Polypoid Endometriosis in A Postmenopausal Woman Treated With Tamoxifen Due to DCIS: A Case Report And Review of the Literature
Article Information
Schwab Roxana1, Boutas Ioannis1,5*, Dappa Evelyn2, Kohlwes Elke3, Bossart Michaela4, Woll Joerg4, Potiris Anastasios5, Künzel Klaudija1, Hasenburg Annette1
1Department of Gynecology, Johannes Gutenberg University Medical Center, Mainz, Germany.
2Department of Diagnostic and Interventional Radiology, Universitätsmedizin Mainz, Mainz, Germany.
3Institute of Pathology, University of Mainz, Mainz, Germany.
4Department of Obstetrics and Gynaecology, Freiburg Medical School, University of Freiburg, Freiburg, Germany.
5Third Department of Obstetrics and Gynaecology, National and Kapodistrian University of Athens, Attikon University Hospital, Athens, Greece.
*Corresponding author: Ioannis Boutas, Department of Gynecology, Johannes Gutenberg University Medical Center, Mainz, Germany
Received: 23 August 2019; Accepted: 05 September 2019; Published: 18 June 2020
Schwab Roxana, Boutas Ioannis, Dappa Evelyn, Kohlwes Elke, Bossart Michaela, Woll Joerg, Potiris Anastasios, Künzel Klaudija, Hasenburg Annette. Polypoid Endometriosis in A Postmenopausal Woman Treated With Tamoxifen Due to DCIS: A Case Report And Review of the Literature. Archives of Clinical and Biomedical Research 4 (2020): 239-253.
Citation: Schwab Roxana, Boutas Ioannis, Dappa Evelyn, Kohlwes Elke, Bossart Michaela, Woll Joerg, Potiris Anastasios, Künzel Klaudija, Hasenburg Annette. Polypoid Endometriosis in A Postmenopausal Woman Treated With Tamoxifen Due to DCIS: A Case Report And Review of the Literature. Archives of Clinical and Biomedical Research 4 (2020): 239-253.
View / Download Pdf Share at FacebookAbstract
Patient: We present a case of polypoid endometriosis in a postmenopausal 58 years old patient presenting with a pelvic retroperitoneal nodule mimicking a neoplasm. Additionally, the patient reported uterine bleeding three years after starting tamoxifen for ductal carcinoma in situ (DCIS) of the breast. Preoperative magnetic resonance imaging suggested the nodule as an endometriotic lesion.
Interventions: Imaging results described a combined solid and liquid adnexal mass close to the right ovary. At laparoscopy, a 5-centimeter large parauterine retroperitoneal mass was identified medial to the right ureter. The suspected mass was completely resected and a subsequent hysterectomy was performed because of recurrent postmenopausal bleeding.
Main outcome measures: Exclusion of malignancy (and resolution of postmenopausal bleeding).
Results: Pathologic diagnosis revealed polypoid endometriosis and a uterine polyp. Conclusion: Polypoid endometriosis is a very rare disease but should be taken into account in postmenopausal women with use of tamoxifen or hormonal therapy. This case emphasizes the importance of broad differential diagnosis for an adnexal mass in the menopause. To our knowledge this is the first case of polypoid endometriosis in a postmenopausal woman treated with Tamoxifen for DCIS.
Keywords
Polypoid endometriosis; Postmenopause; Tamoxifen; DCIS
Article Details
Introduction
Endometriosis is a chronic inflammatory disease that affects approximately 10% of fertile women [1], up to 50% of women with infertility problems and up to 70% of women with chronic pelvic pain [2]. It is caused by the growth of ectopic endometrial tissue outside the uterine cavity. To date, three clinical distinct phenotypes of endometriosis of the pelvis are recognized: ovarian cysts (endometrioma), peritoneal implants of endometriotic lesions and deep infiltrating endometriosis [1]. Deep infiltrating endometriosis is defined by fibrous infiltration of endometriotic tissue into organs or anatomical structures [3]. The primary localization is the pelvis, extrapelvic lesions are extremely rare. The pathophysiology of endometriosis remains controversial, although three distinct mechanisms are widely accepted: the Sampsons theory of backflow menstruation, the coeleomic-metaplasia hypothesis and the venous and/or lymphatic metastatic dissemination theory [1]. Current concepts of therapy imply surgical resection and/or endocrine treatment creating a hypo-estrogenic environment [2]. The choice of treatment should respect current symptoms and individual patient preferences. Therapeutic goals are dependent on the location of the disease.
The incidence of endometriosis in postmenopausal women is estimated to be 2-4 % of all patients with endometriosis [4-7]. Delay of diagnosis is typical in these patients and may be more accentuated in postmenopausal women, because of rarity of the disorder in this specific age group. Due to its nature as an estrogen dependent disease, endometriosis usually resolves in menopause because of decreasing hormone levels. However, symptoms persist in some women. Moreover, some women experience recurrent disease due to estrogen biosynthesis in peripheral tissue by aromatization of androgens [1, 5], due to hormonal treatment of climacteric symptoms with unopposed estrogens [5, 8, 9] or, exceptionally rare, as a consequence of tamoxifen treatment [10].
Polypoid endometriosis is a distinct form of endometriosis and was first defined by Mostoufizadeh and Scully as ectopic endometrial tissue, which histologically resembles an endometrial polyp [11]. Polypoid endometriosis, especially occurring in postmenopausal women, can mimic neoplasia and must be differentiated from pathologies like adenofibroma, adenosarcoma, rectal cancer or disseminated ovarian cancer [12, 13]. Polypoid endometriosis should be considered in postmenopausal women treated with tamoxifen [14].
Tamoxifen is a nonsteroidal triphenylene compound with dual action on estrogen receptors and belongs therefore to the group of selective estrogen receptor modulators (SERM). It exerts antagonistic estrogen effects in breast tissue and is used as adjuvant treatment in women with breast cancer but also in a preventive manner in women with ductal carcinoma in situ (DCIS) of the breast. Tamoxifen reduced the incidence of invasive breast cancer and of carcinoma in situ of the breast by 49% and 50%, respectively [15]. In contrast, in the postmenopausal period, tamoxifen can exert agonist effects onto the female genital tract due to low serum estradiol levels [16]. Side effects induced by tamoxifen are based on its partial agonist actions on the uterus, respectively on the endometrium: endometrial hyperplasia, endometrial polyps, endometrial carcinoma, endometriosis, adenomyosis and cervical polyps [10, 17, 18]. Occurrence of estrogen-related disease in postmenopausal women taking tamoxifen suggests a causal relationship between the drug and the disease.
Case report
A 58-year-old woman presented to our hospital with a history of postmenopausal bleeding. She was third gravida and third para. She entered the menopause 5 years ago. A pre-existing essential hypertension was managed by oral medication. On examination she was generally well with normal blood pressure and pulse rate.
Past medical history was significant for DCIS (ductal carcinoma in situ) of the left breast three years earlier. She subsequently underwent conservative surgery of the left breast followed by radiotherapy of the breast with a total dose of 50.4 Gy. She was following the medical advice to complete tamoxifen treatment for 5 years at a dose of 20 mg daily and until presentation to our hospital she remained well without relapse or progression to invasive breast cancer. Besides the postmenopausal bleeding, she reported no major side effects in respect to Tamoxifen.
She denied any history of dysmenorrhea, dyspareunia, dyschezia or dysuria during her fertile years. She reported no other current changes to her baseline health: no abdominal bloating, increase in size and no abdominal pain. Normal appetite and no feeling of fullness were reported.
The patient´s body mass index was 23.0 kg/m2 at presentation. She had an up-to-date cervical cancer screening showing no cytologic alterations. Full blood count was within normal range. The bimanual examination revealed an adnexal nodule lateral to the uterus.
A transvaginal ultrasound examination of the pelvis reveled an anteflexed uterus with a width of 51 mm and an endometrial width of 25 mm. The endometrium was thickened and a hyperechogenic tissue with cystic enlargements in the uterine cavity was suggestive of Tamoxifen-associated endometrial hyperplasia or of an endometrial polyp. The ovaries appeared normal and were approximately 20x20 mm in diameter, bilaterally. A unilocular 41x36 mm nodular, partly hypoechoic, partly isoechoic thickening was located in the right lateral compartment, adjacent to the uterus and the right lateral pelvic wall. The nodule was partly solid, partly of liquid appearance and of honeycomb-like inner structure and with smooth, irregular contours. The proportion of solid tissue was 80%. No septa were identified within the cysts on sonographic imaging. The nodule showed no mobility by ultrasound examination when assessed in respect to the uterus and to the pelvic wall. Color flow mapping revealed a vast prefusion of the tumor and Doppler flow showed a rich vascularization, both arterial and venous, within the adnexal mass increasing the suspicion of malignancy. No peritoneal nodular implants or ascites were detected. A transabdominal kidneys scan was normal.
She underwent an additional examination by MRI. A large circumscribed, bilobulated mass within the pouch of Douglas was detected, adjacent to the posterior uterine contour and the right pelvic wall (max. 3.7 x 5 x cm). On T2-weighted sequences, the mass showed a hyperintense signal intensity with multiple cystic lesions, most likely representing ectopic endometrial glands. On T1-weighted sequences, the largest lesion showed an intratumoral, hyperintense cyst, indicative of hemorrhagic or proteinaceous content. After intravenous application of contrast medium, the mass showed vivid enhancement with “swiss cheese” appearance due to lack of enhancement of dilated cystic glands.
The endometrium was thickened and showed a heterogeneous, hyperintense signal intensity on T2-weighted images with multiple intratumoral cysts, suggestive of endometrial hyperplasia or an endometrial polyp (Figure 2A). Furthermore, multiple uterine leiomyomas were detected, which showed characteristic low signal intensity on T2-weighted images.
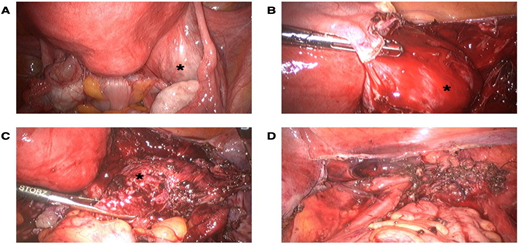
Considering the history of postmenopausal bleeding, the presence of a uterine mass and a suspicious right adnexal mass, a laparoscopy with hysterectomy and bilateral salpingo-oophorectomy was consented with the patient. At laparoscopy, a distorted anatomy of the pelvic organs with severe pelvic adhesions and a complete obstruction of the pouch of Douglas were detected, which justified the diagnosis of frozen pelvis. On gross examination, both ovaries looked normal. A retroperitoneal mass adjacent right to the uterus was detected (Figure 1A). No ascites was seen and there was no evidence of intraabdominal metastatic disease. The pelvic adhesions were lysed. The suspected retroperitoneal mass was removed and a preexisting cyst within retroperitoneal lesion spontaneously teared during excision revealing a dark brown content, corresponding to a chocolate cyst (Figures 1B and 1C). The hysterectomy and bilateral salpingo-oophorectomy were performed (Figure 1D).
Figure 1: Intraoperative view of the pelvis. (A) Intraoperative view of the protruding retroperitoneal mass (asterisk) and severe pelvic adhesions (black arrow), (B) Intraoperative view of the retroperitoneal mass after incision of the peritoneum (asterisk), (C) Intraoperative view of the retroperitoneal mass before removing the mass showed dark brown content outpouring the mass (asterisk) and (D) Intraoperative situs after laparoscopic hysterectomy with bilateral salpingo-oophorectomy and removal of all endometriotic lesions
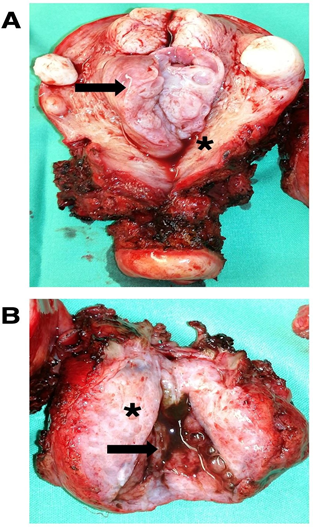
On cross examination, the uterine cavity showed an endometrial polyp (Figure 2A). The removed retroperitoneal mass was 55x40x40 mm and showed variable shades of white-grey and was partly cystic (Figure 2B). The largest cyst contained “chocolate- colored” fluid on sectioning (Figure 2B).
Figure 2: (A) Macroscopic view of the removed uterus with an endometrium polyp and several myoma. Endometrial polyp (black arrow), atrophic endometrium (asterisk) and (B) Macroscopic view of the removed retroperitoneal mass. White-grey, partly cystic gross section through the retroperitoneal mass (aterisk) and a chocolate colored containing cysts within the removed retroperitoneal mass (black arrow).
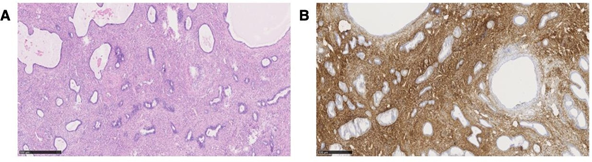
Histological examination revealed an atrophic endometrium with a width of 0.2 cm, an endometrial polyp of 35x25x15 mm and adenomyosis uteri besides uterine myoma. The microscopic sections of formalin- fixed paraffin-embedded tissue of the retroperitoneal lesion showed after hematoxylin and eosin (H&E) staining a biphasic proliferation with both benign appearing endometrial cells with highly prismatic morphology and benign appearing, focally fibrous, partly edematous and partly spindle-shaped endometriotic stroma (Figure 3A). Thick-walled blood vessels were embedded within the stroma.
Immunohistochemical analysis showed CD10 expression but no expression of inhibin, SMA or WT1 (Figure 3B). The retroperitoneal lesion was diagnosed as polypoid endometriosis. Other endometriotic lesions were diagnosed in the pouch of Douglas, on the dorsal uterine wall and adjacent to the rectum wall. The final histopathological diagnosis was polypoid endometriosis in the pelvis besides adenomyosis of the uterus, myoma and an endometrium polyp.
Currently, the patient is doing well, at ultrasound examination no recurrent disease was detected.
Figure 3: (A) Microscopic view of the removed parauterine mass H&E staining reveal blue staining of the ectopic endometrial glands and reddish staining of the adjant stroma and (B) CD 10 Immunochemistry of the removed parauterine mass Confirmation of the diagnosis by brown staining of the ectopic endometrial stroma but no staining of the endometrial glands
Discussion
Incidence of endometriosis in the menopause is extremely rare and is estimated by 2-4% [4-7] as endometriosis normally is suppressed by the low estrogen state of menopause. It is unclear if endometriosis diagnosed in the menopause is a de novo development of endometriotic lesions, an activation of already preexisting endometriotic foci or a continuation of an already preexisting disease [5]. The series of Morotti et al. described in only 15.3% of cases of endometriosis in menopause a preexisting endometriosis [19].
Conditions or medication leading to increased estrogen levels can trigger or reactivate dormant endometriotic lesions. Our patient was of normal weight, an estrogen excess due to aromatization in peripheral fat tissue was unlikely. However, in postmenopausal women with a history of endometriosis or in women taking tamoxifen as in this case, recurrence of endometriosis should be taken into account. Due to various different symptoms or even lack of symptoms, the clinical diagnosis of endometriosis might be a great challenge. It is assumed that about 10 % of all women have asymptomatic endometriosis [20]. Our patient reported no symptoms related to endometriosis during her fertile life span. Dysmenorrhea, as leading symptom of endometriosis, is absent in menopausal women. Other symptoms, as dyspareunia, may be misled as a consequence of age- related alterations of the urogenital tract. Therefore, in menopause, active endometriosis is often diagnosed because of severe symptoms as postrenal failure or as incidental finding in surgery to exclude malignancy [10], as described in the current case.
Hormone replacement may increase the risk of recurrence of endometriosis in postmenopausal women [5]. Matorras et al. showed increased risk of recurrence in women with bilateral salpingo-ovarectomy and hormone replacement with transdermal estrogen and micronized oral progesterone compared to no hormone replacement of 1% versus 0% recurrence risk per year [21]. In women with preexisting endometriosis and definite surgery (hysterectomy and bilateral salpingo- oophorectomy) the recurrence rate of endometriosis was 2% in women when taking unopposed estrogens [9]. In contrast, tibolone showed a decreased recurrence risk compared to transdermal estrogen and cyclic medroxyprogesterone acetate [8].
The pathophysiological mechanisms leading to endometriosis are not fully understood. Endometriosis is an estrogen dependent and estrogen driven disease [1]. In women of fertile age, estrogen is mainly a product of ovarian biosynthesis and promotes growth of endometriotic lesions through systemic effects [1]. On the other hand, estrogen released with the follicular fluid during ovulation can locally stimulate the peritoneal implants, whereas in postmenopausal women estrogen is mainly synthesized in peripheral tissue (adipose tissue or skin) by aromatization of steroids produced within the adrenal glands [1, 5]. Estron, the leading postmenopausal estrogen can be converted within the endometriotic lesion via 17- β hydroxylase into estradiol, which for its part is not metabolized into its degradation products due to lack of 17- β hydroxyl steroid dehydrogenase type 2 [5]. Additionally, local estrogen biosynthesis in endometrial stromal cells may support growth of the lesions and sustains its own synthesis by inducing the expression of local aromatase by binding to ER-β (estrogen receptor β) [1, 22] in both women of fertile age and women in menopause. Estrogen acts through ER-β and complementary through VEGF (vascular endothelial growth factor) and to IL-1β (interleukin 1 β) on induction of COX-2 (cyclooxygenase-2) expression and PGE2 (prostaglandin E2) synthesis, which mediates inflammation and pain generation on one hand, on the other, it leads to a vicious circle, which promotes its one local synthesis [1, 22] and thus proliferation and long- term survival of ectopic endometrial tissue. Additionally, due to epigenetic changes in endometriotic tissue, ER-β levels are 142 times the levels in normal endometrium and lead to suppression of ER-α (estrogen receptor α) and to progesterone resistance [1]. Another crucial step in the establishment of endometriosis lesions is characterized by neoangiogenesis, thus endometriosis is generally referred to as benign cancer [23]. VEGF is expressed in an estrogen dependent manner in the epithelium of endometrial implants and in activated peritoneal macrophages and neutrophils [24]. There is a direct correlation between VEGF expression, microvessel density and proliferative activity of endometriotic lesions [25].
Tamoxifen binds to the estrogen receptor and forms a complex, which binds irreversibly to DNA of the target cells, representing the classical pathway of gene expression mediated by estrogen receptor. In tissue, in which tamoxifen exerts agonist action, like endometrial derived tissue [26], DNA binding of the tamoxifen- estrogen receptor complex leads to increased synthesis of estrogen receptor, leading to locally increased tamoxifen action due to high levels of binding substrate [27]. Moreover, tamoxifen exerts its tissue specific estrogen antagonist or agonist effects dependent on the promoter of the specific genes and the particular intracellular context [28]. The concentration and availability of coactivators of gene transcription, like the steroid receptor coactivator (SRC-1), influence the level of impact tamoxifen exerts to a specific tissue [29]. Shang and Brown described a second pathway of tamoxifen driven gene transcription: transcription activation in an endometrial cancer cell line was stimulated by tamoxifen in spite of lack of estrogen response elements within the gene promoter of specific genes like c-Myc and IGF-1 (insulin growth receptor 1), both genes responsible for cellular proliferation and gene expression [29]. Taken together, these two pathways may explain the effect of tamoxifen on endometriotic lesions in some patients, like in this case. Our patient had no previous history of endometriosis, but it seems probable, due to the extent of intraabdominal adhesions and endometriotic lesions, that tamoxifen had stimulated pre-existing enometriotic foci.
Polypoid endometriosis was first defined by Mostoufizadeh and Scully as ectopic endometrial tissue, which histologically resembles an endometrial polyp [11]. Nonetheless, in the series published by Parker et al., polypoid endometriosis resembled in almost 50% of cases an intrauterine endometrial polyp [12]. The involved organs were ovaries, adnexal soft tissue, uterine serosa, cervix, vagina, ureter and omentum [12]. Because of the rarity of this condition, other cases of polypoid endometriosis might have been misinterpreted.
The polypoid endometriosis could mimic malignancy on intraoperative evaluation and histological examination or on preoperative imaging, as in the presented case [12]. Histological examination of polypoid endometriotic lesion can be difficult and demanding as up to 50% of all cases were misinterpreted as low-grade or well-differentiated neoplasm in the first place and proliferative benign-like, endometroid-type glands were seen in polypoid endometriosis as well as in adenofibroma and in adenosarcoma [12]. The series of cases described by Parker et al. included women aged 23 to 78 years and lesions, which ranged from 0,4 to 14cm in maximal size [12]. 60% of them were postmenopausal, in contrast to typical endometriosis, arising almost always in premenopausal women [12]. Polypoid endometriosis was associated with typical endometriosis in 75% of cases and a personal medical history of endometriosis was reported in 29% of cases [12]. Multilocular presentation of polypoid endometriosis was reported in up to 39% of cases [12]. The manifestation of the disease in our patient suits the previous reported cases, as the dimensions of the lesion was 4cm, the histopathological diagnosis revealed multilocular presentation and was associated with typical endometriotic lesions.
Association of polypoid endometriosis with endocrine therapy was described in 4 cases in the series of Parker et al.: one patient was diagnosed during tamoxifen therapy, one patient developed polypoid endometriosis after GnRH agonist discontinuation and two patients were using unopposed estrogen [12]. Two other cases regarding the association of polypoid endometriosis with tamoxifen were described by Choi et al. and Kraft et al. Both patients had a previous history of breast cancer [30, 31]. One premenopausal woman with a history of DCIS and tamoxifen treatment as breast cancer prevention and subsequent endometriosis exacerbation was described in the literature. To our knowledge, the current case report of polypoid endometriosis arising in a postmenopausal woman with a history of DCIS and tamoxifen intake as preventive therapy is the first reported in literature.
Polypoid endometriosis is a benign disease with potential to malignant transformation. The malignant transformation seems to be a function of age and hormone effect. To date, clear cell carcinoma of the ovary, the most frequent malignancy related to endometriosis, as 70% of patients had concurrent histological findings [32], has not been associated to polypoid endometriosis [12]. Malignant transformation was described in less than 10% of cases in the series described by Parker et al. and included an endocervical mucinous borderline tumor and a complex atypical hyperplasia with grade 1 endometroid adenocarcinoma within an ovarian endometriotic cyst, while metaplastic epithelia were found in 75% of cases [12]. Takeuchi reported a case of a 52 years old perimenopausal women with no history of endocrine therapy with histological diagnosis of atypical endometrial hyperplasia and malignant transformation of polypoid endometriosis to well-differentiated endometroid carcinoma of a nodule located posterior to the uterus [33].
In contrast, malignant transformation of typical endometriotic lesions during tamoxifen therapy has been reported more often. There is substantial evidence, that benign or malignant transformation of endometroid foci can occur similar to transformation of eutrophic endometrium, regardless of affected anatomical structure, and is associated with endocrine therapy and age of the patient [12, 34]. Duration of tamoxifen treatment until malignant transformation was one to four years with gradual transition of the endometriotic lesion from typical to atypical endometriosis into neoplasia [18, 35]. There are several case reports describing malignant transformation related to endometriotic lesions and tamoxifen intake. Cohen at al. reported a case of a postmenopausal woman with a history of invasive breast cancer and tamoxifen therapy who showed serial plasma 17 β estradiol levels within normal range of menopause (<20pg/ml) and who developed an endometroid ovarian cancer within foci of ovarian endometriosis [34]. Okugawa described a case of a 67-year-old woman with a history of breast cancer who was diagnosed with an endometroid adenocarcinoma of the left ovary FIGO Ia after 4 years of tamoxifen treatment [35]. McCluggage documented a case of a 50-year-old breast cancer survivor and tamoxifen use for 3 years, who developed an endometroid adenocarcinoma of the ovary [18]. Bese et al. described a case of a 74-year-old woman with a history of tamoxifen therapy and subtotal hysterectomy with bilateral salpingo-oophorectomy, who developed a supracervical pelvic mass 14 months after hysterectomy. Histopathological analysis revealed endocervical adenocarcinoma [36]. Bardi et al. reported a case of a 57-year-old woman with diagnosis of pelvic endometriosis, adenomatose hyperplasia and endometroid carcinoma G1 two years after hysterectomy and bilateral salpingo-oophorectomy with a history of tamoxifen use due to breast cancer [37]. Schlesinger and Silverberg reported a case of a 62-year- old with multiple arising tamoxifen-associated endometriotic foci and subsequent endometroid adenocarcinoma [10].
The preoperative diagnosis of postmenopausal endometriosis is challenging. Deep infiltrating endometriosis affects up to 20% of women with endometriosis and 1% of women of fertile age [38, 39]. For the postmenopausal life span, data regarding incidence of DIE are scarce. A thorough physical examination can reveal tenderness and palpable pelvic masses as manifestation of deep infiltrating endometriosis (DIE) and can lead to the diagnosis [2]. Nevertheless, the sensitivity of routine clinical examination to detect DIE, even performed by specialists, is limited, as only 73.5% of uterosacral ligament endometriosis, 50% of vaginal endometriosis and only 46% of intestinal endometriosis are detected by this procedure [40].
Ultrasound is the first-line approach for pathologies of the pelvis on the imaging work-up, both for pre- and postmenopausal women. Nisenblat et al. showed a sensitivity of 79% and a specificity of 94% [3] for detection of DIE on transvaginal ultrasound. Rectal sonography showed a sensitivity of 48% and a specificity of 44% for detection of endometriosis [3]. A nodular hypoechoic thickening may be suggestive for endometriosis, as in our case. Transvaginal color and power-Doppler sonography allow in vivo assessment of vascularity of suspect pelvic masses. Intense vascularization of deep infiltrating endometriosis might indicate an elevated activity of the endometriotic lesions, analogous to enhanced vascularity in highly active ovarian endometrioma [41]. Transvaginal ultrasound meets the criteria for a triage test in combination with patient history but is not recommended as replacement test for diagnostic surgery [3]. Surgery remains the gold standard for diagnosis, both for premenopausal and for postmenopausal women, even if the postmenopausal women might have more comorbidities and thus an elevated operative risk.
MRI is used as an additional imaging modality in complex cases with unclear risk/benefit ratio for surgery or in women with significant or relevant comorbidities [3, 42]. MRI is a noninvasive imaging method without the use of ionizing radiation which has an excellent soft tissue contrast. MRI is highly accurate in the evaluation of endometriosis with a sensitivity of 94 % and specificity of 77 % [3] as it can evaluate areas which are difficult to assess on ultrasound or laparoscopy making it possible to detect endometriotic lesions on multiple sides of the abdomen [43]. T2-weighted sequences without fat suppression are the best sequences for detecting pelvic endometriosis [44]. Endometriotic lesions show a low signal intensity on T2-weighted imaging and intermediate signal intensity on T1- weighted imaging, sometimes with high signal intensity foci due to hemorrhage in ectopic endometrial glands [42]. In contrast, polypoid endometriosis often shows high signal intensity on T2-weighted images [13] indicative of edematous tissue and a high proportion of endometrial glandular tissue. The signal intensity is dependent on quantity and age of hemorrhage and on the proportion between endometrial and stromal cells [44].
Furthermore, MRI can be useful to evaluate operative steps, perioperative risks and possible complications [3]. We propose a multidisciplinary approach to optimize preoperative planning. Computed tomography (CT) has a limited role in the evaluation of endometriosis due to its poor soft tissue contrast and provides therefore only minimal additional information [43].
There is no gold standard for surgical treatment of endometriosis in postmenopausal women. The first line therapy in postmenopausal patients with a pelvic mass resembling a malignant lesion on imaging is radical resection of the lesion to prove the diagnosis and prevent spreading in case of malignancy. Postmenopausal endometriosis, even without malignant transformation should be resected, because this may prevent subsequent malignant transformation, as endometriosis is acknowledged as a precursor lesion of several gynecologic neoplasms. Almost all patients with polypoid endometriosis in the series of Parker et al. received a complete removal of the lesions and of the affected organs [12].
Successful conservative therapy with aromatase inhibitors (AI) in postmenopausal women with recurrence of an aggressive form of endometriosis was first described in 1998 by Takayama K et al. (oral anastrozole 1mg/d, elemental calcium 1.5 g/d and Vit D 800 U/d) for nine months [22]. Within nine months, they reported a near-complete eradication of a 30 mm implant. AI can sufficiently block local aromatase in the endometrial stromal cells, resulting in a hypo-estrogenic state within the endometriotic lesion [22]. Moreover, aromatase inhibitors can block the aromatization from androgen into estrogen in the adipose tissue, which is markedly increased in adipose women even in the menopause, as the adrenal glands as well as the ovaries produce androgens even at an advanced age. Therefore, aromatase inhibitors are an appropriate therapy for symptomatic adipose women with an history of endometriosis, as they resolve the vicious circle of peripheral and local estrogen impact on endometriotic lesions. Conservative management of tamoxifen induced or reactivated endometriosis is histological diagnosis through biopsy followed by tamoxifen discontinuation or conversion to aromatase inhibitor therapy. The difficulty of conservative management is to obtain a representative probe to assure the benign diagnosis. Nevertheless, oral therapy with aromatase inhibitors could be an adequate therapy for women in the postmenopause diagnosed with endometriosis. Moreover, women with a history of endometriosis and need of endocrine therapy due to breast cancer, could be treated with AI instead of tamoxifen in the first line.
Conclusion
With this case report and review of literature we aim to raise the awareness of the condition of postmenopausal endometriosis as an extremely rare but potentially serious tamoxifen-related complication. Postmenopausal endometriosis requires a high index of suspicion. Tamoxifen may induce or unmask endometriosis in both pre- and postmenopausal women with no prior history of endometriosis [10]. In cases suspicious of malignancy, a histological analysis via biopsy or, if the suspect mass is inaccessible to biopsy, a complete resection of the lesion should be performed. Additionally, aromatase inhibitor treatment should be reconsidered in favor of a therapy with tamoxifen in patients with a history of endometriosis or in patients with disease exacerbation in the menopause.
The present case highlights the importance of broad differential diagnosis, a multidisciplinary approach and it underlines endometriosis not only as a disease of fertile women but as well as a condition of the postmenopausal woman.
Author Contribution
RS: Project development and manuscript writing, IB: Manuscript writing, ED: Ultrasound and MRI scan assessment, EK: Tissue analysis, MB: Tissue analysis, JW: Data collection and review, AP: Manuscript editing, KK: Data collection and review and AH: Project development.
Funding
No funding has been given for this study.
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Bulun SE. Endometriosis. N Engl J Med 360 (2009): 268-279.
- Gambone JC, Mittman BS, Munro MG et al. Consensus statement for the management of chronic pelvic pain and endometriosis: proceedings of an expert-panel consensus process. Fertil Steril 78 (2002): 961-972
- Nisenblat V, Bossuyt PM, Farquhar C et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2 (2016):
- Haas D, Chvatal R, Reichert B et al. Endometriosis: a premenopausal disease? Age pattern in 42,079 patients with endometriosis. Arch Gynecol Obstet 286 (2012): 667-670.
- Inceboz U. Endometriosis after menopause. Womens Health (Lond) 11 (2015): 711-715.
- Nikkanen V, Punnonen R. External endometriosis in 801 operated patients. Acta Obstet Gynecol Scand 63 (1984): 699-701
- Sasson IE, Taylor HS. Aromatase inhibitor for treatment of a recurrent abdominal wall endometrioma in a postmenopausal woman. Fertil Steril 92 (2009): 1170 e1171-1174.
- Fedele L, Bianchi S, Raffaelli R et al. Comparison of transdermal estradiol and tibolone for the treatment of oophorectomized women with deep residual endometriosis. Maturitas 32 (1999): 189-193.Rattanachaiyanont M, Tanmahasamut P, Angsuwatthana S et al. Hormonal replacement therapy in surgical menopause with underlying endometriosis. J Med Assoc Thai 86 (2003): 702-707
- Schlesinger C, Silverberg SG. Tamoxifen- associated polyps (basalomas) arising in multiple endometriotic foci: A case report and review of the literature. Gynecol Oncol 73 (1999): 305-311.
- Mostoufizadeh M, Scully RE. Malignant tumors arising in endometriosis. Clin Obstet Gynecol 23 (1980): 951-963
- Parker RL, Dadmanesh F, Young RH et al. Polypoid endometriosis: a clinicopathologic analysis of 24 cases and a review of the literature. Am J Surg Pathol 28 (2004): 285- 297
- Yamada Y, Miyamoto T, Horiuchi A et al. Polypoid endometriosis of the ovary mimicking ovarian carcinoma dissemination: a case report and literature review. J Obstet Gynaecol Res 40 (2014): 1426-1430.
- Jaegle WT, Barnett JC, Stralka BR et al. Polypoid endometriosis mimicking invasive cancer in an obese, postmenopausal tamoxifen user. Gynecol Oncol Rep 22 (2017): 105-107.
- Fisher B, Costantino JP, Wickerham DL et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90 (1998): 1371-1388
- MacNab MW, Tallarida RJ, Joseph R. An evaluation of tamoxifen as a partial agonist by classical receptor theory--an explanation of the dual action of tamoxifen. Eur J Pharmacol 103 (1984): 321-326.
- Chalas E, Costantino JP, Wickerham DL et al. Benign gynecologic conditions among participants in the Breast Cancer Prevention Trial. Am J Obstet Gynecol 192 (2005): 1230- 1237.
- McCluggage WG, Bryson C, Lamki H et al. Benign, borderline, and malignant endometrioid neoplasia arising in endometriosis in association with tamoxifen therapy. Int J Gynecol Pathol 19 (2000): 276- 279
- Morotti M, Remorgida V, Venturini PL et al. Endometriosis in menopause: a single institution experience. Arch Gynecol Obstet 286 (2012): 1571-1575.
- Ballard KD, Seaman HE, de Vries CS et al. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case- control study--Part 1. BJOG 115 (2008): 1382- 1391.
- Matorras R, Elorriaga MA, Pijoan JI et al. Recurrence of endometriosis in women with bilateral adnexectomy (with or without total hysterectomy) who received hormone replacement therapy. Fertil Steril 77 (2002): 303-308
- Takayama K, Zeitoun K, Gunby RT et al. Treatment of severe postmenopausal endometriosis with an aromatase inhibitor. Fertil Steril 69 (1998): 709-713
- Cao Y, Ye Q, Zhuang M et al. Ginsenoside Rg3 inhibits angiogenesis in a rat model of endometriosis through the VEGFR-2-mediated PI3K/Akt/mTOR signaling pathway. PLoS One 12 (2017):
- Taylor RN, Yu J, Torres PB et al. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod Sci 16 (2009): 140-146.
- Bourlev V, Volkov N, Pavlovitch S et al. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction 132 (2006): 501-509.
- Jordan VC. The secrets of selective estrogen receptor modulation: cell-specific coregulation. Cancer Cell 1 (2002): 215-217
- Muldoon TG. Steroid hormone receptor dynamics: The key to tissue responsiveness. Molecular mechanism of steroid hormone action 2011: 377-397
- Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti- oestrogen 4-hydroxytamoxifen. EMBO J 9 (1990): 2811-2818
- Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science 295 (2002): 2465-2468.
- Choi IH, Jin SY, Jeen YM et al. Tamoxifen- associated polypoid endometriosis mimicking an ovarian neoplasm. Obstet Gynecol Sci 58 (2015): 327-330.
- Kraft JK, Hughes T. Polypoid endometriosis and other benign gynaecological complications associated with Tamoxifen therapy-a case to illustrate features on magnetic resonance imaging. Clin Radiol 61 (2006): 198-201.
- Ogawa S, Kaku T, Amada S et al. Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecol Oncol 77 (2000): 298-304.
- Takeuchi M, Matsuzaki K, Bando Y et al. A case of polypoid endometriosis with malignant transformation. Abdom Radiol (NY) 41 (2016): 1699-1702.
- Cohen I, Altaras MM, Lew S et al. Ovarian endometrioid carcinoma and endometriosis developing in a postmenopausal breast cancer patient during tamoxifen therapy: a case report and review of the literature. Gynecol Oncol 55 (1994): 443-447.
- Okugawa K, Hirakawa T, Ogawa S et al. Ovarian endometrioid adenocarcinoma arising from an endometriotic cyst in a postmenopausal woman under tamoxifen therapy for breast cancer: a case report. Gynecol Oncol 87 (2002): 231-234
- Bese T, Simsek Y, Bese N et al. Extensive pelvic endometriosis with malignant change in tamoxifen-treated postmenopausal women. Int J Gynecol Cancer 13 (2003): 376-380
- Bardi M, Arnoldi E, Pizzocchero G et al. Endometrioid carcinoma in pelvic endometriosis in a postmenopausal woman with tamoxifen adjuvant therapy for breast cancer. A case report. Eur J Gynaecol Oncol 15 (1994): 393-395
- Cosma S, Salgarello M, Ceccaroni M et al. Accuracy of a new diagnostic tool in deep infiltrating endometriosis: Positron emission tomography-computed tomography with 16alpha-[18F]fluoro-17beta-estradiol. J Obstet Gynaecol Res 42 (2016): 1724-1733.
- Koninckx PR, Meuleman C, Demeyere S et al. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril 55 (1991): 759-765
- Dos Bispo AP, Ploger C, Loureiro AF et al. Assessment of pelvic floor muscles in women with deep endometriosis. Arch Gynecol Obstet 294 (2016): 519-523.
- Alcazar JL, Garcia-Manero M. Ovarian endometrioma vascularization in women with pelvic pain. Fertil Steril 87 (2007): 1271-1276.
- Bazot M, Darai E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril 108 (2017): 886-894.
- Hoyos LR, Johnson S, Puscheck E. Endometriosis and Imaging. Clin Obstet Gynecol 60 (2017): 503-516.
- Foti PV, Farina R, Palmucci S et al. Endometriosis: clinical features, MR imaging findings and pathologic correlation. Insights Imaging 9 (2018): 149-172.