Positive End-Expiratory Pressure During Helmet Ventilation in Critically Ill Bronchiolitis: A Multicenter Randomized Clinical Trial
Article Information
Emanuele Rossetti MD, PhD1*, Zaccaria Ricci, MD2, Daniele Bonacina, MD3, Cristina Giugni MD2 , Ezio Bonanomi MD3, Marina Franci MN1, Marta Ciofi degli Atti MD4, Manuela L’Erario MD2, Daniela Perrotta MD1, Corrado Cecchetti MD, PhD1, Linda Appierto MD1, Luigi Dei Giudici MD1, Alessandro Germani MD1, Francesco Polisca MD1, Francesca Tortora MD1, Ivano Farinelli MD1, Fabrizio Chiusolo MD1, Paola Serio MD2, Roberto Bianchi MD1, Sergio G. Picardo MD1 on behalf of the SARNePI (Italian Society for Pediatric and Neonatal Anaesthesia and Intensive Care).
1Bambino Gesù Children’s Hospital, Emergency, Anesthesia and Intensive Care Department, Pediatric Intensive Care Unit, Rome, Italy
2Meyer Hospital, Anesthesia Department, Pediatric Intensive Care Unit, Florence, Italy
3Papa Giovanni XXIII Hospital, Anesthesia Department, Pediatric Intensive Care Unit, Bergamo, Italy
4Bambino Gesù Children’s Hospital, Epidemiology Unit, Rome, Italy
*Corresponding Author: Emanuele Rossetti, Bambino Gesù Children’s Hospital, Emergency, Anesthesia and Intensive Care Department, Pediatric Intensive Care Unit, Rome, Italy.
Received: 26 April 2023; Accepted: 25 May 2023; Published: 12 May 2023
Citation:
Emanuele Rossetti, Zaccaria Ricci, Daniele Bonacina, Cristina Giugni, Ezio Bonanomi, Marina Franci, Marta Ciofi degli Atti, Manuela L’Erario, Daniela Perrotta, Corrado Cecchetti, Linda Appierto, Luigi Dei Giudici, Alessandro Germani, Francesco Polisca, Francesca Tortora, Ivano Farinelli, Fabrizio Chiusolo, Paola Serio, Roberto Bianchi, Sergio G. Picardo, on behalf of the SARNePI (Italian Society for Pediatric and Neonatal Anaesthesia and Intensive Care). Positive End-Expiratory Pressure During Helmet Ventilation in Critically Ill Bronchiolitis: A Multicenter Randomized Clinical Trial. Journal of Pediatrics, Perinatology and Child Health. 7 (2023): 103-110.
View / Download Pdf Share at FacebookAbstract
Objectives: To evaluate the effects of two different levels of Positive End-Expiratory Pressure (PEEP) during Helmet Continuous Positive Airway Pressure (HCPAP) support on the intubation rate in infants with bronchiolitis admitted to Pediatric Intensive Care Units (PICUs).
Design: Multicentric prospective, randomized, open clinical trial.
Setting: Four tertiary PICUs in Italy.
Participants: Infants admitted to PICUs due to severe bronchiolitis and requiring respiratory support.
Interventions: The enrolled patients were randomly assigned one of two PEEP levels for HCPAP support: high (P10 group: 10 cmH2O) or low (P5 group: 5 cmH2O).
Measurements and Main Results: In total, 64 patients were randomly assigned to the P10 group, and 60 to the P5 group. The intubation rate was 9/60 (15%) and 9/64 (14%) in P5 and P10 groups (OR 0.94, 95% CI 0.36- 2.46, p=0.99). Of the patients in the P5 group, 47 (78%) were escalated to a PEEP level of 10 cmH2O. PEEP level was not associated to intubation rate (OR 0.69, 95% CI 0.19 to 2.40, p=0.57), after adjustment for age, gestational age, high flow nasal cannula application, bronchiolitis severity score and pediatric index of mortality 3. No cases of pneumothorax were observed in this study.
Conclusions: In this trial on infants with severe bronchiolitis, a PEEP level of 10 cm H2O during HCPAP in comparison to an initial level of 5 cm H2O did not show to prevent intubation. These results are not conclusive due to the premature stopping.
Keywords
Bronchiolitis; Non-invasive ventilation; Helmet Continuous Positive Airway Pressure; Positive End Expiratory Pressure; Intubation; Pediatric Intensive Care
Article Details
1. Introduction
Bronchiolitis is one of the most common causes of admission to Pediatric Intensive Care Units (PICUs) [1]. In the first year of life, 2-3% of infants are admitted in hospital for bronchiolitis [1]. Newborns and infants with severe bronchiolitis admitted to PICUs are exposed to a high risk of being supported with invasive mechanical ventilation. The costs of health care support for children under 2 years of age with bronchiolitis exceeded €1.7 billion in the United States [2]. While the rate of mortality due to severe bronchiolitis in PICUs has dropped from 20% to less than 1%, in developed countries, it is usually associated with severe cardiac and respiratory comorbidities [2,3].
After the first 48-72 hours, upper respiratory tract infection symptoms (i.e., fever, congestion, rhinorrhea, irritability, poor feeding development) may evolve into lower respiratory tract symptoms in about one third of patents. These manifest as a typical bronchiolitis pattern (i.e., cough, tachypnea, wheezing, grunting, nasal flaring, thoracic retractions, and hyperinflation of the lungs) [4]. In such clinical condition, air trapped in the alveoli is reabsorbed, resulting in localized distal obstruction. This eventually leads to increased work of breathing, reduction of lung compliance, ventilation and perfusion mismatch, and hypoxemia, therefore leading to life–threatening failure of respiratory function. In such cases, the first-line respiratory support includes High Flow Nasal Cannula (HFNC) with delivery of high inspired oxygen concentration. Non-Invasive Ventilation (NIV) delivered through a mechanical ventilator and Continuous Positive Airway Pressure (CPAP) delivered through specific devices utilizing only pressure deriving from gas flows are considered second-line incremental respiratory treatments, reserved for non-responder hypoxemic patients, who generally require PICU admission.
No recommendations are currently available regarding timing and modality of the administration of different types of respiratory support for these patients [5,6]. Furthermore, there are not evidence-based criteria to indicate when invasive ventilation should be provided [7]. Retrospective and prospective observational studies have indicated that NIV may significantly reduce intubation rates in infants with bronchiolitis 8-10. However, available reports on severe bronchiolitis in critically ill children have not evaluated different levels of Positive End Expiratory Pressure (PEEP) to determine which concentration provides optimal respiratory support during Helmet Continuous Positive Airway Pressure (HCPAP) administration [11-14].
This randomized study aimed to evaluate the impact of two different levels of PEEP during HCPAP support on the intubation rate and requirement of mechanical ventilation in infants with severe bronchiolitis admitted to PICUs.
2. Materials and Methods
2.1 Trial design
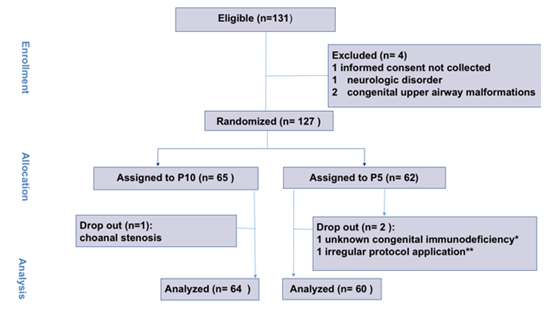
This was a multicenter, prospective, randomized, open, clinical trial. The study protocol was registered at ClinicalTrials.gov ID, NCT02977585, “Helmet Study”. The Bambino Gesù Hospital Ethics Committee approved this study (protocol number 1239_OPBG_2016). The Institutional Ethics Committee of each study site approved the study protocol. The Consolidated Standards of Reporting Trials (CONSORT) checklist was followed. The study was interrupted after the enrollment of the first 127 patients due to a low enrollment rate.
2.2 Participants
Four PICUs in Italy contributed to the study - Bambino Gesù Children’s Hospital, General and Emergency Department PICU, Rome; Papa Giovanni XXIII Hospital PICU, Bergamo; and Meyer Hospital PICU, Florence.
The inclusion criteria for this study were as follows: severe bronchiolitis diagnosed according to a bronchiolitis severity score (BSS) [15] greater than 2; admission to a PICU for respiratory support; corrected gestational age between 38 weeks and 18 months; no more than 72 hours of HFNC or O2 therapy by nasal cannulas; arterial blood line in place. Patients’ parents were required to provide written consent prior to study participation.
Exclusion criteria for this study were the presence of congenital malformations in upper airways, congenital inborn errors of metabolism, cyanotic congenital heart diseases, immunodeficiencies, neonatal or postnatal neurologic disorders, and the presence or suspicion of pneumothorax on chest radiograph or lung ultrasound.
2.3 Interventions
After the initial screening, all patients were randomized into either of the two study groups: patients in the P5 group received 5 cmH2O PEEP during HCPAP therapy within 24 hours from PICU admission, and patients in the P10 group received 10 cmH2O PEEP. HCPAP was initially set with a gas of flow rate of 50 L/min and a fraction of inspired oxygen (FiO2) level of 0.5. Clinicians could proceed with endotracheal intubation if more than two of the following clinical signs worsened after initiation of HCPAP: thoracic or intercostal efforts with patients’ discomfort on attending clinician evaluation, increase in BSS, pH <7.10, PaO2/FiO2 <100, rising of lactate level on two consecutive blood gas analyses. If clinicians indicated to escalate the support of a patient in the P5 group before endotracheal intubation (i.e., an increase in PEEP to a level of 10 cmH2O), as well as they indicated to de-escalate from P10 to P5 (i.e., due to intolerance of the patient) this was considered a protocol violation, but the patient was still analyzed in the original group per intention-to-treat. Vital parameters (pH, respiratory rate, heart rate, PaO2/FiO2 ratio, and partial pressure of carbon dioxide -PaCO2-) were recorded at the start of HCPAP, and after 1, 12, 24 and 48 hours. Outcomes were recorded until the patient was discharged from the PICU. All enrolled patients were managed according to a standard treatment protocol for severe bronchiolitis (Supplementary Table 1). A nasal polymerase chain reaction swab for the research of respiratory viruses and common bacteria was performed at admission in all patients. HCPAP was discontinued to HFNC if oxygen saturation was kept above 95% for more than 24 hours with a FIO2<0.3.
2.4 Outcomes
The primary outcome was the evaluation of whether the intubation rate was significantly lower for P10 patients than for P5 patients. The secondary outcomes were compared between the two groups: a) the impact of PEEP levels on cardiorespiratory parameters over time; b) the pneumothorax rate, c) enteral feeding intolerance (defined as the need to stop enteral nutrition due to excessive gastric residual volumes and abdominal distension); d) the length of stay in the PICUs.
2.5 Sample size
An initial sample size of 488 patients was determined based on the results of a retrospective study conducted from 2011 to 2015 [16]. According to the hypothesis that the intubation rate with 5 cmH2O PEEP was 15% and that the application of 10 cmH2O PEEP might reduce it by 50%, the study would have required a sample of 244 patients in each arm to have a significance of 5% and a power of 80%. Unfortunately, the study sites collected fewer patients than initially planned. Due to the Coronavirus Disease-19 pandemic, enrollments essentially stopped in 2020 due to resource limitations and a significant decrease in bronchiolitis incidence. Hence, according to Ethics Committee at the coordinating center (Bambino Gesù) and based on the data available for the first patients (unplanned interim analysis), the study was interrupted.
2.6 Randomization
A computer-generated block randomization was designed to provide each site with a blinded randomized sequence assigning the PEEP level during HCPAP therapy. An initial sequence of 50 patients was sent to the study coordinator at each site, with an equal number of patients in each group at all sites. The subsequent planned blocks were not sent after study interruption. After patients were randomly assigned to the study groups and achievement of informed consent, the PEEP level enrollment group was revealed, and it was open to all caregivers due to the impossibility of blinding it.
2.7 Statistical analysis
Categorical data are presented as numbers, percentages, and rates, while continuous data as mean and standard deviation, or median and range, when appropriate. A chi square test or Fisher’s exact test were performed to analyze categorical data, while unpaired Student T test or Mann Whitney test were applied depending on patients’ distribution. Two-way analysis of variance for repeated measures was applied to verify respiratory differences in the two groups. Multivariate Logistic Regression Analysis (MLRA) was used to assess the effect of PEEP on intubation, adjusted for the covariates listed as baseline features. Variation Inflation Factor (VIF) was used to evaluate for collinearity. Variables with VIF>2 were considered colinear and excluded from the analysis. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed using the GraphPad Prism 8.0 software package (GraphPad Software, San Diego, CA).
3. Results
Overall, 131 infants were considered eligible. After four of them were excluded, 127 patients were randomized into the P10 arm (65 patients) and into the P5 arm (62 patients). Three patients (one from the P10 and two from the P5 group) were dropped out after randomization; this was due to the presence of undiagnosed choanal stenosis, to irregular study protocol application and to previously unrecognized congenital immunodeficiency (Figure 1). Patients were enrolled between November 2016 and December 2019. The study was prematurely interrupted thereafter due to low enrollment rate. The study was prematurely ended after the first 127 patients and did not enroll the planned 488 cases due to an almost complete drop in bronchiolitis occurrence in 2020. Table 1 summarizes baseline features of the enrolled patients: age, gestational age, weight, BSS, bacterial coinfection, HFNC support prior to PICU admission and PIM3 score.
|
Baseline features |
P5 (n. 60) |
P10 (n. 64) |
|
Age (days) |
53 (35-119) |
65 (39-127) |
|
Gestational Age at birth (w) |
39 (38-40) |
39.5 (38-40) |
|
Weight (kg) |
4.5 (4-6) |
5 (3.9-6) |
|
BSS at PICU admission |
6 (4-7.12) |
6 (4-7.5) |
|
PIM3 |
0.5 (0.4-0.5) |
0.45 (0.3-0.6) |
|
HFNC before Helmet (%) |
45 (75%) |
51 (80%) |
|
Viral Pathogens (number, type) |
47, RSV |
45, RSV |
|
12, Rhinovirus |
14, Rhinovirus |
|
|
3, Bocavirus |
1, Bocavirus |
|
|
2, Adenovirus |
2, Adenovirus |
|
|
4, Coronavirus |
1, Coronavirus |
|
|
2, Metapneumovirus |
3, Metapneumovirus |
|
|
1, HPIV |
2, HPIV |
|
|
Viral coinfection n. (%) |
8 (13%) |
5 (5%) |
|
Bacterial Pathogens (n., type) |
1, Moraxella |
3, Haemphilus Influentiae |
|
3, Haemophilus Influentiae |
5, Streptococcus Pneumoniae |
|
|
4, Streptococcus Pneumoniae |
2, Staphilococcus Aureus |
|
|
2, Staphilococcus Aureus |
2, Bordetella Pertussis |
|
|
1, Klebsiella Pneumoniae |
||
|
Bacterial coinfection n. (%) |
7 (12%) |
10 (16%) |
Table 1: Baseline features of patients: age, gestational age at birth in weeks (w), weight in kilograms (kg), bronchiolitis severity score (BSS), viral pathogens isolated (including viral coinfections), bacterial coinfections, High Flow Nasal Cannula (HFNC) support in the 72 hours prior to pediatric intensive care unit (PICU) admission and Pediatric Index Of Mortality 3 (PIM3) score. Data are expressed as medians (25th-75th quartiles) or as absolute numbers (percentage). RSV: respiratory syncytial virus, HPIV: human parainfluenza virus.
Patients were admitted to the PICU after a median time of 1 (0-3) days after hospital admission with no difference between the two groups (p=0.88). HCPAP was started after 13 (6-18) hours in the P5 group and 11 (7-15) in the P10 group (p=0.24). Patients were treated with HCPAP for a median time of 3 days (1-5) and there was no difference between groups (p=0.15). Pathogens responsible of respiratory insufficiency in the two groups are listed in Table 1.
All patients survived to PICU discharge.
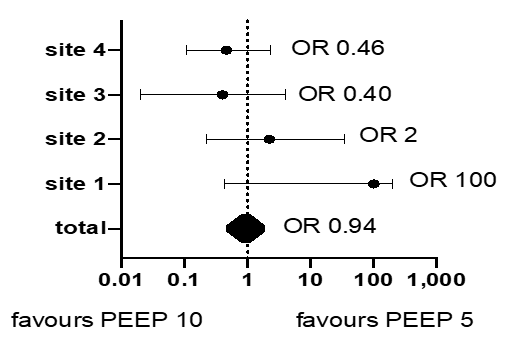
The overall intubation rate of study population was 18/124 (14.5%). The intubation rates were 9/60 (15%) and 9/64 (14%) in P5 and P10 groups, respectively (OR 0.94, 95% CI 0.36 to 2.46, p=0.99). Of the patients in the P5 group, 47 (78%) were escalated to a PEEP level of 10 cmH2O. Respiratory symptoms were successfully stabilized in 38 of them (81%), while the remaining 9 “escalated” patients were ultimately intubated. In this group, 13 patients who did not require escalation nor intubation. No patient in the P10 group needed de-escalation to a PEEP level of 5 for intolerance. PEEP level was not associated to intubation rate (OR 0.69, 95% CI 0.19 to 2.40, p=0.57), after adjustment for age, gestational age, HFNC application, BSS and PIM3. The intubation rate was heterogeneous across sites (i.e., 37%, 14%, 13.6 % and 3.7%, respectively, among 27, 25, 22 and 50 patients) (Figure 2). However, the initial PEEP level was not significantly associated with intubation in any center. Length of mechanical ventilation was 7 days (5-10) with no difference between groups (p=0.51).
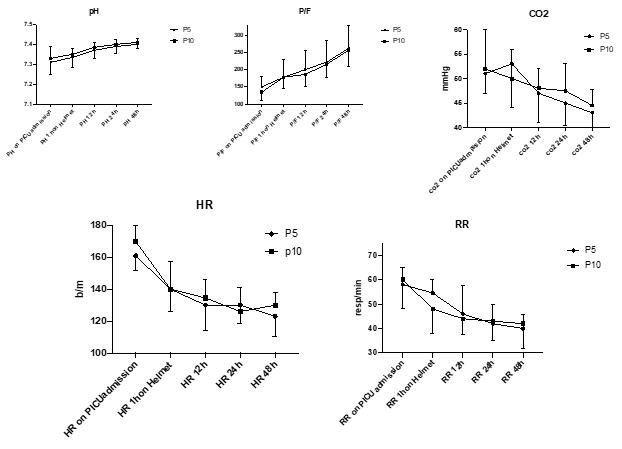
None of the clinical or respiratory parameters differed significantly between groups; however, they all showed a significant improvement over time (p<0.001 in all cases) (Figure 3).
No cases of pneumothorax were described during the study, and the impact of HCPAP on enteral feeding was not significant (2/60 in P5 and 0/64 in P10 group, OR 0.00, 95% CI 0.0- 2.0, p=0.23). Length of PICU stay was similar for both groups, 7 days (5-10) for the P5 group and 7 days (5-8.7) for the P10 group (p=0.94). However, both intubation (OR 1.9; 95% CI 1.5-2.7; p=0.0001) and PEEP escalation (OR 1.27, 95% CI 1.22-1.47; p=0.0003) were associated with PICU length of stay.
4. Discussion
Critically ill newborns and infants, admitted to the PICU for severe bronchiolitis, are typically affected by severe hypoxemic respiratory failure, generally refractory to HFNC oxygen administration [17,18]. Even if invasive mechanical ventilation can be considered the most aggressive approach for the severe cases [19], NIV support has been repeatedly proposed in order to assist such patients and to reduce intubation rate [20,21]. The Italian Network of Pediatric Intensive Care Units (TIPNET) reported a national intubation rate of 15% among critically ill children with bronchiolitis in 2010-2016 [22], regardless which NIV interface was applied16. These findings were confirmed by the present study. Nevertheless, there is still a lack of multicenter, prospective, and randomized studies to determine the most effective and well-tolerated type of non-invasive support for these patients. Furthermore, among many studies on NIV in children with bronchiolitis admitted to the PICU, information about which optimal inspiratory and expiratory pressure levels should be administered is currently lacking [23]. Finally, HCPAP treatment in children with respiratory failure has rarely been evaluated, potentially due to the fact that few centers are specifically skilled at this technique and routinely apply it [24].
Although this study was interrupted due to organizational issues further complicated by the Coronavirus Disease 2019 pandemic, results carry several implications. We found that during HCPAP a PEEP level of 10 is not likely to reduce intubation rates compared to PEEP 5. However, children with respiratory insufficiency due to severe bronchiolitis in the PICU seem to require intubation when bacterial coinfection worsens respiratory insufficiency. These patients likely represent a subgroup of particularly ill subjects who may be resistant to CPAP support. The theoretical premise of this study was that the use of a PEEP level of 5 cmH2O might be ineffective as a respiratory strategy in comparison to a level of 10 cmH2O. As a matter of fact, PEEP escalation proved effective in 38 over 47 P5 patients (75%) and clinicians reported improvement of symptoms in all such cases. The remaining 9 patients who did not show a benefit from escalation were all eventually intubated. These patients may be represented by a subgroup of particularly severe cases and were interestingly a very similar number then the P10 group, mostly being bacterial coinfections. There are many potential explanations for this finding. Positive pressure during HCPAP is delivered through a high flow of an air/oxygen mixture and a mechanical spring valve and without the use of the ventilator. Furthermore, the plastics of the helmet have relatively high compliance that, in part, explain the high tolerance of this method [25]. With these premises, the system may not accurately deliver the exact pressure level that is set with the valve. Therefore, it is possible that a setting of 5 cmH2O does not confer enough pressure to effectively recruit distal bronchioles. The rapid relief provided by an adequate HCPAP setting is due to the resolution of ‘air trapping’, after provision of a positive pressure level equivalent to intrinsic PEEP. Oxygenation, CO2 levels and respiratory efforts rapidly improve; hence, the weakening of respiratory muscles and patients’ exhaustion are avoided. Since the clinicians optimized the respiratory parameters of the two groups in this study, it is not surprising that, over time, collected clinical variables were not different.
Another point that is worth remarking about our partial results is that despite being insufficient in most patients with a failure rate of 78%, an initial PEEP of 5 was not detrimental in the sense that it did not lead to a higher intubation rate. In other words, this study suggests that although it is likely not enough support, starting with a low PEEP and titrating the support may be at least not harmful.
This study also confirmed that HCPAP, in general, and a PEEP level of 10 cmH2O, in particular, are not associated to pneumothorax. Furthermore, enteral feeding intolerance was low. The efficacy of a PEEP above 6 cmH2O to manage acute respiratory failure has been previously described 26, and its use in the pediatric population has been also confirmed [27]. However, several concerns have been expressed regarding side effects of high PEEP on smaller children; our study provides a significant contribution against them. It can be speculated that a lower level of PEEP during HCPAP could be delivered to non-critical bronchiolitis children in pediatric wards or emergency rooms in a timely manner and that ‘escalated therapy’ could be reserved for cases of clinical worsening [28]. Further studies exploring this strategy are warranted to explore this strategy in order to improve the treatment of these patients and anticipate the ventilatory support [28].
5. Limitations
This study has some limitations to acknowledge. The lower sample size than initially planned may have caused a beta error (absence of significance in the intubation rate due to a low number of patients). Failure of therapy with a PEEP of 5 was extremely high (78%) and it is possible that further continuation of the study might have required an interim analysis to discuss eventual amendments. Furthermore, even if not pre-designed by our protocol, we were not able to identify any specific subgroup of patients who might have benefit by different PEEP regimes. However, it must be remarked that patients with respiratory insufficiency due to bronchiolitis have frequently very similar clinical characteristics and those with bacterial coinfection be well represent a subgroup of more severe patients, refractory to CPAP approach. This study is also lacking a third “NIV arm” (applying a different interface, i.e., face mask) that may be a potentially interesting comparator, especially in most severe cases. However, the initial intention of this study was to apply helmet in all bronchiolitis. HCPAP support requires a learning curve for optimization of the effectiveness. In this study, we recruited centers with proven and skilled experience in HCPAP treatment for bronchiolitis, thus limiting the external validity of the study. It is also likely that this contributed to the early interruption of the trial, given the unexpectedly low enrollment rate with respect to the planned schedule.
Furthermore, the intubation rate was heterogeneous across study sites, likely representing the challenge of the non-invasive respiratory management of these patients and the potential differences in HCPAP setup. However, in all centers, the primary outcome was not associated with initial PEEP level. Another potential weakness of the study was the absence of control regarding the timing of admission to PICU for bronchiolitis; this may vary significantly among different centers due to organizational aspects and availability of PICU beds. However, the ideal timing for PICU transfer is currently unknown, this did not appear to be different in the two groups and even if proactive admission to a PICU may affect the outcomes, this has not yet been proven. Furthermore, among the inclusion criteria, the maximum duration of 72 hours of HFNC therapy partially limited this weakness. Finally, bacterial coinfection was diagnosed through a nasal swab, which do not allow a definitive diagnosis of actual lung infection.
6. Conclusions
In this trial on infants with severe bronchiolitis, a PEEP level of 10 cm H2O during HCPAP in comparison to an initial level of 5 cm H2O did not show to prevent intubation. These results are not conclusive due to the premature stopping.
Acknowledgments
We sincerely thank all PICU nurses for their passionate care of our patients and active support in this trial.
Conflict of interests
The authors have no conflicts of interest to disclose.
Competing interests:
The authors declare that they have no competing interests.
Funding:
No external funds were required.
References
- Ingelfinger Viral Bronchiolitis in Children. N Engl J Med 374 (2016): 62-72.
- Ralston SL, Lieberthal AS, Meissner HC, et al. American Academy of Pediatric et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics134 (2014): e1474-1502.
- Bem AR, Bont LJ, van Woensel JBM. Life-threatening bronchiolitis in children: eight decades of critical care. Lancet Respir Med 8 (2020): 142-144.
- Lieberthal AS, Bauchner H, Hall CB, et al. Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and Management of Bronchiolitis. Pediatrics 118 (2006): 1774-1793.
- Schlapbach LJ, Straney L, Gelbart B, et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur Respir J49 (2017): 1601648.
- Mecklin M, Heikkilä P, Korppi M. The change in management of bronchiolitis in the intensive care unit between 2000 and 2015. Eur J Pediatr177 (2018): 1131-1137.
- Hasegawa K, Pate BM, Mansbach JM, et al. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad Pediatr 15 (2015): 77-81.
- Donlan M, Fontela SF, Puligandla PS: Use of Continuous Positive Airway Pressure (CPAP) in acute viral bronchiolitis: a systematic review. Pediatr Pulmonol 46 (2011): 736-746.
- Habra B, Janahi IA, Dauleh H, et al. A comparison between high-flow nasal cannula and noninvasive ventilation in the management of infants and young children with acute bronchiolitis in the PICU. Pediatr Pulmonol 55 (2020): 455-461.
- Clayton JA, McKee B, Slain KN, et al. Outcomes of Children With Bronchiolitis Treated With High-Flow Nasal Cannula or Noninvasive Positive Pressure Ventilation. Pediatr Crit Care Med 20 (2019): 128-135.
- Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr 105 (2016): e368-72.
- Mayfield S, Bogossian F, O’Malley L, et al. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health 50 (2014): 373-378.
- Pierce HC, Mansbach JM, Fisher ES, et al. Variability of intensive care management for children with bronchiolitis. Hosp Pediatr 5 (2015): 175-184.
- Essouri S, Baudin F, Chevret L, et al. Variability of Care in Infants with Severe Bronchiolitis: Less-Invasive Respiratory Management Leads to Similar Outcomes. J Pediatr 188 (2017): 156-162.
- Diana M Duarte-Dorado, Danitza S Madero-Orostegui, Carlos E Rodriguez-Martinez, et al. Validation of a scale to assess the severity of bronchiolitis in a population of hospitalized infants. J Asthma 50 (2013): 1056-1061.
- Rossetti E, De Galasso L, Appierto L, et al. Retrospective study found that helmet continuous positive airway pressure provided effective support for severe bronchiolitis. Acta Paediatrica 109 (2020): 2671-2673.
- Trevisanuto D, Camiletti L, Doglioni N: Noise exposure is increased with neonatal helmet CPAP in comparison with conventional nasal CPAP. Acta Anaesthesiol Scand 55 (2011): 35-38.
- Milési C, Essouri S, Pouyau R, et al. High Flow Nasal Cannula (HFNC) versus nasal Continuous Positive Airway Pressure (nCPAP) for the initial respiratory management of acute viral bronchiolitis in young infants: a multicenter randomized controlled trial (TRAMONTANE study). Intensive Care Med 43 (2017): 209-216.
- De Luca D, Piastra m, Chidini G, et al. The use of the Berlin definition for acute respiratory distress syndrome during infancy and early childhood: multicenter evaluation and expert consensus. Intensive Care Med 39 (2013): 2083-2091.
- Ganu SS, Gautam A, Wilkins B, et al. Increase in use of non-invasive ventilation for infants with severe bronchiolitis is associated with decline in intubation rates over a decade. Intensive Care Med 38 (2012): 1177-1183.
- Milési C, Ferragu F, Jaber S, et al. Continuous positive airway pressure ventilation with helmet in infants under 1 year. Intensive Care Med 36 (2010): 1592-1596.
- http://tipnet.cineca.it/report/TIPNET-Report 2010-2014.pdf
- Combret Y, Prieur G, le Roux P, et al. Non-invasive ventilation improves respiratory distress in children with acute viral bronchiolitis: a sistematic review. Minerva Anest 83 (2017): 624-637.
- Vitaliti G, Vitaliti MC, Finocchiaro MC, et al. Randomized comparison of Helmet continuous positive airway pressure versus high flow nasal cannula oxygen in pediatric respiratory distress. Respir Care 62 (2017): 1036-1042.
- Chidini G, Piastra M, Marchesi T, et al. Continuous positive airway pressure with helmet versus mask in infants with bronchiolitis: a RCT. Pediatrics 135 (2015): e868-875.
- Patroniti N, Foti G, Manfio A, et al. Head helmet versus face mask for non-invasive continuous positive airway pressure: a physiological study. Intensive Care Med 29 (2003): 1680-1687.
- Essouri S, Durand P, Chevret L, et al. Optimal level of nasal continuous positive airway pressure in severe viral bronchiolitis. Intensive Care Med 37 (2011): 2002-2007.
- Heikkilä P, Forma L, Korppi M: Hospitalisation costs for infant bronchiolitis are up to 20 times higher if intensive care is needed. Acta Paediatr 04 (2015): 269-273.
- Franklin D, Fraser JF, Schibler A: Respiratory support for infants with bronchiolitis, a narrative review of the literature. Paediatr Respir Rev 30 (2019): 16-24.
Supplementary Table 1:
|
STANDARD TREATM ENT PROTOCOL FOR SEVERE BRONCHIOLITIS ADMITTED IN PICU |
|
- Registration of the modified Wood’s Clinical Asthma score mWCAS (BSS) |
|
- Recruitment and randomization to 5/10 cmH2O PEEP level, 50 L/min gas flow, FiO2 0.5 on PICU admission |
|
- Clarithromycine prophylaxis for newborns (up to 30 days old), or I generation cephalosporin for |
|
older infants, if high fever, lung opacities or high inflammation markers occurs (PCR, procalcitonin, white body cells) |
|
- Volume replacement (20 ml/kg of saline solution NaCl 0.9% or albumine 5%) in 60 minute |
|
- Morphine ev bolus: 20 mcg/kg in 2 min and following 5 mcg/kg/h infusion |
|
- Nasogastric tube placement and early enteral feeding 4-6 hours after admission (5-10 ml/h) |
|
- Dexamethasone 0.2 mg/kg x 3/day ev |
|
- Proton pump inhibitors |
|
- Aerosol therapy 4 time/day: NaCl 3% (or NaCl 0.9%) 2 ml with ipratropium bromide |
|
- ABE before HCPAP application and in the following 1, 12, 24, a nd 48 hours |
|
- Pharingeal swab for molecular analysis to detect viral DNA |
|
- Occipital and neck skin protection to avoid Helmet CPAP pressure sores |
|
- Each Helmet will be provided of a filter to reduce noise inside it |
|
- Helmet gas will flow through heat-moisture device switched on 5 min/hour |