Pleiotropic Antithrombotic Effects of Cardiovascular Drugs
Article Information
Alexander von Ehr, Christoph Bode, Ingo Hilgendorf*
Department of Cardiology and Angiology I, University Heart Center Freiburg-Bad Krozingen, Faculty of Medicine, University of Freiburg, Freiburg, Germany
*Corresponding Author: Ingo Hilgendorf, Department of Cardiology and Angiology I, University Heart Center Freiburg-Bad Krozingen, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Received: 15 October 2020; Accepted: 28 October 2020; Published: 20 November 2020
Citation: Alexander von Ehr, Christoph Bode, Ingo Hilgendorf. Pleiotropic Antithrombotic Effects of Cardiovascular Drugs. Cardiology and Cardiovascular Medicine 4 (2020): 702-714.
View / Download Pdf Share at FacebookAbstract
Cardiovascular drugs are cornerstones of treatment of various cardiovascular diseases in clinical and outpatient practice. Commonly used cardiovascular drugs such as statins and antihypertensive medication rank among the top 10 prescribed drugs in the United States. Although primarily administered to lower blood lipid, blood sugar or blood pressure levels, many of the drugs, administered to cardiovascular patients, have been reported to exert ancillary effects that contribute to their favorable risk-benefit profiles. The aim of this non-systematic review is to give an overview about the pleiotropic antithrombotic effects of cardiovascular drugs, and to discuss the potential underlying biochemical mechanisms and their clinical relevance.
Keywords
Pleiotropic; Antithrombotic effects; Cardiovascular drugs; Statins; Aantidiabetic drugs
Pleiotropic articles; Antithrombotic effects articles; Cardiovascular drugs articles; Statins articles; Aantidiabetic drugs articles
Article Details
1. Introduction
Antithrombotic drugs such as aspirin, clopidogrel, prasugrel, ticagrelor among others are standardly used in patients with acute or chronic coronary syndrome, peripheral artery disease or cerebral stroke. Regarding prototypical antiplatelet drugs, there is plenty of knowledge and awareness about the underlying mode of action and the potential complications, predominantly major bleedings. Atherosclerotic cardiovascular disease (ASCVD) however, is a multifactorial disorder. Hyperlipidemia, arterial hypertension and diabetes represent important and treatable cardiovascular risk factors. In addition, ASCVD is often accompanied by atrial fibrillation and chronic heart failure, that may require concomitant drug treatment. In this non-systematic review we want to summarize the pleiotropic antithrombotic effects and the underlying mechanism of the most common drugs used in cardiovascular patients, foremost statins, antihypertensive, antiarrhythmic and antidiabetic drugs.
2. Methods
A comprehensive literature search was conducted, mainly using PubMed database. The search terms were adjusted due to our clinical experience and included the terms: ”pleiotropic antithrombotic effects, antithrombotic effects or anti platelet effects” and ”statins, antihypertensive drugs, antiarrhythmic drugs, antiarrhythmics or antidiabetic drugs”. Moreover, we enlarged our findings upon articles that gave further insight in the underlying biochemical mechanisms of the pleiotropic antithrombotic effects. This non-systematical review makes no pretense to completeness and ought to provide an overview about the preexisting data.
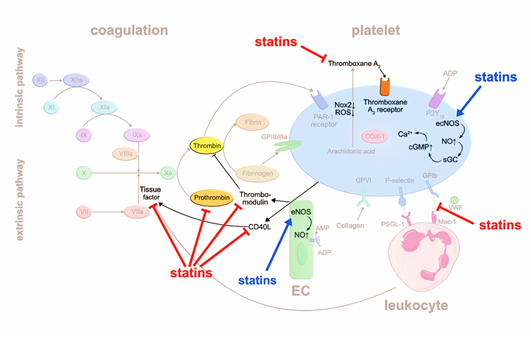
3. Statins
The JUPITER trial, published more than 10 years ago, was a primary prevention trial in mid-aged individuals without previously known cardiovascular disease and normal LDL-cholesterol levels of less than 130 mg/dL but an elevated high sensitive CRP above 2 mg/L. 17,802 participants were enrolled and randomized to rosuvastatin versus placebo. As a first result the occurrence of a first cardiovascular event, as a mixed endpoint of nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from cardiovascular causes, was significantly reduced by rosuvastatin when the study was terminated after a median follow-up of 1.9 years [1]. The marked reduction of the mixed endpoint was driven by fewer atherothrombotic complications. Interestingly, the study revealed that lowering of high sensitive CRP seemed to convey additional and partially independent benefits over lowering LDL-cholesterol alone [2]. Another analysis of the same study focused on venous thrombosis and pulmonary embolism and showed that rosuvastatin reduced venous thromboembolic events as well. The venous thrombotic events occurred independently of cardiovascular atherothrombotic complications, i.e. patients that did not experience a reduction in cardiovascular events still experienced less venous thromboembolic events [3]. The reduction of venous thromboembolic events and the effect of lowering high sensitive CRP suggest that there is more to statins than just cholesterol lowering. There are several potential mechanisms by which statins influence pathways of platelet aggregation and blood coagulation.
Statins were reported to interfere with coagulation factors. They can suppress the release of tissue factor (TF) from inflammatory and endothelial cells [4], which may otherwise trigger the extrinsic blood coagulation cascade by forming the active complex of TF and factor VIIa [5]. A randomized trial including 24 patients showed that treatment with simvastatin for eight weeks significantly decreases TF activity compared to dietary restriction alone [4]. Another study demonstrated that atorvastatin reduces the expression of CD40 ligand (CD40L) on platelets and circulating levels of soluble (s) CD40L, independent of its lipid lowering effects [6]. CD40L is a member of the tumor necrosis factor family, expressed by various cell types such as endothelial cells (EC), smooth muscle cells, and platelets, and is associated with proinflammatory and prothrombotic effects [7]. CD40L stimulates thrombus formation partly via CD40-mediated induction of TF generation. Thus, atorvastatin, by reducing CD40L expression, may inhibit TF release and activation of the extrinsic coagulation pathway. Atorvastatin and simvastatin were reported to reduce prothrombin (FII) and thrombin (FIIa) formation, respectively [6,8]. Additionally, statins stimulate thrombomodulin (TM) expression on EC that scavenges FIIa, resulting in inhibition of fibrin formation and platelet activation [9]. The authors suggested that statins inhibit protein prenylation of Rho GTPases, which then lose their inhibitory effect on TM generation in EC. Besides affecting fibrin formation, TM-bound FIIa may activate protein C which in turn degrades procoagulant factor Va (FVa) and factor VIIIa (FVIIIa). In summary, statins impact thrombus formation by interfering with the production and function of various coagulation factors.
Another possible mechanism that may be relevant for the antithrombotic effects of statins is the suppression of platelet-leukocyte interaction. For example, CD40 expressing immune cells can interact with CD40L-expressing platelets, whereby statins reduce CD40L expression on platelets7. In line with this hypothesis, patients admitted for acute coronary syndrome (ACS) demonstrate less circulating platelet-leukocyte aggregates in the first 24h after early administration of rosuvastatin [10]. Whether reduced platelet-leukocyte aggregates are linked to antithrombotic effects remains to be determined.
Intracellular oxidative stress activates platelets. Accordingly, deficiency in glutathione peroxidase-3, an enzyme that scavenges reactive oxygen species, leads to increased platelet-dependent thrombosis [11]. Statins may have an early antioxidant effect independent of cholesterol-lowering by downregulating NADPH oxidase 2 [12]. Thromboxane A2 (TxA2) activates platelets in an auto- and paracrine fashion, and is inactivated within seconds through hydrolyzation to thromboxane B2 (TxB2) [13]. Pignatelli et al. [12] measured reduced level of the TxB2 metabolite in blood as a surrogate for reduced TxA2 production, proposing that statins may inhibit phospholipase A2 upstream the arachidonic acid pathway.
Another mechanism by which statins are postulated to impair platelet aggregation is the increase in intraplatelet nitric oxide (NO). NO is produced by endothelial nitric oxide synthase (eNOS) in EC and platelets. NO activates the soluble guanylate cyclase (sGC) that increases cyclic guanosine monophosphate (cGMP) levels and intracellular calcium (Ca2+) levels, resulting in inhibition of platelet adhesion [14]. Daily intake of atorvastatin for three weeks stimulates eNOS production in human platelets [15]. Taken together, statins suppress platelet activation via multiple pathways (Figure 1).
4. Antihypertensive drugs
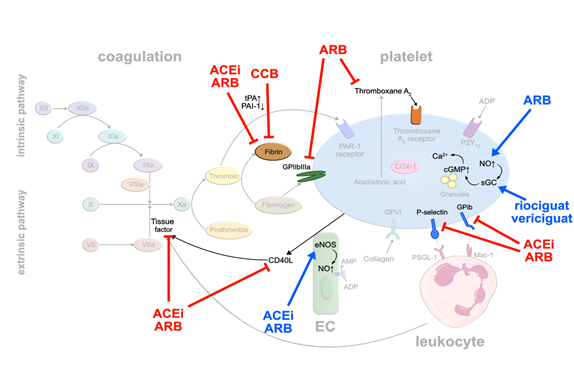
RAS inhibitors: Arterial hypertension represents one of the major cardiovascular risk factors. Several studies have demonstrated that some of the daily used antihypertensive drugs may exert pleiotropic antithrombotic effects. Initially, the focus was on drugs interfering with the renin-angiotensin system (RAS). Ridker et al. [16] injected angiotensin II into healthy volunteers to explore links between hypertension and thrombotic complications. He observed that increasing doses of angiotensin II elevated plasma levels of plasminogen activator inhibitor (PAI-1) while decreasing free tissue plasminogen activator (tPA). PAI-1 forms an inactivating complex with tPA, thereby preventing the generation of fibrinolytic plasmin [17]. Elevated levels of PAI-1 in blood have been linked to thromboembolic events [18,19]. Interestingly, angiotensin converting enzyme (ACE)-inhibitors and angiotensin II receptor (AT1)-blockers (ARB) led to an increase in tPA and a decrease in PAI-1 production [20]. Angiotensin II stimulates PAI-1 release from EC, counteracted by ACE-inhibitors and ARB [21]. In addition, ACE-inhibitors increase bradykinin levels that stimulate tPA release [22]. The inhibitory effect of ACE-inhibitors on PAI-1 activity has been confirmed by an analysis of a subgroup of 120 post-MI patients in the HEART study treated with ramipril. Paired blood samples from the index event and at 2 weeks follow-up were available. Compared with the placebo group, PAI-1 antigen and PAI-1 activity levels were significantly lower at day 14 in the group of ramipril-treated patients. In contrast, plasma tPA levels were not significantly different between the placebo-treated and ramipril-treated groups [23] (Figure 2).
Similar to statins, blocking of TF release from monocytes, and stimulation of endothe- lial and platelet NO release have been observed with ACE inhibitors and ARB, resulting in suppressed platelet activation [24]. Activation of eNOS was induced dose-dependently by losartan and valsartan, but not by candesartan suggesting that this mode of action may not apply to all ARB [25].
Some ARB seem to inhibit the thromboxane receptor, directly. Guerra-Cuesta et al. [26] described that losartan reduces the binding capacity of TxA2 to its receptor on platelets. This effect seems to be dose dependent, however, supraphysiological doses of losartan need to be applied. Clinical data of the randomized ‘Valsartan Inhibits Platelets‘-trial showed that valsartan exerts platelet inhibitory effects already after 5 weeks of treatment independent of the doses used (80, 160 or 320 mg). This observation was based on multiple platelet surface receptors being downregulated upon valsartan therapy, in particular GPIb and activated GP IIb/IIIa, P-selectin and CD40-ligand. The authors hypothesize that these changes result from valsartan modulating complex endothelial-platelet interactions, rather than affecting a single receptor or pathway [27] (Figure 2).
Calcium antagonists: Another frequently used class of antihypertensive drugs is dihydropyridine calcium antagonists. A systematic review including 22 studies showed in 2006 that calcium antagonists significantly enhanced fibrinolysis caused by an increase in tPA antigen level and a decrease in PAI-1 [28] (Figure 2). The reservation must be made, however, that fibrinolytic activity of dihydropyridine calcium antagonists is weak compared to the commonly used fibrinolytic drugs like urokinase or recombinant tissue plasminogen activator (rtPA), and is only functional in patients with impaired fibrinolysis but not in healthy individuals.
sGC stimulators: Of note, a novel drug class of direct, NO-independent sGC stimulators is making its way into heart failure treatment. Vericiguat was reported to reduce cardiovascular death and hospitalization for heart failure events in patients with heart failure with reduced ejection fraction (HFrEF) [29]. A similar drug, riociguat, currently marketed for treatment of pulmonary arterial hypertension, was evaluated for its effects on platelets. While direct stimulation of sGC by riociguat did inhibit activation of isolated platelets (Figure 2), it failed to do so in whole blood which was speculated to result from binding to serum proteins. Whether this applies to vericiguat remains to be tested [30].
5. Antiarrhythmic drugs
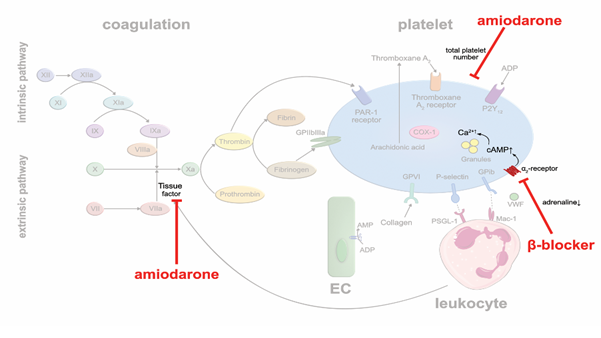
Class II agents: β-blockers are frequently used in coronary heart disease, heart failure and arrhythmia. By blocking beta adrenergic receptors, they lower heart rates and decrease blood pressure and myocardial oxygen demand. In addition, β-blockers may also affect platelet aggregation. A meta-analysis of 31 clinical studies in which a total of 454 patients were treated with β-blockers, and platelet aggregation was tested ex vivo, described a modest antithrombotic effect [31]. The lipophilic, nonselective β-blocker carvedilol showed a bigger impact on platelet aggregation than selective β-blockers. Yet, the underlying mechanism responsible for the antithrombotic effect of β-blockers is likely indirect. There are many more α2-receptors expressed on the platelet surface than β2-receptors. Probably, decreased generation of plasma catecholamines due to β-blocker therapy, leads to lower activation of α2-receptors, increased intraplatelet levels of cyclic adenosine monophosphate (cAMP) and increased Ca2+ availability, resulting in reduced platelet activation [32] (Figure 3). The hypothesis is supported by the observation that nonselective β-blockers are more efficient in reducing sympathetic activity and in suppressing platelet aggregation [33]. Nevertheless, the antithrombotic effects of β-blockers seem rather modest and of subordinate importance, clinically.
Class III agents: Amiodarone is a commonly used antiarrhythmic drug in patients with atrial fibrillation to establish and sustain sinus rhythm, or to suppress ventricular arrhythmias. A meta-analysis of 13 randomized studies in 6,553 patients with symptomatic, compensated, congestive heart failure with a mean left-ventricular ejection fraction of 31% or prior acute myocardial infarction showed that amiodarone reduced the incidence of sudden cardiac death (SCD) [34]. Whether these beneficial outcomes are solely based on preventing arrhythmias, or whether prevention of lethal thromboembolic complications may have contributed, is unknown. Beside its multiple unfavorable side effects, there is preclinical evidence that amiodarone prevents thrombus formation. Vascular TF activity and total platelet numbers were reduced in a mouse model of photochemical injury in vivo upon amiodarone treatment [35] (Figure 3). In human blood (ex vivo), dronedarone was shown to exert direct anticoagulant and antithrombotic effects [36]. These antithrombotic pleiotropic effects may contribute to the reduction of stroke and transient ischemic attacks in patients with persistent or paroxysmal atrial fibrillation under treatment with class III antiarrhythmic agents [37].
6. Antidiabetic drugs
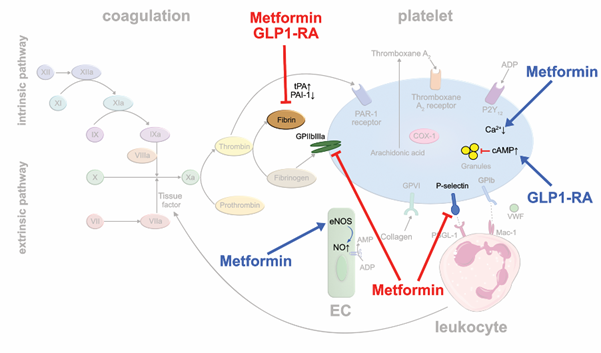
Biguanides: Metformin is the most widely used oral antidiabetic drug in type II diabetes mellitus (T2D). It is speculated that metformin may improve mortality by reducing diabetes-associated thrombotic events [38,39]. In metformin-treated rats, experimentally induced arterial and venous thrombus formation were significantly reduced, and the susceptibility to pulmonary embolism markedly decreased [40]. Mechanistically, reduced platelet prothrombinase activity, and αIIbβ3 and P-selectin expressions were made responsible. Moreover, metformin increases the phosphorylation of eNOS in EC resulting in an enhanced production of platelet-inhibiting NO [41]. Metformin reduces PAI-1 levels that are elevated in patients with T2D to balance out prothrombotic and fibrinolytic activity [42] (Figure 4). In the 10-year follow-up of the United Kingdom Prospective Diabetes Study (UKPDS), long-term use of metformin was associated with reductions in myocardial infarctions independent of glycemic control in obese diabetics [43]. Interestingly, metformin-treated mice did not show any bleeding complications and the bleeding time did not change significantly suggesting that metformin therapy might be a safe option of antithrombotic treatment [40].
GLP-1 receptor agonists and DPP-4 inhibitors: Novel antidiabetic drugs, such as glucagon-like peptide 1 receptor agonists (GLP-1RA), carry now a Class IA recommendation for treating patients with T2D and predominantly atherosclerotic cardiovascular disease according to the 2019 ESC guidelines [44]. GLP-1RA were shown to lower the rates of atherothrombotic complications such as myocardial infarction and stroke in T2D patients with coronary heart disease. Regarding potential antithrombotic actions of GLP-1RA, liraglutide reduced PAI-1 plasma levels in clinical trials in contrast to other antidiabetic drug classes [45] (Figure 4). Experimental studies showed that exenatide inhibits platelet aggregation in murine and human blood samples stimulated with thrombin, ADP or collagen [46]. Using a laser injury mouse model and intravital microscopy, the authors showed that exenatide treatment markedly reduced thrombus formation. In contrast, glucagon-like peptide 1 receptor-deficient mice featured increased platelet aggregability, suggesting that the drugs act via their canonical receptor. Binding of GLP-1 analogs to the GLP-1 receptor increases intraplatelet cAMP which controls a large number of distinct platelet functions and suppresses platelet activity [47]. Similar effects have been reported for dipeptidyl peptidase 4 (DPP-4) inhibitors such as linagliptin or sitagliptin, that inhibit the degradation of endogenous GLP-1, lending further support to the atheroprotective ancillary actions of these drugs beyond their effects on glycemic control [48,49] (Figure 4).
7. Concluding remarks
Cardiovascular disease and atherothrombotic complications are among the leading causes of morbidity and mortality in the world. Thus, cardiovascular drugs represent highly effective options to treat millions of patients. Beside their main mode of action, they exert ancillary effects such as pleiotropic antithrombotic effects. While not increasing the risk of bleeding, these pleiotropic antithrombotic effects may contribute to the overall cardiovascular benefits of statins and certain antihypertensive, antiarrhythmic and antidiabetic drugs. Clinicians and scientists may thus want to consider the ancillary effects in their daily practice and research.
Conflict of Interest:
The authors declare no relevant conflicts of interest.
Acknowledgments:
I.H. acknowledges funding support by the German Research Foundation (HI1573/2-1)
Author contribution:
A.E. and I.H. contributed equally to writing the manuscript. C.B. discussed and commented the research.
Abbreviations
ACE - Angiotensin converting enzyme
ACS - Acute coronary syndrome
ARB - AT1-blockers
ASCVD - Atherosclerotic cardiovascular disease
AT1 - Angiotensin II receptor
Ca2+ - Calcium
CD40L - CD40 ligand
cAMP - Cyclic adenosine monophosphate
cGMP - Cyclic guanosine monophosphate
DPP-4 - Dipeptidyl peptidase 4
EC - Endothelial cells
eNOS - Endothelial nitric oxide synthase
FII - Prothrombin
FIIa - Thrombin
FVa - Factor Va
FVIIIa - Factor VIIIa
GLP-1R - Glucagon-like peptide 1 receptor
GLP-1RA - Glucagon-like peptide 1 receptor agonists
HFrEF - Heart failure with reduced ejection fraction
NO - Nitric oxide
PAI-1 - Plasminogen activator inhibitor
RAS - Renin-angiotensin system
rtPA - Recombinant tissue plasminogen activator
SCD - Sudden cardiac death
sGC - Soluble guanylate cyclase
T2D - Type II diabetes mellitus
TF - Tissue factor
TM - Thrombomodulin
tPA - Tissue plasminogen activator
TxA2 - Thromboxane A2
TxB2 - Thromboxane B2
UKPDS - United Kingdom Prospective Diabetes Study
References
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England Journal of Medicine 359 (2008): 2195-2207.
- Ridker PM. The JUPITER trial: results, controversies, and implications for prevention. Circulation: Cardiovascular Quality and Outcomes 2 (2009): 279-285.
- Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. New England Journal of Medicine 360 (2009): 1851-1861.
- Ferro D, Basili S, Alessandri C, et al. Simvastatin reduces monocyte-tissue-factor expression type IIa hypercholesterolaemia. The Lancet 350 (1997): 1222.
- Rauch U, Nemerson Y. Circulating tissue factor and thrombosis. Current Opinion in Hematology 7 (2000): 273-277.
- Sanguigni V, Pignatelli P, Lenti L, et al. Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation 111 (2005): 412-419.
- Schönbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cellular and Molecular Life Sciences CMLS 58 (2001): 4-3.
- Undas A, Celinska-Lowenhoff M, Brummel-Ziedins KE, et al. Simvastatin given for 3 days can inhibit thrombin generation and activation of factor V and enhance factor Va inactivation in hypercholesterolemic patients. Arteriosclerosis, Thrombosis, and Vascular Biology 25 (2005): 1524-1525.
- Masamura K, Oida K, Kanehara H, et al. Pitavastatin-induced thrombomodulin expression by endothelial cells acts via inhibition of small G proteins of the Rho family. Arteriosclerosis, Thrombosis, and Vascular Biology 23 (2003): 512-517.
- Sexton TR, Wallace EL, Macaulay TE, et al. The effect of rosuvastatin on platelet-leukocyte interactions in the setting of acute coronary syndrome. Journal of the American College of Cardiology 65 (2015): 306-307.
- Jin RC, Mahoney CE, Anderson L, et al. Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation 123 (2011): 1963-1973.
- Pignatelli P, Carnevale R, Pastori D, et al. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 126 (2012): 92-103.
- Helgadóttir H, Ólafsson Í, Andersen K, et al. Stability of thromboxane in blood samples. Vascular Health and Risk Management 15 (2019): 143.
- Gkaliagkousi E, Ferro A. Nitric oxide signalling in the regulation of cardiovascular and platelet function. Front Biosci 16 (2011): 1873.
- Tannous M, Cheung R, Vignini A, et al. Atorvastatin increases ecNOS levels in human platelets of hyperlipidemic subjects. Thrombosis and Haemostasis 82 (1999): 1390-1394.
- Ridker PM, Gaboury CL, Conlin PR, et al. Stimulation of plasminogen activator inhibitor in vivo by infusion of angiotensin II. Evidence of a potential interaction between the renin-angiotensin system and fibrinolytic function. Circulation 87 (1993): 1969-1973.
- Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. British Journal of Haematology 157 (2012): 291-298.
- Hamsten A, Walldius G, Szamosi A, et al. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. The Lancet 330 (1987): 3-9.
- Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. New England Journal of Medicine 342 (2000): 1792-1801.
- Vaughan DE. Angiotensin and vascular fibrinolytic balance. American Journal of Hypertension 15 (2002): 3S-8S.
- Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. The Journal of Clinical Investigation 95 (1995): 995-1001.
- Brown NJ, Gainer JV, Stein CM, et al. Bradykinin stimulates tissue plasminogen activator release in human vasculature. Hypertension 33 (1999): 1431-1435.
- Vaughan DE, Rouleau JL, Ridker PM, et al. Effects of ramipril on plasma fibrinolytic balance in patients with acute anterior myocardial infarction. Circulation 96 (1997): 442-447.
- Napoleone E, Di Santo A, Camera M, et al. Angiotensin-converting enzyme inhibitors downregulate tissue factor synthesis in monocytes. Circulation Research 86 (2000): 139-143.
- Kalinowski L, Matys T, Chabielska E, et al. Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertension 40 (2002): 521-527.
- Guerra-Cuesta JI, Montón M, Rodríguez-Feo JA, et al. Effect of losartan on human platelet activation. Journal of Hypertension 17 (1999): 447-452.
- Serebruany VL, Pokov AN, Malinin AI, et al. Valsartan inhibits platelet activity at different doses in mild to moderate hypertensives: Valsartan Inhibits Platelets (VIP) trial. American Heart Journal 151 (2006): 92-99.
- Di Vergouwen M, Vermeulen M, De Haan RJ, et al. Dihydropyridine calcium antagonists increase fibrinolytic activity: a systematic review. Journal of Cerebral Blood Flow & Metabolism 27 (2007): 1293-1308.
- Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. New England Journal of Medicine 382 (2020): 1883-1893.
- Reiss C, Mindukshev I, Bischoff V, et al. The sGC stimulator riociguat inhibits platelet function in washed platelets but not in whole blood. British Journal of Pharmacology 172 (2015): 5199-5210.
- Bonten TN, Plaizier CE, Snoep JJ, et al. Effect of β-blockers on platelet aggregation: a systematic review and meta- British Journal of Clinical Pharmacology 78 (2014): 940-949.
- Anfossi G, Trovati M. Role of catecholamines in platelet function: pathophysiological and clinical significance. European Journal of Clinical Investigation 26 (1996): 353-370.
- Azevedo ER, Kubo T, Mak S, et al. Nonselective versus selective β-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation 104 (2001): 2194-2199.
- Connolly SJ. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: Meta-analysis of individual data from 6500 patients in randomised trials. Lancet 350 (1997): 1417-1424.
- Breitenstein A, Stampfli SF, Camici GG, et al. Amiodarone inhibits arterial thrombus formation and tissue factor translation. Arteriosclerosis, Thrombosis, and Vascular Biology 28 (2008): 2231-2238.
- Zafar MU, Santos-Gallego CG, Smith DA, et al. Dronedarone exerts anticoagulant and antiplatelet effects independently of its antiarrhythmic actions. Atherosclerosis 266 (2017): 81-86.
- Connolly SJ, Crijns HJGM, Torp-Pedersen C, et al. Analysis of stroke in ATHENA: A placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial . Circulation 120 (2009): 1174-1180.
- Roussel R, Travert F, Pasquet B, et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Archives of Internal Medicine 170 (2010): 1892-1899.
- Lu DY, Huang CC, Huang PH, et al. Metformin use in patients with type 2 diabetes mellitus is associated with reduced risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. BMC Cardiovascular Disorders 14 (2014): 187.
- Xin G, Wei Z, Ji C, et al. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release. Scientific Reports 6 (2016): 36222.
- Sambe T, Mason RP, Dawoud H, et al. Metformin treatment decreases nitroxidative stress, restores nitric oxide bioavailability and endothelial function beyond glucose control. Biomedicine & Pharmacotherapy 98 (2018): 149-156.
- Grant PJ. Beneficial effects of metformin on haemostasis and vascular function in man. Diabetes & Metabolism 29 (2003): 6S44-6S52.
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. New England Journal of Medicine 359 (2008): 1577-1589.
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). European Heart Journal 41 (2020): 407-477.
- Davidson MH. Cardiovascular effects of glucagonlike peptide–1 agonists. The American Journal of Cardiology 108 (2011): 33B-41B.
- Cameron-Vendrig A, Reheman A, Siraj MA, et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 65 (2016): 1714-1723.
- Raslan Z, Aburima A, Naseem KM. The spatiotemporal regulation of cAMP signaling in blood platelets—Old friends and new players. Frontiers in Pharmacology 6 (2015): 266.
- Steven S, Jurk K, Kopp M, et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. British Journal of Pharmacology 174 (2017): 1620-1632.
- Gupta AK, Verma AK, Kailashiya J, et al. Sitagliptin: anti-platelet effect in diabetes and healthy volunteers. Platelets 23 (2012): 565-570.
- Bäuml M, Hilgendorf I. Future antithrombotic therapies in cardiology. Hämostaseologie 38 (2018): 236-239.