
<0.001** |
|
Em/Am ratio |
0.70±0.04 |
0.78±0.08 |
<0.001** |
|
Sm/PSM ratio |
0.62±0.29 |
1.52±0.48 |
<0.001** |
Table 4: Tissue Doppler Imaging - MID Posterior Inter Ventricular Septum.
(Sm - myocardial systolic velocity, Em - myocardial early diastolic velocity, Am - myocardial late diastolic velocity and PSM – post systolic motion)
Table- 4 shows the results of pulsed tissue doppler analysis of mid posterior interventricular septum. Patients with LAD stenosis had higher amplitude of PSM (p<0.001), lower Sm peak velocity (p<0.001) and higher Em and Am peak velocities when compared to patients without LAD stenosis. Both Sm/PSM ratio and Em/Am ratio were significantly lower in patients with LAD stenosis group compared to the other.
|
Tissue Doppler Imaging - Lateral Mitral Annulus |
Group I (With LAD stenosis) |
Group II (Without LAD stenosis) |
P value |
|
Sm (cm/s) |
7.88±0.94 |
7.94±0.86 |
0.825 |
|
Em (cm/s) |
8.40±1.35 |
8.60±1.23 |
0.592 |
|
Am (cm/s) |
9.47±1.32 |
11.74±1.12 |
<0.001** |
|
Em/Am ratio |
0.89±0.03 |
0.73±0.04 |
<0.001** |
Table 5: Tissue Doppler Imaging - Lateral Mitral Annulus.
(Sm - myocardial systolic velocity, Em - myocardial early diastolic velocity, Am - myocardial late diastolic velocity and PSM – post systolic motion)
Table- 5 shows the results of pulsed tissue doppler analysis at lateral mitral annulus. Patients with LAD stenosis had lower Am peak velocity (p<0.001) and higher Em/Am ratio compared to patients without LAD stenosis.
|
Cut-off score |
AUC |
P value |
Sensitivity |
Specificity |
LR+ |
LR- |
|
|
PSM (cm/s) |
>6.3 |
0.908 |
<0.001** |
77.27 |
96.55 |
22.41 |
0.24 |
|
Em/Am RATIO |
≤0.73 |
0.847 |
<0.001** |
86.36 |
75.86 |
3.58 |
0.28 |
|
Sm/PSM RATIO |
≤0.81 |
0.936 |
<0.001** |
77.27 |
96.55 |
22.41 |
0.24 |
Table 6: ROC curve analysis to predict LAD stenosis in patients with LBBB using PSM, Em/Am ratio and Sm/PSM ratio.
The ideal cut – off values of PSM, Em/Am ratio and Sm/PSM to predict LAD stenosis were >0.63, ≤0.73 and ≤0.81. The positive likelihood ratios of PSM and Sm/PSM ratio variables were higher than other variables and the negative likelihood ratios of PSM and Sm/PSM variables were lower than other variables. An Sm/PSM ratio of ≤0.81 was found in 77% of patients with LAD stenosis and only 3% of patients without LAD stenosis with a sensitivity of 77%, specificity of 96%, positive predictive value of 94% and negative predictive value of 85%.
4. Discussion
PSM is a delayed ejection motion of myocardium occurring in iso-volumic relaxation time (IVRT) about 100 milli seconds after aortic valve closure. Gibson et al, first described PSM by cine-ventriculograms 4-hours post myocardial infraction [9]. PSM is an early and sensitive marker of ischemia [10,11]. PSM is seen in akinetic, hypokinetic and dyskinetic segments. But the mechanism of PSM slightly varies among these segments. In dyskinetic segments, PSM is due to passive inward movements caused by adjacent normally contracting segments. In hypokinetic segments, it is due to delayed active contraction appearing after LV unloading and decrease in regional wall stress [12]. PSM can also be seen in non-ischemic myocardium i.e. in patients free of CAD, such as LVH, LV volume overload and even in healthy subjects [13,14,15]. In our study also PSM is seen not only in patients with LBBB with LAD stenosis but also in patients with LBBB without LAD stenosis. So, when we analyze the differences, PSM occurring in patients with LAD stenosis is of higher amplitude, delayed (> 100ms from aortic valve closure) and associated with reduced myocardial systolic velocities. In our study, PSM value of > 6.3 m/s was found in 77% of LBBB patients with LAD stenosis and 3% in patients without LAD stenosis. So when we set of a cut-off value for PSM >6.3 m/s, it had 77% sensitivity and 96% specificity in detecting LBBB patients with LAD stenosis. Systolic phase and early diastolic relaxation phases of cardiac cycle are high energy consuming processes and are very sensitive to ischemia. So systolic (Sm) and early diastolic (Em) myocardial velocities are reduced in patients with ischemia [16,17,18,19]. Similarly late diastolic phase of cardiac cycle is relatively passive and less energy consuming compared to the above phases. Late diastolic (Am) myocardial velocities are increased in patients with ischemia [20]. In our study also Em/Am ratio is reversed in LBBB patients with ischemia. In this study, Sm/Psm ratio < 0.8 LAD stenosis in LBBB patients with 77% sensitivity and 96% specificity and negative predictive value of 85%. Similar results were obtained by Citro et al, in his study [21]. Limitations of the present study was that the TDI sampling was limited to IVS and LV lateral mitral annulus assuming dyskinesia due to LBBB is predominantly evident at the septal level. The study, would have been more comprehensive if we had evaluated other LV segments also. Another limitation is that in clinical setting, measurement of PSM needs patience, technical feasibility and additional time to acquire and interpret during routine echo evaluation.
5. Conclusions
Evidence of CAD in LBBB patients is difficult to unmask using traditionally non-invasive tools. Present study demonstrate the use of TDI to identify CAD in LBBB patients. PSM value of > 6.3 m/s and Sm/PSM ratio ≤ 0.8 detects ischemia in LBBB patients with high sensitivity and specificity. So, TDI can be used as a routine non-invasive tool to assess ischemia in LBBB patients.
Conflict of interest
Authors have no conflict of interest.
References
- Hardarson T, Arnason A, Eliasson GJ, et al, Left bundle branch block: prevalence, incidence, follow-up and outcome, Eur. Heart J 8 (1987): 1075-1079.
- Mulcahy R, Hickey N, Maurer B, Aetiology of bundle-branch block, Br. Heart J 30 (1968): 34-37.
- Schneider JF, Thomas HE, Sorlie P, et al. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 47 (1983): 931-940.
- Takayama M, Norris RM, Brown MA, et al. Post-systolic shortening of acutely ischemic canine myocardium predicts early and late recovery of function after coronary artery reperfusion. Circulation 78 (1988): 994-1007.
- Skulstad H, Edvardsen T, Urheim S, et al. Postsystolic shortening in ischemic myocardium: active contraction or passive recoil? Circulation 106 (2002): 718-724.
- Hosokawa H, Sheean FH, Suzuky T. Measurement of postsystolic shortening to assess viability and predict recovery of left ventricular function after acute myocardial infarction. J Am Coll Cardiol 35 (2000): 1842-1849.
- Jamal F, Kukulski T, D’hooge J, et al. Abnormal postsystolic thickening in acutely ischemic myocardium during coronary angioplasty: a velocity, strain, and strain rate Doppler myocardial imaging study. J Am Soc Echocardiogr 12 (1999): 994-996.
- Isaaz K, Thompson A, Ethevenot G, et al. Doppler echocardiographic measurement of low velocity motion of the left ventricular posterior wall. Am J Cardiol 64 (1989): 66-75
- Gibson D, Mehmel H, Schwarz F, et al: Changes in left ventricular asynchrony after intracoronary thrombolysis in patients with impending myocardial infarction. Br Heart J 56 (1986): 121-130.
- Kerber RE, Abboud FM. Echocardiographic detection of regional myocardial infarction: an experimental study. Circulation 47 (1973): 997-1005.
- Barletta G, Del Bene R, Lo Sapio P, et al. Post-ejection thickening as a marker of viable myocardium. An echocardiographic study in patients with chronic coronary artery disease. Basic Res Cardiol 93 (1998): 313-324.
- Wiegner AW, Allen GJ, Bing OHL: Weak and strong myocardium in series: Implication for segmental dysfunction. Am J Phisiol 1978; 235 (1978): H776-H783 .
- Pai GR, Gill KS. Amplitudes, durations, and timings of apical directed left ventricular myocardial velocities: II. Systolic and diastolic asynchrony in patients with left ventricular hypertorphy. J Am Soc Echocardiogr 11 (1998): 112-118.
- Galderisi M, Petrocelli A, D’Errico A, et al: Clinical value of post systolic motion in patients affected by aortic regurgitation: Analysis by pulsed tissue doppler. Eur J Echocardiogr 3 (2002): S79.
- Voigt Ju, Lindenmeier G, Exener B, et al: Incidence and characteristics of segmental post systolic longitudinal shortening in normal, acutely ischemic and scarred myocardium. J Am Soc Echocardiogr 16 (2003): 415-423.
- Hoffmann S, Jensen JS, Iversen AZ, et al. Tissue Doppler echocardiography improves the diagnosis of coronary artery stenosis in stable angina pectoris. Eur Heart J Cardiovasc Imaging 13 (2012): 724-729.
- Nikitin NP, Loh PH, de Silva R, et al. Prognostic value of systolic mitral annular velocity measured with Doppler tissue imaging in patients with chronic heart failure caused by left ventricular systolic dysfunction. Heart 92 (2006): 775-779.
- Hoffmann S, Mogelvang R, Olsen NT, et al. Tissue Doppler echocardiography reveals distinct patterns of impaired myocardial velocities in different degrees of coronary artery disease. Eur J Echocardiogr 11 (2010): 544-549.
- Hoffmann S, Mogelvang R, Sogaard P, et al. Tissue Doppler echocardiography reveals impaired cardiac function in patients with reversible ischemia. Eur J Echocardiogr 12 (2011): 628-634.
- Garcia-Fernandez MA, Azevedo J, Moreno M, et al. Regional diastolic function in ischaemic heart disease using pulsed wave Doppler tissue imaging. Eur Heart J 20 (1999): 496-505.
- Citro R, Galderisi M, Guarini P, et al. Post-systolic motion in left bundle branch block with and without coronary artery disease: Analysis by pulsed tissue Doppler. Ital Heart J 4 (2003): 706-712.